Abstract
Objective
To examine associations of foot symptoms with self-reported and performance-based measures of physical function in a large, bi-racial, community-based sample of individuals ≥ 45 years old.
Methods
Data from 2,589 Johnston County participants (evaluated 1999–2004) were used in cross-sectional analyses. Presence of foot symptoms was defined as pain, aching, or stiffness of at least one foot on most days. Physical function was assessed by the Stanford Health Assessment Questionnaire (HAQ; 0, >0 but < 1, ≥ 1), timed 5 repeated chair stands (completion time <12 seconds (s), ≥ 12s, unable), and 8-foot walk time (<3.35s, ≥ 3.35 s). Separate multivariable logistic regression models examined associations between foot symptoms and physical function measures, controlling for age, race, gender, body mass index (BMI), radiographic knee osteoarthritis (OA), radiographic hip OA, knee symptoms, hip symptoms, and depressive symptoms. Interaction terms between each of the 3 physical function measures and each demographic and clinical characteristic were examined.
Results
The prevalence of foot symptoms was 37%. Participants with foot symptoms were more likely than those without symptoms to have higher HAQ scores (adjusted odds ratio [aOR]=1.79, 95% confidence interval [CI] 1.50–2.12). Among obese participants, those with foot symptoms had longer chair stand (aOR=1.38, 95% CI 1.04–1.87) and 8-foot walk times (aOR=1.61, 95% CI 1.21–2.15) than those without symptoms.
Conclusions
Foot symptoms were independently and significantly associated with 2 of 3 measures of poorer physical function. Interventions for foot symptoms may be important for helping patients prevent or deal with an existing decline in physical function.
Keywords: foot, pain, osteoarthritis
Introduction
Foot pain is common among older adults, affecting approximately 20–30% of community-dwelling adults aged 65 years and older. [1–3] An association between foot pain and decreased functional abilities has been demonstrated in several studies. [2–8] Most prior studies of foot symptoms (i.e., pain, aching, and stiffness) and functional abilities have focused on adults 62 years and older, [2, 3, 5, 6, 8] but this relationship has been explored less in middle-aged adults [9, 10] and important public health subsets, such as obese adults. Further, few studies have examined these associations controlling for the effects of osteoarthritis (OA). Identifying adults at higher risk of functional limitation related to foot symptoms earlier in the life course may aid in preventing or slowing functional loss. Additionally, prior investigations of the association between foot symptoms and disability have focused primarily on self-report or physical assessments of lower extremity function. [3, 5, 6, 8] However, both lower and upper extremity task performance may be affected by lower extremity symptoms, as illustrated by a prior study of knee pain and lower and upper extremity function. [11]
The purpose of the present study was to assess the association between foot symptoms and self-reported and performance-based measures of physical function in a large, bi-racial, community-based sample of individuals that includes younger individuals (45 years of age and older) than the populations usually studied. These associations were examined to determine whether they were independent of knee and hip radiographic OA and knee and hip symptoms, and whether these associations varied by age, race/ethnicity, gender, body mass index (BMI), and depressive symptoms.
Methods
Study Participants
This cross-sectional sample was composed of individuals enrolled in the Johnston County Osteoarthritis Project, an ongoing, community-based study of the occurrence of knee and hip OA in African American and Caucasian residents in a rural county in North Carolina. Details of this study have been reported previously. [12] Briefly, this study involved civilian, non-institutionalized adults aged 45 years and older who resided in six townships in Johnston County. Participants were recruited by probability sampling using 1990 census data, with over-sampling of African Americans. The sampling design consisted of selection of primary sampling units by stratified simple random sampling of streets, and households were counted on each street. Age, sex, ethnic group, and marital status of each household were collected, and age and ethnicity information was used to determine eligibility of each household. A total of 14,297 households were identified, and 4,866 were eligible. From May 1991 to December 1998, first home baseline interviews were completed by 3,690 participants (76% of the eligible 4,866), and 83% (3,068 participants) of those who completed the interview were examined at the local clinic.
The present analysis used data collected from individuals who had participated in the clinic visit arms of either the first follow-up study (n = 1,699) in 1999–2004, or the new enrollment study (n = 1,007) in 2003–4 (total study sample=2,706). The new enrollment aimed to enrich the sample for African Americans and younger individuals; thus, these participants were younger (mean age 59.6 vs. 66.0 years) and more likely to be African American (39.9% vs. 27.9%), as compared to participants of the first follow-up study.
Foot Symptoms
Participants completed an interviewer-administered questionnaire in which they answered “Yes” or “No,” separately for their right and left feet: "On most days, do you have pain, aching, or stiffness in your right/left foot?" Participants were considered to have foot symptoms if they answered affirmatively to the foot symptoms question for at least one foot.
Knee and Hip Symptoms
Participants were asked separately for left and right knees and left and right hips: “On most days do you have pain, aching or stiffness in your [left/right] [knee/hip]?” Participants were considered to have knee symptoms if they answered affirmatively to the knee symptoms question and to have hip symptoms if they answered affirmatively to the hip symptoms question.
Radiographic Assessment
All participants completed bilateral anteroposterior radiography of the knee in weight bearing. Women over 50 years of age and all men completed supine anteroposterior pelvic radiography. Radiographs were rated by a single musculoskeletal radiologist (JBR) using the Kellgren-Lawrence (K-L) radiographic atlas for overall knee and hip radiographic grades [13]. As previously described, interrater reliability (comparison of radiograph readings between JBR and another radiologist) and intrarater reliability (comparison of radiograph readings completed by JBR at two separate times) for the radiologist were high (weighted kappa for interrater reliability 0.9; kappa for intrarater reliability 0.9). [12] Radiographs were classified using the K-L scale as having radiographic OA if they had a grade of 2–4 (showing an osteophyte or having joint space narrowing). [14] Associations between foot pain and radiographic OA in areas other than the foot have been shown in previous studies. [5, 7]
Measurement of Upper- and Lower-Extremity Function
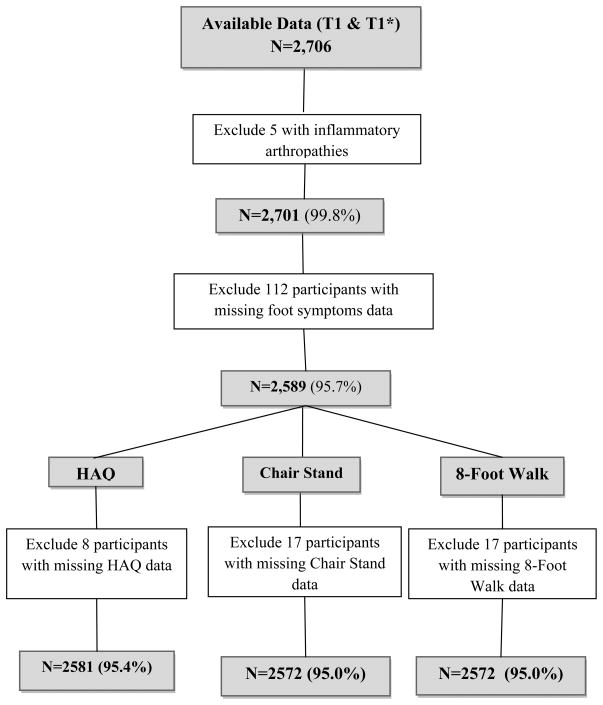
Self-reported functional status was assessed with the Stanford Health Assessment Questionnaire (HAQ) Disability Index. [15] Twenty individual functions covering 8 domains (dressing, arising, eating, walking, reaching, gripping, chores, and hygiene) were scored on a scale of 0–3(0=with no difficulty, 1=with little difficulty,2=only with much difficulty, and 3=unable to do). A score of 2 was assigned for an individual function if the participant needed an assistive device to perform that activity. The highest score of the individual functions in each domain determined the score for that domain. HAQ scores were calculated by averaging the scores of the 8 domains. HAQ scores were available for 2,581 participants with foot symptoms data (Figure 1).
Figure 1.
Description of Participants included in Analyses.
Performance-based functional status was assessed using timed5 chair stands and a timed 8-foot walk [16]. The chair stand test is considered an assessment of lower extremity strength, and the 8-foot walk assesses gait velocity, a component of walking functional ability [17]. Although most studies have assessed the validity of the chair stand test among elderly adults, one study of adults aged 55–70 years reported good validity for a chair stand test for assessing lower extremity strength when compared to a 1 repetition maximum leg press (Pearson r= .68, p < .05 [18]). Gait velocity has been examined across all ages of adults, and walking speed does decline with age [19]. Participants were asked to rise from a chair of standard height consecutively 5 times as quickly and comfortably as possible without using their arms while a study evaluator measured performance time with a stop watch. If a participant completed fewer than 5 stands, the performance was recorded as “unable”. If a participant completed 5 stands, the time of completion to the nearest tenth of a second was recorded. For the timed 8-foot walk, participants completed two timed trials of walking 8 feet at their usual walking pace and using walking aids if needed. Times were measured with a stop watch and recorded to the nearest tenth of a second for each trial. For analyses, the timed 8-foot walk was calculated as the average of the two trials.
Demographic and Clinical Characteristics
The following participant characteristics were examined as potential covariates in our analyses because they may be associated with foot symptoms and physical function: gender; self-reported race (African American or Caucasian); age (continuous variable in years); BMI at baseline (continuous variable calculated as weight in kilograms/height in meters squared), and depressive symptoms (reported using the Centers for Epidemiologic Studies-Depression Scale (CES-D)[20]). Menz and Morris[6] reported that female gender and higher BMI were each strongly associated with self-reported disabling foot pain. Height without shoes was measured in centimeters and weight was measured in kilograms using a balance beam scale.
Statistical Analysis
Cochran–Mantel–Haenszel statistics for categorical variables and one-way ANOVA testing of linear trend for continuous variables were used to compare all demographic and clinical characteristics (gender, race, age, BMI [continuous variable], knee radiographic OA, hip radiographic OA, knee symptoms, and hip symptoms, and CES-D Scale) across the three levels of the HAQ score. Three functional outcomes were considered: total HAQ score, timed 5 chair stands, timed 8-foot walk. The total HAQ score was categorized into 3 levels (0; greater than0 but less than 1; and >=1). The median value of each physical performance measure was used as a cutpoint to create categorical variables. Timed 5 chair stands was coded as a 3-level variable (<median completion time of 12 seconds (s) (referent), ≥ 12s, and unable). Timed 8-foot walk was coded as a dichotomous variable setting the cutpoint at the median completion time (<3.35s and >=3.35 s). Participants with missing exposure or outcome data were excluded from analyses (Figure 1).
Associations between foot symptoms and each physical function measure were examined in separate logistic regression models, adjusting first for age, gender, race, and BMI, then additionally for knee and hip symptoms and knee and hip radiographic OA, and finally for CES-D score. The proportional odds assumption was tested for the 3-level outcomes of the HAQ and the timed 5 chair stands. A proportional odds model assumes that the relationship between the independent variable of interest and the 3 levels of outcome is similar across successively more severe levels of the outcome. A single odds ratio was calculated to describe the comparison between: 1) the best physical function (referent) category (e.g., HAQ= 0) and the 2 other categories combined and 2) between the best physical function category plus the middle category (e.g., 0<HAQ<1) compared with the worst category (e.g., HAQ ≥ 1). A generalized logit model was used if the proportional odds assumption was violated. Therefore, a proportional odds model was used to analyze associations for the HAQ score outcome, and a generalized logit model was used for timed 5 chair stand analyses. A generalized logit model generates an odds ratio for each comparison between the referent (<12 s) and the middle physical function category (≥12 s) and between the referent and the worst physical function category (unable). Interactions between the foot symptom variable and race, gender, BMI (categorized as >30 [obese] and ≤ 30 kg/m2 [non-obese]for analyses of interactions), hip symptoms, and knee symptoms were tested jointly in each model. In models with significant interactions (p-values <0.05), appropriate sub-groups were examined, and odds ratios and 95% confidence intervals were calculated separately for each sub-group. The Hochberg-Benjamini method[21] was used to adjust for multiple comparisons for each outcome. All statistical computations were performed using SAS Version 9.1 software (SAS Institute, Cary, NC).
Results
Of the initial 2,706 participants clinically evaluated, all had data available for analyses, but 5 (0.2%) with evidence of an inflammatory arthropathy on knee or hip radiographs were excluded, leaving 2,701 for analysis (Figure 1). Foot symptoms data were available for 2,589 participants (95.9%; Figure 1), and chair stand and 8-foot walk data were available for 2,572 participants (99.3%) with foot symptoms data (Figure 1). Foot symptoms were reported by 37.0% (N=957) of participants. Knee symptoms were reported by 50.1% (N=1296) and hip symptoms by 38.3% (N=991). Radiographic knee OA was present in 34.8% (N=901) of participants, and 30.6% (N=793) had radiographic hip OA. The characteristics of most variables of the study population are shown in Table 1. Obesity, defined as a BMI > 30 kg/m2, was present in 46.6% (N=1206) of participants. Approximately 18.8% (N=484) were unable to perform the timed 5 chair stands, and the median completion times for 5 chair stands and the 8-foot walk were 12 seconds and 3.35 seconds, respectively. Compared to participants without foot symptoms, those with foot symptoms were more likely to have a higher BMI, be female, have radiographic knee OA, have knee or hip symptoms, report higher HAQ scores, have slower chair stand and 8-foot walk completion times, and report depressive symptoms (Table 1).
Table 1.
Characteristics of the Johnston County Osteoarthritis Project Study Sample, 1999–2004 (N=2589).
| Variables | Total N=2589 | Foot Symptoms | p-value* | ||
|---|---|---|---|---|---|
| Yes N=957 (37.0%) | No N=1632 (63.0%) | ||||
| Mean Age (±SD) (in years) | 63.2 (10.4) | 63.4 (10.4) | 63.3 (10.5) | 0.8264 | |
| Mean BMI (±SD) (kg/m2) | 30.6 (6.7) | 31.9 (7.0) | 29.8 (6.4) | <0.0001 | |
| African–American, n/N (%) | 843/2589 (32.6) | 297/957 (31.0) | 546/1632 (33.4) | 0.2083 | |
| Women, n/N (%) | 1686/2589 (65.1) | 670/957 (70.0) | 1016/1632 (62.3) | <0.0001 | |
| Radiographic hip OA, n/N (%) | 793/2589 (30.6) | 311/957 (32.5) | 482/1632 (29.5) | 0.1190 | |
| Radiographic knee OA, n/N (%) | 901/2589 (34.8) | 372/957 (38.9) | 529/1632 (32.4) | 0.0008 | |
| Hip symptoms, n/N (%) | 991/2588 (38.3) | 536/957 (56.0) | 455/1631 (27.9) | <0.0001 | |
| Knee symptoms, n/N (%) | 1296/2588 (50.1) | 669/957 (69.9) | 627/1631 (38.4) | <0.0001 | |
| HAQ, n/N (%): | >=1 | 571/2581 (22.1) | 344/952 (36.1) | 227/1629 (13.9) | <0.0001 |
| >0 but < 1 | 935/2581 (36.2) | 372/952 (39.1) | 563/1629 (34.6) | ||
| 0 | 1075/2581 (41.7) | 236/952 (24.8) | 839/1629 (51.5) | ||
| Chair Stand, n/N (%): unable | 484/2572 (18.8) | 271/949 (28.5) | 213/1623 (13.1) | <0.0001 | |
| >=12s | 875/2572 (34.0) | 329/949 (34.7) | 546/1623 (33.6) | ||
| <12 s | 1214/2572 (47.2) | 349/949 (36.8) | 865/1623 (53.3) | ||
| 8-footWalk, n/N (%): | >=3.35s | 1285/2572 (50.0) | 563/949 (59.3) | 722/1623 (44.5) | <0.0001 |
| <3.35 s | 1287/2572 (50.0) | 386/949 (40.7) | 901/1623 (55.5) | ||
| Mean CESD Score (±SD) | 6.6 (8.5) | 8.5 (9.5) | 5.5 (7.7) | <0.0001 | |
p-values determined by Cochran-Mantel-Haenszel statistics for categorical variables and one- way ANOVA for continuous variables.
Those with higher HAQ scores were more likely to be older adults, women, or African American, and to have a higher mean BMI, radiographic hipor knee OA, knee or hip symptoms, or depressive symptoms (Table 2). These same groups required longer times to complete the timed 5 chair stands and timed 8-foot walk (results not shown).
Table 2.
Characteristics of the sample according to Health Assessment Questionnaire (HAQ) categories (N=2581).
| Covariate | HAQ = 0 N=1,075 (41.7%) | 0<HAQ<1 N=935 (36.2%) | HAQ≥1 N=571 (22.1%) | p-value |
|---|---|---|---|---|
| Mean age (SD) | 62.7 (9.9) | 63.4 (10.4) | 64.5 (11.3) | <0.0001 |
| Age groups, n/N (%) | ||||
| 44 to <55 | 280/1075 (26.1) | 237/935 (25.3) | 135/571 (23.6) | |
| 55 to <65 | 371/1075 (34.5) | 303/935 (32.4) | 186/571 (32.6) | <0.0001 |
| 65 to <75 | 283/1075 (26.3) | 253/935 (27.1) | 126/571 (22.1) | |
| 75+ | 141/1075 (13.1) | 142/935 (15.2) | 124/571 (21.7) | |
| African–American, n/N (%) | 307/1075 (28.6) | 306/935 (32.7) | 228/571 (39.9) | <0.0001 |
| Women, n/N (%) | 612/1075 (56.9) | 622/935 (66.5) | 445/571 (77.9) | <0.0001 |
| Mean BMI (SD) | 29.2 (5.4) | 30.7 (6.7) | 33.1 (8.0) | <0.0001 |
| Radiographic hip OA, n/N (%) | 307/1075 (28.6) | 281/935 (30.0) | 202/571 (35.4) | 0.0037 |
| Radiographic knee OA, n/N (%) | 279/1075 (26.0) | 346/935 (37.0) | 274/571 (48.0) | <0.0001 |
| Hip symptoms, n/N (%) | 216/1075 (20.1) | 387/935 (41.4) | 382/571 (66.9) | <0.0001 |
| Knee symptoms, n/N (%) | 308/1075 (28.7) | 522/935 (55.8) | 460/571 (80.6) | <0.0001 |
| Median CES-D score (SD) | 3.8 (5.9) | 6.6 (7.7) | 12.1 (11.1) | <0.0001 |
Participants with foot symptoms were more likely than those without symptoms to have higher total HAQ scores after adjusting for the study covariates (adjusted odds ratio [aOR]=1.79, 95% confidence interval [CI] 1.50–2.12; Table 3). Statistically significant interactions were observed between foot symptoms and BMI for the logistic regression models of chair stand and 8-foot walk outcomes. Among obese (BMI >30 kg/m2) participants, those with foot symptoms required more time to complete the chair stands (unable vs. <12 s: aOR 2.12, 95% 1.46–3.07; ≥ 12s vs. <12s: aOR=1.38, 95% CI 1.04–1.87) and the 8-foot walk (aOR=1.61, 95% CI 1.21–2.15) than those without symptoms, a difference not seen for non-obese participants (unable vs. < 12 s chair stands: aOR 1.21, 95% CI 0.81–1.79; ≥ 12s vs. <12s chair stands: aOR=0.88, 95% CI 0.66–1.18; 8-foot walk: aOR=1.13, 95% CI 0.86–1.50; Table 4).
Table 3.
Adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for associations between foot symptoms and Health Assessment Questionnaire score categories.*
| Foot symptoms aOR (95%CI) | P-value | |
|---|---|---|
| Crude | 3.34 (2.86–3.90) | <0.001 |
| Adjusted 1† | 3.05 (2.61–3.57) | <0.001 |
| Adjusted 2‡ | 1.95 (1.65–2.31) | <0.001 |
| Adjusted 3§ | 1.79 (1.50–2.12) | <0.001 |
HAQ scores (0,>0 and <1, ≥ 1)
Adjusted 1: Adjusted for age, race, sex, BMI
Adjusted 2: Adjusted as in model 1 plus knee symptoms, hip symptoms, and radiographic knee and hip OA
Adjusted 3: Adjusted as in model 2 plus CES-D score.
Table 4.
Adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for associations between foot symptoms and functional performance measures, by BMI category.
| Foot symptoms* aOR (95%CI) | |||
|---|---|---|---|
| Outcomes | BMI† ≤ 30 kg/m2 (53.4%) | BMI† >30 kg/m2 (46.6%) | p-value for Interaction |
| Timed 5 chair stands | |||
| Unable vs. <12‡ seconds | 1.21 (0.81–1.79) | 2.12 (1.46–3.07) | 0.0110 |
| ≥12§ s vs. <12‡ seconds | 0.88 (0.66–1.18) | 1.38 (1.04–1.87) | 0.0290 |
| Timed 8-foot walk | |||
| ≥3.35 vs <3.35‡ seconds | 1.13 (0.86–1.50) | 1.61 (1.21–2.15) | 0.0314 |
Adjusted for age, sex, race/ethnicity, hip symptoms, knee symptoms, radiographic hip OA, radiographic knee OA and CES-D score.
BMI=body mass index.
Referent group.
Does not include individuals who were unable to complete timed 5 chair stand activity.
Discussion
Thirty-seven percent of participants in the present study reported foot symptoms, slightly higher than the proportion reported in studies of adults 65 years of age and older, signifying that foot symptoms are also a common condition in non-elderly adults. Participants with foot symptoms were more likely than those without foot symptoms to report greater difficulty with self-reported and performance-based physical function (as indicated by the 8 foot walk), even when controlling for important covariates. These results among participants aged 45 years and older are consistent with prior studies that have examined adults 62 years and older, demonstrating a persistent relationship association even at younger ages. [2, 3, 5, 6, 8]
The measure of self-reported physical function, the HAQ, includes questions about both upper and lower extremity function. A direct relationship between foot symptoms and lower extremity function can be easily appreciated. However, foot symptoms may affect ones overall confidence in his or her ability to complete physical tasks (functional self-efficacy). Thus, inclusion of a measure like the HAQ was important to attempt to reflect the functional self-efficacy associated with foot symptoms. Self-efficacy was not assessed specifically in the present study, but the results were independent of depressive symptoms, which have been shown to contribute to self-efficacy for general physical tasks. [22]
Foot symptoms may affect physical function by contributing to altered gait patterns, ultimately reducing gait speed. This may explain the slower completion times noted among participants with foot symptoms during the 8-foot walk. In general, no association was observed between foot symptoms and timed chair stands comparing ≥ 12 to < 12 seconds. However, the presence of obesity positively modified this association; obese participants (BMI >30 kg/m2) with foot symptoms required more time to complete the chair stand task than those without symptoms, but this association was not observed among non-obese participants with and without foot symptoms. Thus, weight reduction may assist in diminishing the impact of foot symptoms on some functional activities.
Strengths of this study include that it is community-based, consists of African American and Caucasian men and women, and includes radiographic OA data and symptoms data of the knee and hip. Additionally, this is the largest study to date that includes assessment of foot symptoms and self-reported and physical function. One limitation of the present study is that conditions contributing to foot symptoms in this population are not known. Foot radiographs were not obtained, but it is unlikely that participants had arthritic and rheumatic conditions other than OA because participants with inflammatory conditions on knee or hip radiographs were excluded. Other conditions that may have contributed to foot symptoms, such as calluses, corns, hallux deformities, toe deformities, pes planus, heel spurs, plantar fasciitis, and neuropathy, were not included in these analyses, but will be the topic of future research. A comprehensive physical assessment of the foot may help identify musculoskeletal origins of foot symptoms in this population, but previous studies have demonstrated that some foot disorders, such as hallux valgus and toe deformities and especially if mild disease, may not be associated with foot symptoms or functional limitations. [5, 23] Another possible limitation is that results for women are only generalizable to women over the age of 50 because hip radiographs were obtained only for women who were at least 50 years of age due to radiation concerns with reproductive health. However, in contrast to prior comparable studies, [2, 5] the present study included middle-aged adults (at least 45 years of age). Additionally, whether the foot symptoms were acute or chronic in this population is not known because the duration of foot symptoms was not collected during study interview. Furthermore, a measure of self-reported physical function that was specific to the foot was not collected at baseline. Finally, due to the cross-sectional study design, a temporal relationship between foot symptoms and physical function cannot be inferred. A longitudinal analysis would assist in determining the possible contribution of foot symptoms to functional decline.
In summary, foot symptoms play an important role in poor physical function, independent of knee and hip symptoms and OA. Interventions for foot symptoms, including weight loss, foot orthotics, foot care strategies, and patient education on proper footwear, may be important for helping patients prevent or manage an existing decline in perceived and performance-based functional abilities. Screening strategies may be helpful in identifying patients with foot symptoms who are at risk of the development or progression of functional decline.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Funding was made possible in part by the Centers for Disease Control and Prevention/Association of Schools of Public Health S043 and S3486.
Funding: Jordan/Renner: Centers for Disease Control and Prevention/Association of Schools of Public Health S043 and S3486, Multipurpose Arthritis and Musculoskeletal Diseases Center grant 5-P60-AR-30701 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases; Golightly: Arthritis and Immunology T-32 Training grant AR-07416 from the National Institute of Health
Footnotes
Competing Interests: The authors report no conflicts of interest in relation to this work.
References
- 1.Dunn JE, Link CL, Felson DT, Crincoli MG, Keysor JJ, McKinlay JB. Prevalence of foot and ankle conditions in a multiethnic community sample of older adults. Am J Epidemiol. 2004;159:491–8. doi: 10.1093/aje/kwh071. [DOI] [PubMed] [Google Scholar]
- 2.Benvenuti F, Ferrucci L, Guralnik JM, Gangemi S, Baroni A. Foot pain and disability in older persons: an epidemiologic survey. J Am Geriatri Soc. 1995;43:479–84. doi: 10.1111/j.1532-5415.1995.tb06092.x. [DOI] [PubMed] [Google Scholar]
- 3.Menz HB, Tiedemann A, Kwan MMS, Plumb K, Lord SR. Foot pain in community-dwelling older people: an evaluation of the Manchester Foot Pain and Disability Index. Rheumatology. 2006;45:863–7. doi: 10.1093/rheumatology/kel002. [DOI] [PubMed] [Google Scholar]
- 4.Bowling A, Grundy E. Activities of daily living: changes in functional ability in three samples of elderly and very elderly people. Age Ageing. 1997;26:107–14. doi: 10.1093/ageing/26.2.107. [DOI] [PubMed] [Google Scholar]
- 5.Leveille SG, Guralnik JM, Ferrucci L, Hirsch R, Simonsick E, Hochberg MC. Foot pain and disability in older women. Am J Epidemiol. 1998;148:657–665. doi: 10.1093/aje/148.7.657. [DOI] [PubMed] [Google Scholar]
- 6.Menz HB, Morris ME. Determinants of disabling foot pain in retirement village residents. J Am Podiatr Med Assoc. 2005;95:573–579. doi: 10.7547/0950573. [DOI] [PubMed] [Google Scholar]
- 7.Gorter KJ, Kuyvenhoven MM, DeMelker RA. Nontraumatic foot complaints in older people: a population-based survery of risk factors, mobility, and well-being. J Am Podiatr Med Assoc. 2000;90:397–402. doi: 10.7547/87507315-90-8-397. [DOI] [PubMed] [Google Scholar]
- 8.Keysor JJ, Dunn JE, Link CL, Badlissi F, Felson DT. Are foot disorders associated with functional limitation and disability among community-dwelling older adults? J Aging Health. 2005;17:734–752. doi: 10.1177/0898264305280998. [DOI] [PubMed] [Google Scholar]
- 9.Hill CL, Gill TK, Menz HB, Taylor AW. Prevalence and correlates of foot pain in a population-based study: the North West Adelaide health study. J Foot Ankle Res. 2008;1:2. doi: 10.1186/1757-1146-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrow AP, Silman AJ, Macfarlane GJ. The Chesire Foot Pain and Disability Survey: a population survey assessing prevalence and associations. Pain. 2004;110:378–384. doi: 10.1016/j.pain.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Jordan JM, Luta G, Renner J, Dragomir A, Hochberg M, Fryer J. Knee pain and knee osteoarthritis severity in self-reported task specific disability: the Johnston County Osteoarthritis Project. J Rheumatol. 1997;24:1344–9. [PubMed] [Google Scholar]
- 12.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis Care Res. 1995;8:242–250. doi: 10.1002/art.1790080407. [DOI] [PubMed] [Google Scholar]
- 13.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172–180. [PubMed] [Google Scholar]
- 14.Kellgren JH, Lawrence JS. The epidemiology of chronic rheumatism, atlas of standard radiographs. Oxford: Blackwell Scientific; 1963. [Google Scholar]
- 15.Fries JF, Spitz PW, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Simonsick EM, Ferucci L, Glynn RJ, Berkman LG, Blazer DG. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 17.Hayes KW, Johnson ME. Measures of adult general performance tests. Arthritis Rheum. 2003;49(5S):S28–S42. [Google Scholar]
- 18.Ritchie C, Trost SG, Brown W, Armit C. Reliability and validity of physical fitness field tests for adults aged 55 to 70 years. J Sci Med Sport. 2005;8(1):61–70. doi: 10.1016/s1440-2440(05)80025-8. [DOI] [PubMed] [Google Scholar]
- 19.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing. 1997;26:15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 20.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 21.Hochberg Y, Benjamini Y. More power procedures for multiple significance testing. Stat Med. 1990;9(7):811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 22.Maly MR, Costigan PA, Olney SJ. Determinants of self efficacy for physical tasks in people with knee osteoarthritis. Arthritis Rheum. 2006;55:94–101. doi: 10.1002/art.21701. [DOI] [PubMed] [Google Scholar]
- 23.Badlissi F, Dunn JE, Link CL, Keysor JJ, McKinlay JB, Felson DT. Foot musculoskeletal disorders, pain, and foot-related functional limitation in older persons. JAGS. 2005;53:1029–1033. doi: 10.1111/j.1532-5415.2005.53315.x. [DOI] [PubMed] [Google Scholar]