Abstract
Objectives
Our primary goals were to determine whether pre-existing fear of pain and pain sensitivity contributed to post-exercise pain intensity.
Methods
Delayed onset muscle pain was induced in the trunk extensors of 60 healthy volunteers using an exercise paradigm. Levels of fear of pain and experimental pain sensitivity were measured before exercise. Pain intensity in the low back was collected at 24 and 48 hours post-exercise. Participants were grouped based on pain intensity. Group membership was used as the dependent variable in separate regression models for 24 and 48 hours. Predictor variables included fear, pain sensitivity, torque lost during the exercise protocol, and demographic variables.
Results
The final models predicting whether a participant reported clinically meaningful pain intensity at 24 hours only included baseline fear of pain at each level of pain intensity tested. The final model at 48 hours included average baseline pain sensitivity and the loss of muscle performance during the exercise protocol for one level of pain intensity tested (greater than 35mm out of 100).
Discussion
Combined, these findings suggest that the initial reports of pain after injury maybe more strongly influenced by fear while the inflammatory process and pain sensitivity may play a larger role for later pain intensity reports.
Keywords: quantitative sensory testing, fear-avoidance model, DOMS
Introduction
Current literature suggests that there is a potentially complex interaction of factors that contribute to chronic LBP. Psychological factors prolong recovery and predict disability for patients with LBP [1]. Cognitive-behavioral models, such as the fear-avoidance model of exaggerated pain perception [2, 3] help explain the development of chronic disability from an episode of acute LBP. In the fear-avoidance model, an individual has elevated fear-avoidance beliefs because of prior pain experience, present anxiety level, pain behavior, and certain personality traits. These beliefs are suggested to lead to avoidance behavior which in turn may result in chronic disability and exaggerated pain perception [2].
Factors such as pain sensitivity are also associated with chronic musculoskeletal pain conditions. Elevated pain sensitivity has been found in patients with chronic conditions such as fibromyalgia syndrome [4-6], temporomandibular disorders [7], and pelvic floor disorders. Additionally, Clauw et al. 1999 observed an association between increased pain sensitivity, lower self-report of physical function and higher pain intensity for individuals with chronic LBP, and George et al. (2006) observed that thermal pain sensitivity uniquely contributed to variance in self-reported disability from chronic LBP [8].
Both the psychological factors and those related to pain sensitivity have been identified in patients with chronic musculoskeletal conditions. Less clear is whether these factors are present before the injury or develop in response to the injury. One method of retrieving this information is to collect data from a large cohort of healthy participants and follow these individuals until some develop chronic pain. These studies are difficult to do, so an alternative methodology is to induce pain in healthy participants. Models of endogenous muscle pain are particularly useful because they mimic musculoskeletal pain, the most common form of chronic pain [9, 10].
In this study, we examined the transition from a pain free state to a state of acute low back pain in participants using an experimental pain model. Experimental pain models allow control of the type, intensity, frequency and duration of the pain stimulus, as well as the area where pain is experienced.[11] These models can be generated using exogenous methods such as electrical, mechanical, and chemical stimuli. [12] Of more interest in the current study, however, are models using endogenous methods of developing muscle pain. Models of muscle pain are particularly important because musculoskeletal pain is the most common form of recurrent pain [9, 10] and the second most common reason for restricted activity and the consumption of medication.[9, 10, 13] For the current study we chose to use an exercise protocol generating delayed onset muscle soreness in the posterior trunk muscles.
The first goal was to determine whether pre-existing levels of fear (before the induction of pain) predicted pain after exercise. We hypothesized that, consistent with the fear-avoidance model, pre-existing fear of pain will amplify the pain response to muscle injury or insult resulting in increased pain and disability. This hypothesis is based on previous work that indicates that fear of pain is predictive of reported clinical pain and evoked pain after exercise at the shoulder,[14] but has not been previously tested in a model of exercise-induced low back pain. The second goal was to determine whether pain sensitivity from quantitative sensory testing (QST) responses (before induction of pain) predicted self report of pain following DOMS. We hypothesized that participants with elevated pain sensitivity would have higher reports of pain intensity after exercise.
Materials and Methods
Participants
60 healthy volunteers were recruited from the general population of Gainesville, Fl using fliers posted in area restaurants. All participants read and signed an informed consent form approved by the University Institutional Review Board. Participants were excluded if they had participated, within the past 6 months, in a conditioning program that included strengthening exercises targeted to muscles of the posterior trunk including the long erector spinae, deep trunk muscles such as multifidus, or if strengthening exercises for latissimus dorsi or gluteus maximus were performed.
Additionally participants were excluded if they met any of the following criteria: any current back pain or within the past 6 months, any chronic medical conditions that may affect pain perception (e.g., diabetes, high blood pressure, fibromyalgia, headaches), kidney dysfunction, muscle damage, or major psychiatric disorder, history of previous injury including surgery to the lumbar spine, renal malfunction, cardiac condition, high blood pressure, osteoporosis, or liver dysfunction.
In addition, subjects were advised that they would be withdrawn from the study for performance of any intervention for symptoms induced by exercise before the termination of their participation in the protocol; for example, cryotherapy, massage, or taking anti-inflammatory medications.
Procedures
After the informed consent process was finished, study participants completed a demographic survey and questionnaires.
Pain Intensity
Pain intensity in the back 24 and 48 hours after exercise was measured using a self-report 100mm visual analog scale (VAS) anchored at one end with ‘none’ and at the other with ‘worst pain imaginable.’ Participants rated their pain intensity by placing a mark along the line. A previous study has indicated that the VAS is a valid ratio measure for pain intensity.[15] In this study we attempted to replicate clinical rehabilitation practices for patients with low back pain regarding collecting information about any pain that they were experiencing. To that end, we did not provide specific instruction to participants other than the instructions to rate the ‘pain intensity now’ that were written on the visual analogue scale that we used.
Fear
The Fear of Pain Questionnaire (FPQ) is a 30-item, 5-point rating scale, with a range from 30 to 150. The FPQ was developed to measure fear about specific situations that may cause pain. We use the total score for the FPQ, as we were most interested in measuring participants' general fear of pain [16]. We chose to measure this psychological factor given the reported influence on pain intensity in both clinical and experimental models of pain [17], [18].
Experimental Pain Sensitivity
We used quantitative sensory testing protocol using thermal stimuli to determine experimental pain sensitivity. We chose thermal QST because thermal stimuli is sensitive to A-delta fiber and C-fiber mediated pain perception allowing us to estimate general pain sensitivity using pain responses to single thermal pulses and examine the plasticity of this pain sensitivity by measuring temporal sensory summation of the ratings of c-fiber mediated responses.[4, 19, 20]. TSS is thought to be a behavioral measure of wind-up, which is a specific, reversible, element of central sensitization.
All thermal stimuli were delivered to the skin of participants using a computer-controlled Medoc Neurosensory Analyzer (TSA-2001, Ramat Yishai, Israel). We had both a male and female examiner present during testing to account for sex and/or gender influence on pain reporting [21].
Before each testing session, participants underwent a practice session. During this session participants experienced the temperatures to which they were to be exposed. Participants practiced using the rating scale to rate the intensity of the first pain experienced in response to each stimulus. In order to standardize the scaling instructions and to clarify the distinction between the sensory intensity and affective dimensions, a standardized instructional set was used for all participants during every exposure to the thermal stimuli. The scale instructions were repeated for every set of ratings within each session [22].
First pain
In this current study, thermal stimuli of 5 seconds duration were applied to the posterior surface of the upper calf below the popliteal fossa, with the subject in a sitting position. The participants experienced a sequence of four thermal pulses that included 45 °C, 47 °C, 49 °C, or 51°C presented randomly. Stimulation sites were varied to prevent carryover effects due to local sensitization. Participants were cued to provide a rating of any pain experienced immediately after the peak of each thermal pulse using a scale ranging from 0 (no pain) to 100 (worst pain imaginable). These response ratings are believed to be primarily A-delta fiber mediated[23],[24] and were categorized as first pain responses. This procedure was performed twice. The interstimulus interval was at least 60 seconds to avoid carryover effects from one stimulus to another. Summation of responses occurs at frequencies faster than 0.33 Hz,[25] and prior work indicates that painful after sensations that occur after thermal testing have dissipated approximately 30 to 60 seconds after testing.[5] Temperature levels were monitored by a contactor-contained thermistor, and returned to a preset baseline of 35°C by active cooling at a rate of 10°C.sec-1. Average pain sensitivity (AvP) was represented by the average of the ratings at each of the temperatures assessed [26].
Temporal sensory summation
For this study, a train of 10 heat pulses peaking at 49°C was applied at a frequency of approximately 0.5Hz to the posterior surface of the upper calf below the popliteal fossa. All stimulation sites were varied to prevent carryover effects due to local sensitization. Temperature levels were monitored by a contactor-contained thermistor, and returned to a preset baseline by active cooling at a rate of 10°C.sec-1. The participants were asked to rate the magnitude of their second pain sensation following each heat pulse. This increase in the delayed or second pain intensity rating that occurs from early to later inputs is referred to as temporal sensory summation (TSS). The phenomenon is believed to be primarily C-fiber mediated.[25] We used a simple slope calculation as our measure of TSS.
LBP induction exercise protocol
Prior to exercise all participants completed a submaximal effort warm-up session consisting of riding the stationary bicycle at a speed of 50-60 RPM and 1 Kp of resistance and static passive stretching of the lower extremities. Each participant performed an isometric (static) test of total torque of the trunk extensor muscles through their available trunk flexion range of motion (ROM) using a MedX lumbar extension exercise machine following the standardized protocol [27]. The repeatability of isometric torque production is well-established in participants without pain [27] and groups of patients with LBP [28]. Participants were seated in the MedX machine and the stabilizing straps attached across the pelvis and knees. The participant was moved through the range of motion of the machine in lumbar flexion and extension to determine their available range. The device was locked into place in maximal flexion and the subject instructed to build up force gradually against a pad in contact with the mid and lower back. The torque generated by the participant was displayed graphically on the data collection computer. Once peak effort was observed by the research assistant, the participant was instructed to relax, the device released and the subject returned to an upright position for at least 10 seconds. Isometric testing was administered twelve times in positions that ranged from the participant's maximum available trunk extension to maximal trunk flexion. The isometric torque collected at each test angle was summed to give measure of total torque.
After baseline total torque was recorded, participants performed bouts of dynamic exercise to the point of volitional fatigue. To perform the dynamic fatiguing exercise bout, the participants were seated and restrained in a MedX lumbar extension exercise machine. Participants performed as many repetitions as possible using a weight load equal to approximately 80% of the peak torque measured during the isometric test. Each repetition was performed through the full available ROM and the participants were encouraged to perform the lifting portion (concentric) in two seconds and the lowering (eccentric) in seven seconds. Repetitions continued until the patient reported being unable to move through a full range of motion (volitional fatigue). Once this occurred, the isometric torque test was performed again. If the total torque measured during the repeat isometric test was 50% or less of the baseline total torque, the protocol was complete. If this didn't occur, the exercise bout was repeated. Participants repeated this sequence of dynamic exercise and isometric testing until total measured torque decreased to 50% of the baseline measurement. Participants were instructed not to initiate any medication in the next 48 hours, or apply any intervention, such as ice or heating pacs to the lumbar spine.
Average pain sensitivity (AvP) was calculated using the average of the ratings of first pain at each of the temperatures assessed. The loss of muscle performance resulting from the exercise bout was expressed as a percentage calculated by dividing the change in torque from pre to post exercise by the torque measured before exercise.
Statistical Analysis
We planned to use multiple linear regression modeling to examine the contributions of the measured baseline variables to pain intensity. The first step was to examine all the variables for distribution. Our primary outcome variables of interest were pain intensity at 24 and 48 hours. When data were assessed graphically, both variables were noted to be heavily right skewed. Boxcox power transformations suggested a power of 0.2 to assist in transformation of the pain intensity; however, the data remained non-normally distributed after the transformation.
This was also the case for the residuals calculated in a predicted regression models using complete models (age, sex, BMI, FPQ, torque loss, AvP and TSS). In the next step we began to calculate the linear associations between pain intensity and the first two continuous predictors we had planned to use (age and BMI). No such associations were noted; therefore, given violations to at least two of the modeling assumptions for multiple linear regression, we chose to use logistic regression modeling. Also, given the potential for unbalanced groups we chose regression modeling using exact logistic regression over maximum likelihood techniques for estimating the parameters of the model.
Pain intensity ratings were dichotomized into ‘meaningful pain’ and ‘low pain’ groups for both ratings at 24 and 48 hours. Models were built using three different values taken from the literature as a threshold for meaningful pain: 1) greater than 10mm[29], 2) greater than 20mm (twice the error of measurement for the VAS), and 3) pain intensity of 35mm or greater (smallest detectable change in pain described by patients with acute low back pain [30]). The dichotomized variables were used as the outcome variables in subsequent analyses.
We examined the following variables as predictors: TSS and AvP (measures of pain sensitivity), and fear of pain. Additionally, body mass index (BMI), age and sex were added to control for anthropometry of the participants and isometric torque loss experienced by each participant was included to represent the potential amount of muscle change that occurred in response to the exercise protocol. Outliers were assessed using standardized Pearson residuals. Variables with residuals greater than 3 were deleted listwise and the model recalculated. Automatic variable selection using backward elimination was used to choose the parsimonious model. The linearity of the relationship among the response and predictor variables was assessed graphically using the lowess procedure. Fit of the model was examined by determining actual probabilities for each level of the continuous predictor variables and comparing these to the predicted probabilities.
Results
42 participants indicated that they had not performed any type of regular exercise (more than once a week) in the past 6 months. Six indicated participating in weight-training (no training of the trunk extensors or squats), and twelve indicated that they performed aerobic exercise (running, walking, or bicycling). All participants completed the exercise protocol used to induce DOMS as well as the thermal quantitative testing protocol at each testing session. No adverse events were reported and no participants were withdrawn for using interventions for their pain.
Pain intensity at 24 hours
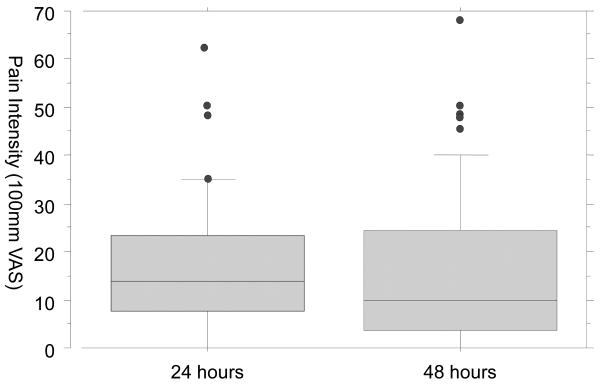
Pain intensity at 24 hours ranged from 0 to 63mm on the VAS. The number of participants reporting pain intensity greater than a specific threshold is summarized in Table 2. Figure 1 plots the distribution of pain intensity reports at 24 hours. No outliers were deleted. The final models predicting whether a participant reported clinically meaningful pain intensity at 24 hours only included baseline fear of pain. The final models are summarized in Table 2.
Table 2.
Parsimonious regression models predicting that pain is greater than a given threshold.
| Time | Threshold | N (pain) | Parameter | Estimate | Odds ratio | 95%CI | Probability > χ2 |
|---|---|---|---|---|---|---|---|
| 24 hours | 10mm | 41 | Fear of pain | 0.031 | 1.032 | 1.003, 1.061 | 0.031 |
| 20mm | 17 | Fear of pain | 0.024 | 1.024 | 1.001, 1.055 | 0.044 | |
| 35mm | 7 | Fear of pain | 0.041 | 1.042 | 1.001, 1.079 | 0.029 | |
| 48 hours | 10mm | 29 | - | - | - | - | - |
| 20mm | 17 | - | - | - | - | - | |
| 35mm | 8 | Average Pain Sensitivity | 0.062 | 1.064 | 1.005, 1.080 | 0.030 | |
| Loss of Torque | 0.133 | 1.142 | 1.106, 1.191 | 0.019 |
Average pain intensity: the average rating of pain reported during quantitative sensory testing.
Loss of torque: isometric torque deficit measured after performing the exercise protocol.
Figure 1. Distribution of pain intensity reports at 24 and 48 hours after exercise.
There were insufficient data points to successfully derive empirical probability estimates for each value of our continuous variables; that is, by way of example, there were not enough participants who had the same fear of pain to calculate probability of experiencing pain for that specific level of fear. Therefore we were unable to adequately assess the exact linearity of the relationship between the response and predictors. However, in the parsimonious models, a one point increase in fear of pain increased the probability of reporting pain between 3 to 4%.
Pain intensity at 48 hours
The pain intensity at 48 hours ranged from 0 to 68mm on the VAS. One outlier was deleted. No significant predictors were identified related to the odds of reporting pain greater than 10 or 20 mm. However, average baseline pain sensitivity and the percentage of torque lost during the exercise protocol were significant predictors of the odds of predicting pain greater than 35mm. The final model is summarized in Table 2. In this model, the overall probability of experiencing pain, if a participant didn't experience any torque loss and rated the experimental thermal stimuli at zero, was 0.04%. When controlling for the percentage of muscle performance lost during exercise, the probability of rating pain intensity as greater than 35 increased 6% with every 1 point increase in average pain sensitivity. Similarly, the probability of reporting pain increased 14% for every 1 unit increase the percentage of muscle performance lost, adjusting for pain sensitivity.
Similar to the previous models, we were unable to derive empirical probability estimates for each value of our continuous variables.
Discussion
Our purpose for this investigation was to examine characteristics of pain free individuals to determine whether these characteristics might be related to reports of pain intensity in the low back after exercise that induces DOMS. We collected demographic, psychological and neurophysiological variables associated with the magnitude of reported pain in both clinical and experimental studies. The first finding of our analyses indicated that the odds of experiencing pain intensity 24 hours after exercise was related primarily to fear of pain, regardless of the threshold used to describe the pain experience as meaningful. This is a novel finding because in our study we followed participants from a pain free state to a condition of acute low back pain. In studies of LBP it is more common that participants already have the LBP when psychological influence is investigated.
Fear of pain is an important element of the fear-avoidance model of musculoskeletal pain [31] and earlier models of exaggerated pain perception [2, 3]. These models suggest that psychological factors lead to hyperalgesia and disability. Additional work has indicated that fear of pain is a strong predictor of pain intensity in experimentally induced pain using the coldpressor test [17] and thermal pain induction [32]. Also, in a study of patients with LBP, elevated fear of pain was predictive of elevated pain intensity ratings during acute pain perception [33]. Our findings in the current study are also similar to another study of DOMS in which FPQ was the primary predictor of shoulder pain intensity at 24 hours after exercise [14]. Together, these findings in experimental and clinical models, including our own, suggest that the immediate or short term pain response after insult may be heavily influenced by psychological factors (in this case fear). These data provide additional evidence that psychological influence on pain perception can occur early in an episode, and need not be the result of prolonged pain.
In contrast, at 48 hours after exercise, we only identified factors that were related to experiencing the highest level of pain we examined (pain intensity >35mm). Our results indicate that the odds of experiencing pain greater than 35mm increase as baseline pain sensitivity increases. This is useful information for those who manage programs in which there is exposure to stimuli that are potentially novel and as a result potentially pain inducing; consider environments in which strenuous fatiguing exercise is performed in novel situations like boot camp in the military, for example. Exercise is also prescribed as an intervention for patients with a variety of disorders. The data from the current study suggest that modifications (such as avoiding high intensity eccentric load, for example) to a proposed program might be made to decrease the risk of experiencing pain greater than 35 out of 100.
Exercise can produce pain during [34] and after activity [35]. Performance of eccentric (lengthening) muscle actions in muscles unaccustomed to such forces causes damage to muscle fibers. Mechanical disruption of protein filaments within muscle fibers occurs along with a secondary inflammatory response [36]. Pain (hyperalgesia and allodynia), edema, and weakness can also result [37-41]. Muscle nociceptors become sensitized after exposure to various mediators of the inflammatory process and this sensitization is a proposed mechanism for exercise-induced muscle pain [42, 43]. Consequently, that the torque loss experienced by participants contributed to the reports of pain intensity is not surprising. This combination of findings may indicate that pain intensity, in this acute model, experienced at 48 hours might reflect the intrinsic muscle physiology of the participant.
Most exercise protocols used to generate DOMS, for example in the shoulder, are far more aggressive than that which was used in the current study with participants providing maximal effort during the lengthening (eccentric) phases of the exercise protocol. When developing this current model of DOMS in the low back we were very cautious about the magnitude of the loading being placed on the participants. Consequently, our protocol was less aggressive than is seen in models developed for the extremities potentially explaining the slightly lower median pain intensity ratings in our model.
None-the-less, these data suggest that there were two responses to the DOMS induction. If a hypothetical participant had elevated fear, its influence on pain response primarily occurred at 24 hours. If the participant had elevated pain sensitivity and had a large reduction in muscle performance in response to the exercise, these influences were reflected in pain reports at 48 hours. The intent of our analysis had not been to specifically determine what characteristics might account for these trajectories of pain development and recovery; however, that only 2 subjects had clinically meaningful pain at both time points might provide information regarding this point. In our sample, both these participants had FPQ scores of more than 100, placing them above the 90th percentile (100), and they experienced a drop in torque production of >56% after exercise potentially indicating that the interactions among these variables could contribute to pain of longer duration.
All of the theoretical predictor variables that we measured were included in the models at both 24 hours and 48 hours. There are several potential reasons why many of the other variables did not significantly contribute to the final model despite prior literature linking these factors to the report of pain intensity. First, we had a relative small sample size within the group reporting clinically meaningful pain, especially greater than 35mm. There is the potential that we were subsequently underpowered to detect true effects if they were present in this analysis. However we used exact logisitic regression minimizing this influence. Related to this we limited the predictors that we did include so as not to saturate the models that we built. For example, possible interactions might have existed between the psychological and the psychophysical measures. Extensions of this work will examine additional interaction factors, as well as including other measures that influence musculoskeletal pain reports such as catastrophizing, for example [31].
DOMS may have more external validity as a musculoskeletal pain model compared to exogenous methods of inducing pain which are of very brief duration [14]. Models of endogenous muscle pain are particularly important because musculoskeletal disorders are the most prevalent cause of chronic health problems, disabilities, and health care utilization and the second most common reason for restricted activity and the consumption of medication [9, 10, 13]. One of the most common forms of musculoskeletal pain experienced is LBP[9, 10]. However, even with DOMS the duration of the pain experience is shorter than most musculoskeletal conditions for which a person might seek intervention. Additionally, DOMS is usually induced in young, healthy individuals (as it was in our study). The individuals are not likely to have the same anatomic changes common to older patients with clinical reports of LBP. For example, autopsy studies on large numbers of subjects have found disc degeneration, facet joint osteoarthritis, or osteophytes in 90–100% of subjects aged over 64 years [44]. Likewise, the prevalence of disc bulges increases with age but not that of protrusions [45]. Consequently we are unable to extend our findings to adults over the age of 40. There is potential for including adults over age of 40 in future studies, however. For example future studies could involve induction of DOMS in older adults, and comparing results of those with anatomic changes to those without.
Additionally, we completed multiple analyses on the data collected from these subjects. Consequently there could be the potential for an inflation of type 1 error, meaning that some of our results could have occurred by chance alone. Despite these limitations, our findings suggest that the initial reports of pain after injury maybe more strongly influenced by psychological factors such as fear. At 24 hours post-exercise, fear was a consistent influence was a consistent influence across all thresholds used to define the development of meaningful pain intensity. After this the inflammatory process and pain sensitivity may play a larger role in explaining pain intensity. These factors were specific to a definition that defined meaningful pain intensity as an increase beyond 35mm.
Table 1. Demographic.
| Mean | Range | |
|---|---|---|
| Age | 22.1 | 18, 40 |
| BMI | 24.0 | 18.0, 48.1 |
| AvPain | 30.9 | 0.8, 87.6 |
| TSS | 10 | -15, 40 |
| Fear of Pain | 75.3 | 30, 113 |
| Sex | ||
| Female | 36 | |
| Male | 24 | |
| Race/Ethnicity | ||
| White | 41 | |
| African American | 5 | |
| Asian | 3 | |
| Hawaiian/Pacific Islander | 1 | |
| More than one race | 10 | |
| Hispanic | 9 |
Acknowledgments
We would like to acknowledge the assistance of Lauren Bernloehr, Nicholas DiSarro, Adrienne Driggers, Carlos Riveros and Sarah Rivard during data collection for this study. We also would like to thank to Ning Li, PhD for help with the SAS code. The work was completed with support from the National Institute for Arthritis and Musculoskeletal and Skin Diseases (AR054331-01A2).
References
- 1.Linton SJ. Do psychological factors increase the risk for back pain in the general population in both a cross-sectional and prospective analysis? Eur J Pain. 2005;9:355–61. doi: 10.1016/j.ejpain.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Lethem J, Slade PD, Troup JD, Bentley G. Outline of a Fear-Avoidance Model of exaggerated pain perception--I. Behav Res Ther. 1983;21:401–8. doi: 10.1016/0005-7967(83)90009-8. [DOI] [PubMed] [Google Scholar]
- 3.Slade PD, Troup JD, Lethem J, Bentley G. The Fear-Avoidance Model of exaggerated pain perception--II. Behav Res Ther. 1983;21:409–16. doi: 10.1016/0005-7967(83)90010-4. [DOI] [PubMed] [Google Scholar]
- 4.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–75. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 5.Staud R, Price DD, Robinson ME, Mauderli AP, Vierck CJ. Maintenance of windup of second pain requires less frequent stimulation in fibromyalgia patients compared to normal controls. Pain. 2004;110:689–96. doi: 10.1016/j.pain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Granot M, Friedman M, Yarnitsky D, Zimmer EZ. Enhancement of the perception of systemic pain in women with vulvar vestibulitis. Bjog. 2002;109:863–6. doi: 10.1111/j.1471-0528.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 7.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 8.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Fear-avoidance beliefs and temporal summation of evoked thermal pain influence self-report of disability in patients with chronic low back pain. J Occup Rehabil. 2006;16:95–108. doi: 10.1007/s10926-005-9007-y. [DOI] [PubMed] [Google Scholar]
- 9.Mantyselka P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamaki H, Halonen P, Takala J. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. 2001;89:175–80. doi: 10.1016/s0304-3959(00)00361-4. [DOI] [PubMed] [Google Scholar]
- 10.Sternbach RA. Pain and ‘hassles’ in the United States: findings of the Nuprin pain report. Pain. 1986;27:69–80. doi: 10.1016/0304-3959(86)90224-1. [DOI] [PubMed] [Google Scholar]
- 11.Graven-Nielsen T, Segerdahl M, Svensson P, Arendt-Nielsen L. Methods for induction and assessment of pain in humans with clinical and pharmacological examples. In: Kruger L, editor. Methods in pain research. CRC Press; Boca Raton: 2001. pp. 264–304. [Google Scholar]
- 12.Staahl C, Drewes AM. Experimental human pain models: a review of standardised methods for preclinical testing of analgesics. Basic Clin Pharmacol Toxicol. 2004;95:97–111. doi: 10.1111/j.1742-7843.2004.950301.x. [DOI] [PubMed] [Google Scholar]
- 13.Badley EM, Rasooly I, Webster GK. Relative importance of musculoskeletal disorders as a cause of chronic health problems, disability, and health care utilization: Findings from the 1990 Ontario Health Survey. J Rheumatology. 1994;21 [PubMed] [Google Scholar]
- 14.George SZ, Dover GC, Fillingim RB. Fear of pain influences outcomes after exercise-induced delayed onset muscle soreness at the shoulder. Clin J Pain. 2007;23:76–84. doi: 10.1097/01.ajp.0000210949.19429.34. [DOI] [PubMed] [Google Scholar]
- 15.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 16.McNeil DW, Rainwater AJ., 3rd Development of the Fear of Pain Questionnaire--III. J Behav Med. 1998;21:389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- 17.George SZ, Dannecker EA, Robinson ME. Fear of pain, not pain catastrophizing, predicts acute pain intensity, but neither factor predicts tolerance or blood pressure reactivity: An experimental investigation in pain-free individuals. Eur J Pain. 2005 doi: 10.1016/j.ejpain.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Picavet HS, Vlaeyen JW, Schouten JS. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156:1028–1034. doi: 10.1093/aje/kwf136. [DOI] [PubMed] [Google Scholar]
- 19.Price DD, Bennett GJ, Rafii A. Psychophysical observations on patients with neuropathic pain relieved by a sympathetic block. Pain. 1989;36:273–88. doi: 10.1016/0304-3959(89)90086-9. [DOI] [PubMed] [Google Scholar]
- 20.Staud R, Robinson ME, Vierck CJ, Jr, Cannon RC, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–22. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 21.Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Rosier EM, I MJ, Coghill RC. Reproducibility of pain measurement and pain perception. Pain. 2002;98:205–216. doi: 10.1016/s0304-3959(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 23.Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68:141–50. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- 24.Djouhri L, Lawson SN. A beta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46:131–145. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. J Invest Dermatol. 1977;69:167–71. doi: 10.1111/1523-1747.ep12497942. [DOI] [PubMed] [Google Scholar]
- 26.Bishop MD, Craggs JG, Horn ME, George SZ, Robinson ME. Relationship of Intersession Variation in Negative Pain-Related Affect and Responses to Thermally Evoked Pain. J Pain. 2009 doi: 10.1016/j.jpain.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graves JE, Pollock ML, Carpenter DM, Leggett SH, Jones A, MacMillan M, F M. Quantitative assessment of full range-of-motion isometric lumbar extension strength. Spine. 1990;15:289–294. doi: 10.1097/00007632-199004000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Robinson ME, Greene AF, O'Connor P, Graves JE, MacMillan M. Reliability of lumbar isometric torque in patients with chronic low back pain. Phys Ther. 1992;72:186–90. doi: 10.1093/ptj/72.3.186. [DOI] [PubMed] [Google Scholar]
- 29.Nelson-Wong E, Flynn T, Callaghan JP. Development of active hip abduction as a screening test for identifying occupational low back pain. J Orthop Sports Phys Ther. 2009;39:649–657. doi: 10.2519/jospt.2009.3093. [DOI] [PubMed] [Google Scholar]
- 30.Ostelo RW, Swinkels-Meewisse IJ, Knol DL, Vlaeyen JW, de Vet HC. Assessing pain and pain-related fear in acute low back pain: what is the smallest detectable change? Int J Behav Med. 2007;14:242–248. doi: 10.1007/BF03002999. [DOI] [PubMed] [Google Scholar]
- 31.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 32.George SZ, Hirsh AT. Psychologic influence on experimental pain sensitivity and clinical pain intensity for patients with shoulder pain. J Pain. 2009;10:293–9. doi: 10.1016/j.jpain.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sieben JM, Vlaeyen JW, T S, Portegijs PJ. Pain-related fear in acute low back pain: the first two weeks of a new episode. Eur J Pain. 2002;6:229–237. doi: 10.1053/eujp.2002.0341. [DOI] [PubMed] [Google Scholar]
- 34.Cook DB, O'Connor PJ, Eubanks SA, Smith JC, Lee M. Naturally occurring muscle pain during exercise: assessment and experimental evidence. Med Sci Sports Exer. 1997;29:999–1012. doi: 10.1097/00005768-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Dannecker EA, Price DD, Robinson ME. An examination of the relationships among recalled, expected, and actual intensity and unpleasantness of delayed onset muscle pain. J Pain. 2003;4:74–81. doi: 10.1054/jpai.2003.7. [DOI] [PubMed] [Google Scholar]
- 36.Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J Sports Med. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- 37.Nosaka K, Clarkson PM. Muscle damage following repeated bouts of high force eccentric exercise. Med Sci Sports Exerc. 1995;27:1263–9. [PubMed] [Google Scholar]
- 38.Bajaj P, Graven-Nielsen T, Wright A, Davies LAI, Arendt-Nielsen L. Muscle hyperalgesia in postexercise muscle soreness assessed by single and repetitive ultrasound stimuli. J Pain. 2000;1:111–121. [Google Scholar]
- 39.Clarkson PM. Exercise-induced muscle damage--animal and human models. Med Sci Sports Exerc. 1992;24:510–1. [PubMed] [Google Scholar]
- 40.Clarkson PM. Eccentric exercise and muscle damage. Int J Sports Med. 1997;18 4:S314–7. doi: 10.1055/s-2007-972741. [DOI] [PubMed] [Google Scholar]
- 41.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 42.Graven-Nielsen T, Mense S. The peripheral apparatus of muscle pain: evidence from animal and human studies. Clin J Pain. 2001;17:2–10. doi: 10.1097/00002508-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Babenko VV, Graven-Nielsen T, Svensson P, Drewes AM, Jensen TS, Arendt-Nielsen L. Experimental human muscle pain induced by intramuscular injections of bradykinin, serotonin, and substance P. Eur J Pain. 1999;3:93–102. doi: 10.1053/eujp.1998.0103. [DOI] [PubMed] [Google Scholar]
- 44.Videman T, Nurminen M, Troup JD. Volvo Award in clinical sciences. Lumbar spinal pathology in cadaveric material in relation to history of back pain, occupation, and physical loading. Spine. 1990;15:728–740. [PubMed] [Google Scholar]
- 45.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]