Abstract
BAFF (BLyS) and APRIL are TNF-like cytokines that support survival and differentiation of B cells. Recent studies have discovered a role for BAFF in augmenting both innate and adaptive immune responses as well as in collaborating with other inflammatory cytokines to promote the activation and differentiation of effector immune cells. BAFF is an important pathogenic factor in lupus mouse models and BAFF inhibition successfully delays disease onset in these mice, although the responsiveness to BAFF inhibition varies among different strains. These results have led to the development of inhibitors targeting BAFF and APRIL in humans. An anti-BAFF antibody has shown significant but modest efficacy in two Phase III clinical trials for moderately active SLE and other inhibitors are being developed or at early stages of clinical testing.
Keywords: Autoimmunity, Systemic lupus Erythematosus, B cells, BAFF
Introduction
BAFF (B cell activating factor belonging to the TNF family), also known as BLyS (B lymphocyte stimulator) is a vital homeostatic cytokine for B cells that helps regulate both innate and adaptive immune responses [1]. Increased serum levels of BAFF and its homolog APRIL (a proliferation inducing ligand) are found in several autoimmune diseases, and both cytokines can be elaborated in inflammatory sites. The successful use of BAFF/APRIL inhibitors in murine models of autoimmunity [2, 3] has led to the rapid development of this class of drugs for clinical testing in humans. The first of these drugs, belimumab, a monoclonal antibody specific for soluble BAFF has demonstrated efficacy in phase III studies of moderately active SLE (R.F. van Vollenhoven, abstract OP0068 presented at EULAR Congress, Rome 2010 and S. Navarra, abstract SAT0204 presented at EULAR Congress, Rome, 2010). A different anti-BAFF antibody that recognizes both soluble and membrane BAFF has exhibited efficacy in phase II studies of rheumatoid arthritis. The dual BAFF/APRIL inhibitor, atacicept (TACI-Ig fusion protein) is undergoing clinical trials in several autoimmune diseases [4-7] and several other antibodies and fusion proteins are in development. In this review we will summarize the physiology of BAFF and its receptors, and discuss both its pathogenic role in autoimmunity and its potential as a therapeutic target with a focus on SLE.
BAFF, APRIL and their receptors
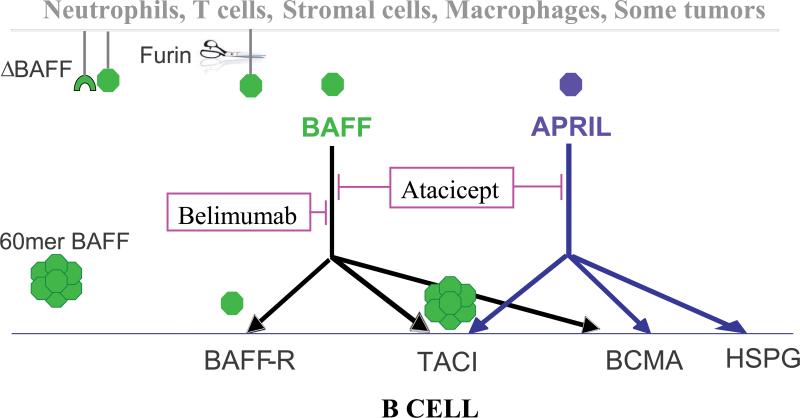
BAFF and its homolog APRIL are members of the tumor necrosis factor family and are expressed by monocytes, DCs, neutrophils, basophils, stromal cells, activated T cells, activated and malignant B cells, and epithelial cells [1, 8-10]. BAFF binds to 3 receptors, BAFF-R, TACI (Transmembrane activator and calcium modulator ligand interactor) and BCMA (B cell maturation antigen) that are expressed by B cells at various times during their ontogeny [2, 11]. BAFF-R is specific for BAFF whereas TACI and BCMA also bind to APRIL [1]. BAFF is mostly cleaved from the cell surface and circulates as a soluble homotrimer that binds to BAFF-R [12]. In contrast, binding of BAFF and APRIL to TACI requires ligand multimerization; BAFF self-associates spontaneously into multimers of twenty trimers whereas APRIL is aggregated on cell surfaces by binding to sulfated proteoglycans [12] (Figure 1).
Figure 1.
The interaction of BAFF/APRIL with their receptors and the targets of belimumab and atacicept. Soluble BAFF shed from the cell surface following protease cleavage, binds to BAFF-R, TACI, or BCMA, whereas APRIL only binds to TACI or BCMA. BAFF trimers bind to BAFF-R and BAFF multimers bind to TACI. APRIL is multimerized on the cell surface by heparan sulfate proteoglycan (HSPG). ΔBAFF, a splice variant of BAFF, forms heterotrimers with full length BAFF thus limiting its availability. Belimumab (anti-BAFF) inhibits the interaction of soluble BAFF with its receptors, leaving APRIL functions intact whereas atacicept (TACI-Ig) blocks the binding of both BAFF and APRIL to their receptors.
Receptor signaling
Each of the three receptors has a different pattern of expression and each mediates distinct functions. BAFF-R is expressed on naïve and memory B cells and, through activation of the alternative NF-κB pathway, enhances B cell survival by upregulating anti-apoptotic proteins [13]. BAFF-R ligation also weakly stimulates the classic NF-κB pathway and transduces signals through mTOR and Pim2 that promote protein synthesis and cell growth [14, 15]. TACI is expressed on most B cell types after the T2 stage and both TACI and BCMA are expressed on plasmablasts and plasma cells [1, 16]. TACI and BCMA signal through the classic NF-κB pathway, as well as through other pathways to counteract apoptosis and to drive class switching [17, 18]. An ongoing puzzle is the mechanism for the regulatory role of TACI, with the development of autoimmunity and lymphomas in TACI deficient mice [19].
Splice variants
Of the several splice variants of the cytokines and their receptors, the best characterized is ΔBAFF, a variant that is missing an exon and is inefficiently cleaved from the cell surface. ΔBAFF does not bind either BAFF-R or TACI in vitro but limits BAFF availability by forming heterotrimers with full length BAFF. ΔBAFF transgenic mice have a mildly reduced B cell pool, a suboptimal antibody response to T cell dependent antigens and more stringent selection of their B cell repertoire [20, 21]. The function of cell surface-expressed ΔBAFF homotrimers is not yet known, nor is it known how differential splicing is regulated. Understanding more about the regulation of expression and function of ΔBAFF is important since a BAFF inhibitor that targets membrane as well as soluble BAFF is in early clinical trials. There is some evidence that signaling through membrane BAFF in monocytes and dendritic cells induces cell activation and expression of inflammatory mediators and costimulatory molecules [22, 23]. For this reason, it needs to be determined whether the membrane BAFF inhibitor will interfere with the regulatory role of ΔBAFF, and how it affects the functions of membrane expressed BAFF.
BAFF and BAFF-R are required for naïve B cell survival and selection
BAFF is crucial both for B cell homeostasis and for the regulation of B cell selection. Early transitional (T1) cells with immature rafts are subject to deletion or anergy induction if they receive a signal through the BCR. In the late transitional stage, BCR signaling through maturing rafts upregulates expression of BAFF-R and also generates p100, a substrate for the non-classical NF-ΔB signaling pathway used by BAFF-R [15, 24]. Autoreactive B cells that have downregulated their BCR as a consequence of antigen stimulation at the T1 stage produce less p100, express less BAFF-R and compete poorly for BAFF as they progress to the T2 stage. When B cell numbers and BAFF levels are normal, stringent deletion of autoreactive B cells occurs. However an increase in serum BAFF levels may result in relaxation of B cell selection, with survival of more autoreactive naïve B cells [25, 26].
BAFF plays an important role in immune responses to pathogens
Innate immunity
BAFF is produced by myeloid DCs in response to type I interferons (IFNs) [27] and it collaborates with cytokines and toll like receptor (TLR) signals to promote Ig class switching and plasma cell differentiation [28, 29]. In SLE, class switching of autoreactive B cells from IgM to more pathogenic IgG is a critical checkpoint in the initiation of clinical disease. Autoreactive B cells in SLE internalize immune complexes or apoptotic material containing nucleic acids that activate TLRs, causing increased expression of the BAFF receptor TACI [28, 30]. High serum levels of BAFF may therefore preferentially support the survival and induce class switching of these cells. In support of this notion, marginal zone B cells undergo T-independent class switching in BAFF transgenic mice and secrete autoantibodies that cause mild SLE [30]. Some SLE patients chronically have 3-4 fold increases in serum BAFF levels; this could be due to B cell lymphopenia, increased type I IFNs, or BAFF production from inflammatory sites. It is not yet clear whether this increase in BAFF levels is responsible for aberrant selection or class switching of naïve B cells in SLE and whether such abnormalities can be reversed by BAFF inhibition.
Antibody responses
T cell independent type II responses and T cell dependent IgM responses require the interaction of BAFF with TACI [1]. BAFF also seems to be involved in germinal center responses as BAFF-deficient mice fail to develop a mature FDC network and have small and unstable germinal centers; class switching and somatic hypermutation still occur, but IgG and secondary responses are diminished [31, 32]. Although germinal centers are similarly small in BAFF-R deficient mice [31], the FDC defect is not seen, indicating that the interaction of BAFF with TACI is most likely involved in FDC maturation.
Effector B cells
Although memory B cells express BAFF-R [33] survival and reactivation of class switched B cell memory cells is BAFF independent under normal physiologic circumstances [34]. BAFF can however, collaborate with inflammatory cytokines such as IL-21 and IL-17 in the reactivation of memory B cells and their differentiation to plasma cells [35, 36]; whether this occurs in vivo is not yet known. Surprisingly, BAFF inhibition with either belimumab or atacicept results in rapid expansion of the memory B cell pool in humans [4, 5, 37]. Whether this is due to homeostatic proliferation of memory B cells or mobilization of cells into the circulation is not yet known.
Plasma cells express TACI and BCMA and their survival can therefore be supported by either BAFF or APRIL that are secreted by stromal cells (BAFF) and dendritic cells (APRIL) within the lymph node or bone marrow microenvironment [38, 39]. This means that blockade of both cytokines is required to deplete plasma cells [3, 40]. Importantly, dual BAFF/APRIL inhibition is associated with a preferential decrease in IgM and IgA producing plasma cells compared with IgG producing plasma cells. In accord with these findings, BAFF/APRIL inhibition has little effect on circulating IgG levels to recall antigens such as tetanus [4].
Regulatory B cells
Recent work suggests a possible role for BAFF in the maintenance of regulatory B cells that produce IL-10 [41] but this needs to be confirmed; the consequence of chronic BAFF inhibition with respect to this cell population is not known.
Unique functions of APRIL
APRIL does not bind to BAFF-R and therefore is not involved with naïve B cell selection or survival. Class switching to IgA appears to be dependent on the interaction of APRIL with TACI suggesting that APRIL functions in the maintenance of mucosal immunity and serum levels of IgA [42]. APRIL is also essential for the survival of plasma cells in neonatal bone marrow and low expression of APRIL by bone marrow stromal cells in early life may be responsible for the rapid waning of IgG antibodies elicited in infancy [38]. As mentioned above, atacicept that blocks both BAFF and APRIL severely depletes serum levels of IgM, thus ablating early humoral responses to bacteria and viruses and potentially increasing infection risk.
Role of BAFF/APRIL in other leukocytes
BAFF-R is expressed on some T cells and may modulate T cell activation and promote IFNγ and IL-17 production [43, 44]. BAFF also induces dendritic cells to produce IL-12 and supports both the survival of monocytes and their differentiation into activated macrophages [45]. In a mouse model of arthritis, synovial dendritic cells transduced with an siRNA that silences BAFF remained in an immature state and failed to produce the IL-6 required for the differentiation of T helper type 17 cells [43]. Activated monocytes and dendritic cells primarily express intracellular TACI, but cell surface TACI expression may be induced during inflammatory conditions [45]. Thus BAFF appears to have a pro-inflammatory role; it helps DCs and monocytes to activate and recruit immune cells and it directly enhances the pro-inflammatory activity of T cells. These functions are important because BAFF is often expressed in target organs in autoimmune diseases and may therefore contribute to amplification of inflammation in these sites.
BAFF and APRIL inhibitors in lupus mouse models
Several strategies have been developed to block BAFF and APRIL in vivo. Selective inhibition of BAFF is achieved with soluble BAFF-R or with antibodies to BAFF; in contrast, soluble TACI blocks both BAFF and APRIL [3, 46]. Because the phenotype of APRIL transgenic and knockout mice is not dramatic there has been little interest in developing APRIL specific drugs for the treatment of autoimmunity.
Extensive studies of the mechanism of action of BAFF and APRIL blockade have been performed in murine models of lupus. In several lupus strains, BAFF-R-Ig and TACI-Ig delay the onset of lupus nephritis and prolong survival with similar efficacy [3, 47], indicating that BAFF blockade alone is sufficient to mediate therapeutic effects. Due to the different ways that the inhibitors were given, a direct comparison between strains is difficult. However, several studies provide evidence supporting the role of genetic background in determining how long and how effectively lupus-prone mice are protected by BAFF/APRIL inhibition. For example, a single injection of an adenovirus expressing BAFF-R-Ig resulted in protein expression for 6-8 weeks but a thirty week delay in time to death in NZB/W F1 mice [3]. In comparison, when NZM2410 mice were treated with the same dose of virus, survival was prolonged for much longer [47]. Moreover, while both BAFF-R-Ig and TACI-Ig reversed established nephritis in NZM 2410 mice, remission induction in NZB/W F1 mice required the addition of a second agent [3, 47].
BAFF/APRIL inhibition does not block the production or the renal deposition of autoantibodies in most mouse lupus strains and even complete deficiency of BAFF does not completely ablate autoantibody production or prevent the eventual development of renal pathology [48]. Plasma cells that produce autoantibodies may arise from germinal centers, extrafollicular foci, or reactivated memory B cells; the source of autoantibodies is variable among mouse strains. In MRL/lpr mice most autoantibody producing plasma cells derive from extrafollicular foci and their survival is largely dependent on TACI and BCMA. TACI-Ig treatment therefore prevents the appearance of both IgM and IgG anti-dsDNA antibodies and renal deposition of immune complexes in this strain [49]. BAFF-R deficiency is not sufficient to inhibit autoantibody formation or prevent lupus nephritis in MRL/lpr mice [50], consistent with the absence of a role for BAFF-R in plasma cell survival.
BAFF/APRIL inhibitors do not prevent the spontaneous formation of germinal centers in the spleens of NZB/W, NZM2410 or NZW/BXSB lupus mice [47, 51, 52], despite the diminished size of B cell follicles caused by B cell depletion. Autoreactive B cells undergo somatic hypermutation and affinity maturation in these germinal centers, and therefore acquire the ability to bind the kidney antigens. In NZB/W and NZW/BXSB mice, TACI-Ig depletes mainly short-lived IgM producing plasma cells with little effect on long-lived plasma cells that produce IgG autoantibodies [3]. These data suggest that, depending on the predominant autoantibody source in an individual strain, BAFF/APRIL blockade may or may not affect autoantibody production.
The contribution of autoreactive memory B cells to the overall pool of autoantibodies either in mice or humans with SLE is not yet fully understood. While BAFF does not support the survival or reactivation of class switched memory B cells under physiologic circumstances [34], it is possible, given the ability of BAFF to collaborate with inflammatory cytokines to promote plasma cell development from memory cells that BAFF inhibition may prevent memory B cells from differentiating into plasma cells in SLE.
Since the therapeutic effects of BAFF/APRIL inhibitors are not necessarily achieved by preventing the formation of autoantibodies in murine models or in humans, questions remain about the precise mechanism of action of these drugs. B cell depletion decreases the non-secretory functions of B cells and leads to diminished numbers of activated T cells and dendritic cells. As a result, the overall load of circulating pro-inflammatory mediators is reduced, preventing the activation of endothelial cells and local inflammation in the kidneys in response to immune complex deposition [3, 47, 52]. Nevertheless in NZB/W mice the therapeutic effect of BAFF inhibition appears to be more robust than that of B cell depletion, an effect also observed in humans.
It is possible therefore that BAFF inhibition has direct effects on cells other than B cells. Notably, in Lyn deficient mice BAFF blockade directly inhibits the activation of T cells and decreases the secretion of IFNγ [44]. BAFF/APRIL blockade may also exert protection through direct inhibition of renal cell activation and local kidney inflammation. Regardless of the precise mechanism, BAFF/APRIL inhibition or even BAFF deficiency does not seem to prevent disease onset completely. Furthermore, it is clear that while BAFF blockade consistently demonstrates the same effects on spleen cell populations and autoantibodies in most murine models of SLE, the clinical effect of BAFF blockade on SLE nephritis varies in the different murine models and with disease stage [3, 47]. This may reflect the presence of other mediators that collaborate with autoantibodies in the effector phase of renal inflammation or with BAFF in supporting plasma cell survival. Thus, in addition to genetic factors, environmental exposures may influence the therapeutic response to BAFF inhibition. Given the heterogeneity of human SLE patients, it will not be surprising to find that certain subsets of patients respond better to BAFF/APRIL inhibition than others.
BAFF and APRIL inhibition for human disease
Despite considerable immunologic differences between rodents and humans the results of the human studies are consistent with those of the mouse studies. In humans, BAFF blockade preferentially depletes naïve and transitional B cells but has little effect on class switched memory B cells and long-lived plasma cells [53]. Selective BAFF blockade has less effect on serum Ig levels than does blockade of both BAFF and APRIL, and both agents preferentially decrease serum levels of IgM. Clinical trials of belimumab and atacicept have been performed in several autoimmune diseases [4-6, 37, 54, 55].
Because increased serum BAFF levels are found in rheumatoid arthritis (RA) patients and both BAFF and APRIL are detected in rheumatoid synovium [56], the first clinical trials of BAFF inhibitors were performed in RA. The effect of both belimumab and atacicept was modest in this disease [5], a disappointing result given the murine studies showing that local BAFF inhibition alters dendritic and T cell function within the joint [43] and the robust effect of anti-CD20 antibodies in RA patients; these studies did not translate into the desired therapeutic effect of BAFF inhibition in human RA. A recent phase II clinical trial of a different anti-BAFF antibody that targets both membrane and soluble BAFF has shown efficacy in RA and further trials are in progress (M Genovese, abstract 1923). Should this drug be effective in larger trials it will be important to understand whether the targeting of membrane BAFF is responsible for the differences in efficacy between this drug and belimumab or atacicept.
Even more disappointing have been preliminary results of a clinical trial of atacicept in multiple sclerosis (MS). Based on the emerging view that B cells are important in the pathogenesis of MS, and the findings that BAFF is expressed within ectopic lymphoid follicles in the meninges and by astrocytes that are closely associated with BAFF-R expressing cells [57], BAFF/APRIL inhibitors were found to ameliorate early experimental allergic encephalomyelitis in mice [58]. Despite these promising findings, a phase II trial of TACI-Ig in human MS was stopped because of disease worsening (www.clinicaltrials.gov). These results, like those in RA, stand in contrast to the long-lasting clinical benefit conferred by B cell depletion with anti-CD20 antibodies.
Given the pathogenic role of BAFF in murine SLE and the urgent need for new drugs that can ameliorate this disease in humans there has been a large effort directed at studying the efficacy of BAFF inhibitors in human SLE. In an initial phase II trial of belimumab, the primary endpoints of the trial were not met, however there appeared to be some improvement in treated patients at 56 and 76 weeks when a post-hoc analysis using a composite disease outcome measure was performed [37]. Given the slow kinetics of B cell depletion mediated by BAFF inhibition in humans, a decrease in clinical activity of SLE might be expected over a long time period. Two subsequent phase III studies were therefore designed to be of long duration (up to 76 weeks) and the composite disease outcome measure was used as the primary outcome. In addition other interventions were allowed during the first 6 months of treatment before the maximal clinical effect of BAFF inhibition was expected. Using this design, both trials met their primary endpoint at 52 weeks, with a modest benefit over standard of care therapy and improvements in secondary outcomes including the frequency of severe flares and ability to decrease steroid dose. Belimumab did not however demonstrate a significant benefit over standard of care therapy at 76 weeks although several secondary outcomes were still achieved (R.F. van Vollenhoven, abstract OP0068 presented at EULAR Congress, Rome, 2010 and S. Navarra, abstract SAT0204 presented at EULAR Congress, Rome, 2010).
Depletion of B cells in belimumab treated patients occurred predominantly in the naive and transitional populations, becoming maximal 6-8 months after starting therapy. In contrast, depletion of plasma cells and memory cells took more than a year and occurred nearly exclusively in the IgM populations with little effect on the IgG populations. Total serum IgG levels decreased by only 10% and autoantibody titers decreased to a modest degree, consistent with the dependence of plasma cells on both BAFF and APRIL. In a few patients however autoantibody titers disappeared entirely, reflecting some heterogeneity in the immunologic response [37, 53]. It is not yet clear whether belimumab alters B cell selection, a question that is important to answer in order to determine potential benefits of long-term belimumab use. The effect of belimumab on T cells and monocytes has not yet been studied.
Similar immunologic effects were observed in phase I study of atacicept in SLE patients [4, 6] with a more profound effect on serum immunoglobulin levels, especially IgM and IgA. Nevertheless, serum IgG levels decreased by only 20% and titers of antibodies to recall antigens were not affected, suggesting that long-lived plasma cells in most humans are not dependent solely on BAFF and APRIL for their survival. However, a combination trial of atacicept with mycophenolic acid in patients with SLE nephritis was halted due to hypogammaglobulinemeia and increased infection risk, indicating that plasma cell survival may be compromised when T cell-derived cytokines are also depleted. Further studies of atacicept in SLE are ongoing. Whether there will be some individuals whose plasma cells are totally dependent on BAFF and APRIL remains to be determined.
Conclusions
Inhibition of BAFF or BAFF and APRIL may be a suitable therapeutic approach for SLE The murine studies in sum show that blockade of BAFF delays disease onset in most models of SLE. However, there is considerable strain and environment dependent variation with respect to efficacy, especially in the later stages of disease, with some strains requiring a second synergistic agent to reverse ongoing inflammatory disease. Furthermore, there is considerable heterogeneity in BAFF or APRIL-dependence of plasma cells in different models; this may depend on the presence or absence of other cytokines able to support plasma cell survival and the nature of the microenvironment in which these cells reside. BAFF blockade also decreases the inflammatory response to immune complex deposition in target organs by mechanisms that remain to be fully elucidated. These studies are consistent with the heterogeneity observed thus far in the response of human SLE patients to BAFF inhibition. As belimumab moves towards the formal approval process, questions remain about which diseases will respond to BAFF inhibition, how to identify drug responders, the appropriate duration of treatment and the use of concomitant immunosuppressive therapies. As new members of the BAFF/APRIL class progress through clinical trials we should learn the relative benefits of blocking APRIL or the BAFF membrane or splice variant forms. Finally, it is important to learn why there are disease specific differences in therapeutic efficacy between BAFF/APRIL inhibition and B cell depletion with anti-CD20 antibodies, in particular their relative effects on IL-10 producing B cells (B regs), on non-B cells and the consequences of the high levels of BAFF that accompany B cell depletion and skew the reconstituting B cell repertoire toward autoimmunity
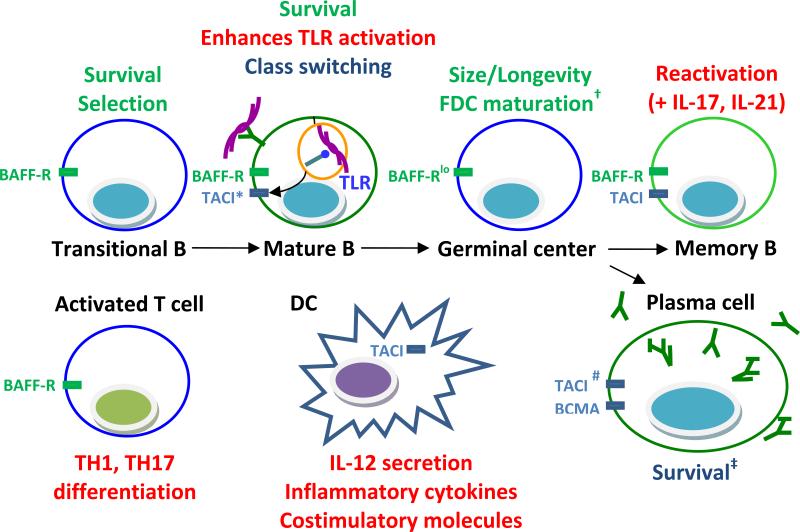
Figure 2.
Effects of BAFF/APRIL on various immune cells in SLE. BAFF supports the survival of transitional and mature B cells and BAFF levels regulate the stringency of selection of naïve B cells. Autoreactive B cells internalize nucleic acids and upregulate TACI as a result of TLR ligation; BAFF/APRIL may therefore preferentially enhance the survival and class switching of these cells and further induce TLR upregulation. BAFF/APRIL also support the long-term survival of plasma cells. In addition, BAFF amplifies the ability of inflammatory cytokines to reactivate memory B cells and induces their differentiation to plasma cells. Finally, BAFF may be involved in survival, activation, differentiation, or cytokine production of T cells and BAFF/APRIL may influence the function of DCs, monocytes, or intrinsic renal cells, contributing directly to local inflammation in the target organs. Functions of BAFF are shown in green. Functions of both BAFF and APRIL are shown in blue. Functions of BAFF or APRIL that occur during inflammation are shown in red.
* the interaction of TACI with APRIL
# TACI is responsible for maintaining Type II T independent immune responses
† Probably mediated via the interaction of BAFF with TACI
‡ IgM plasma cells are more BAFF/APRIL dependent than IgG plasma cells
Acknowledgements
The authors acknowledge grant support from the NIH (R01 AR049938 and RO1 AI082037).
Abbreviations
- SLE
Systemic Lupus Erythematosus
- APRIL
A proliferation inducing ligand
- BAFF
B cell activating factor belonging to the TNF family (also known as BLyS. B lymphocyte stimulator)
- TACI
Transmembrane activator and calcium modulator ligand interactor
- BCMA
B cell maturation antigen
- BAFF-R
BAFF receptor
- HSPG
heparan sulphate proteoglycan
- TLR
toll like receptor
- IFN
interferon
- BCR
B-cell receptor
- RA
rheumatoid arthritis
- MS
multiple sclerosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 2.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 3.Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, Frank D, Rice J, Diamond B, Yu KO, Porcelli S, Davidson A. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dall'Era M, Chakravarty E, Wallace D, Genovese M, Weisman M, Kavanaugh A, Kalunian K, Dhar P, Vincent E, Pena-Rossi C, Wofsy D. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 2007;56:4142–4150. doi: 10.1002/art.23047. [DOI] [PubMed] [Google Scholar]
- 5.Tak PP, Thurlings RM, Rossier C, Nestorov I, Dimic A, Mircetic V, Rischmueller M, Nasonov E, Shmidt E, Emery P, Munafo A. Atacicept in patients with rheumatoid arthritis: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating, single- and repeated-dose study. Arthritis Rheum. 2008;58:61–72. doi: 10.1002/art.23178. [DOI] [PubMed] [Google Scholar]
- 6.Pena-Rossi C, Nasonov E, Stanislav M, Yakusevich V, Ershova O, Lomareva N, Saunders H, Hill J, Nestorov I. An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus. Lupus. 2009;18:547–555. doi: 10.1177/0961203309102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nestorov I, Munafo A, Papasouliotis O, Visich J. Pharmacokinetics and biological activity of atacicept in patients with rheumatoid arthritis. J Clin Pharmacol. 2008;48:406–417. doi: 10.1177/0091270008315312. [DOI] [PubMed] [Google Scholar]
- 8.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 9.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu G, Boone T, Delaney J, Hawkins N, Kelley M, Ramakrishnan M, McCabe S, Qiu WR, Kornuc M, Xia XZ, Guo J, Stolina M, Boyle WJ, Sarosi I, Hsu H, Senaldi G, Theill LE. APRIL and TALL-I and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1:252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- 11.Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, Grewal IS, Cochran AG, Gordon NC, Yin J, Starovasnik MA, Dixit VM. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 12.Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, Scott ML, Maquelin A, Belnoue E, Siegrist CA, Chevrier S, Acha-Orbea H, Leung H, Mackay F, Tschopp J, Schneider P. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111:1004–1012. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 13.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 14.Hatada EN, Do RK, Orlofsky A, Liou HC, Prystowsky M, MacLennan IC, Caamano J, Chen-Kiang S. NF-kappa B1 p50 is required for BLyS attenuation of apoptosis but dispensable for processing of NF-kappa B2 p100 to p52 in quiescent mature B cells. J Immunol. 2003;171:761–768. doi: 10.4049/jimmunol.171.2.761. [DOI] [PubMed] [Google Scholar]
- 15.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 16.Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Semin Immunol. 2006;18:305–317. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18:263–275. doi: 10.1016/j.smim.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19:263–276. doi: 10.1016/j.cytogfr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Gavin AL, Ait-Azzouzene D, Ware CF, Nemazee D. DeltaBAFF, an alternate splice isoform that regulates receptor binding and biopresentation of the B cell survival cytokine, BAFF. J Biol Chem. 2003;278:38220–38228. doi: 10.1074/jbc.M306852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gavin AL, Duong B, Skog P, Ait-Azzouzene D, Greaves DR, Scott ML, Nemazee D. {Delta}BAFF, a Splice Isoform of BAFF, Opposes Full-Length BAFF Activity In Vivo in Transgenic Mouse Models. J Immunol. 2005;175:319–328. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 22.Jeon ST, Kim WJ, Lee SM, Lee MY, Park SB, Lee SH, Kim IS, Suk K, Choi BK, Choi EM, Kwon BS, Lee WH. Reverse signaling through BAFF differentially regulates the expression of inflammatory mediators and cytoskeletal movements in THP-1 cells. Immunol Cell Biol. 2010;88:148–156. doi: 10.1038/icb.2009.75. [DOI] [PubMed] [Google Scholar]
- 23.Diaz-de-Durana Y, Mantchev GT, Bram RJ, Franco A. TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo. Blood. 2006;107:594–601. doi: 10.1182/blood-2004-12-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, Brezski RJ, Treml LS, Jordan KA, Monroe JG, Sen R, Cancro MP. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 27.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like Receptor 9-Dependent and -Independent Dendritic Cell Activation by Chromatin-Immunoglobulin G Complexes. J Exp Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, Cancro MP, Khan WN. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 29.Katsenelson N, Kanswal S, Puig M, Mostowski H, Verthelyi D, Akkoyunlu M. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur J Immunol. 2007;37:1785–1795. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 30.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman ZS, Rao SP, Kalled SL, Manser T. Normal Induction but Attenuated Progression of Germinal Center Responses in BAFF and BAFF-R Signaling-Deficient Mice. J Exp Med. 2003;198:1157–1169. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalled SL. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol. 2006;18:290–296. doi: 10.1016/j.smim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, Brink R, Mackay F, Hodgkin PD, Tangye SG. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 35.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, Cochat P, Pouteil-Noble C, Trolliet P, Durieu I, Tebib J, Kassai B, Ansieau S, Puisieux A, Eliaou JF, Bonnefoy-Berard N. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 36.Ettinger R, Kuchen S, Lipsky PE. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 2008;223:60–86. doi: 10.1111/j.1600-065X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 37.Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, Petri MA, Ginzler EM, Chatham WW, McCune WJ, Fernandez V, Chevrier MR, Zhong ZJ, Freimuth WW. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 2009;61:1168–1178. doi: 10.1002/art.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 39.Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL, Bird R, MacLennan IC. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009;182:2113–2123. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- 40.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O'Neill PJ, Quinn WJ, 3rd, Goenka R, Miller JP, Cho YH, Long V, Ward C, Migone TS, Shlomchik MJ, Cancro MP. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A. 2008;105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, Cao X, Lu L. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 42.Kimberley FC, Hahne M, Medema JP. “APRIL hath put a spring of youth in everything”: Relevance of APRIL for survival. J Cell Physiol. 2009;218:1–8. doi: 10.1002/jcp.21561. [DOI] [PubMed] [Google Scholar]
- 43.Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci U S A. 2008;105:14993–14998. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-{gamma} establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010 doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang SK, Mihalcik SA, Jelinek DF. B lymphocyte stimulator regulates adaptive immune responses by directly promoting dendritic cell maturation. J Immunol. 2008;180:7394–7403. doi: 10.4049/jimmunol.180.11.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramanujam M, Davidson A. BAFF blockade for Systemic Lupus Erythematosus - will the promise be fulfilled? Immunol Rev. 2008;223:156–174. doi: 10.1111/j.1600-065X.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 47.Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Davidson A. Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum. 2010;62:1457–1468. doi: 10.1002/art.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacob CO, Pricop L, Putterman C, Koss MN, Liu Y, Kollaros M, Bixler SA, Ambrose CM, Scott ML, Stohl W. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol. 2006;177:2671–2680. doi: 10.4049/jimmunol.177.4.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu W, Szalai A, Zhao L, Liu D, Martin F, Kimberly RP, Zhou T, Carter RH. Control of spontaneous B lymphocyte autoimmunity with adenovirus-encoded soluble TACI. Arthritis Rheum. 2004;50:1884–1896. doi: 10.1002/art.20290. [DOI] [PubMed] [Google Scholar]
- 50.Ju ZL, Shi GY, Zuo JX, Zhang JW. Unexpected development of autoimmunity in BAFF-R-mutant MRL-lpr mice. Immunology. 2007;120:281–289. doi: 10.1111/j.1365-2567.2006.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, Diamond B, Madaio MP, Davidson A. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173:3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 52.Kahn P, Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Factor SM, Davidson A. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum. 2008;58:2824–2834. doi: 10.1002/art.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobi AM, Huang W, Wang T, Freimuth W, Sanz I, Furie R, Mackay M, Aranow C, Diamond B, Davidson A. Effect of long-term belimumab treatment on b cells in systemic lupus erythematosus: Extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2010;62:201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tak PP, Thurlings RM, Dimic A, Mircetic V, Rishmueller M, Nasanov E, Shmidt E, Emery P, Rossier C, Nesterov I, Hill J, Munafo A. A Phase Ib study to investigate Atacicept (TACI-Ig) in patients with rheumatoid arthritis.. ACR/ARHP Meeting Scientific Meeting; Washington, DC. 2006; (late breaking abstract) [Google Scholar]
- 55.Ginzler E, Furie R, Wallace DJ, Strand V, Lisse J, Stohl W, Merrill JT, Petri M, Aranow C, Weinstein A, Fernandez V, Zhong J, Freimuth W. Novel Combined Response Endpoint Shows that Belimumab (Fully Human Monoclonal Antibody to B-Lymphocyte Stimulator [BLyS]) Improves or Stabilizes SLE Disease Activity in a Phase 2 Trial. EULAR. 2007 [Google Scholar]
- 56.Seyler TM, Park YW, Takemura S, Bram RJ, Kurtin PJ, Goronzy JJ, Weyand CM. BLyS and APRIL in rheumatoid arthritis. J Clin Invest. 2005;115:3083–3092. doi: 10.1172/JCI25265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krumbholz M, Theil D, Derfuss T, Rosenwald A, Schrader F, Monoranu CM, Kalled SL, Hess DM, Serafini B, Aloisi F, Wekerle H, Hohlfeld R, Meinl E. BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med. 2005;201:195–200. doi: 10.1084/jem.20041674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huntington ND, Tomioka R, Clavarino C, Chow AM, Linares D, Mana P, Rossjohn J, Cachero TG, Qian F, Kalled SL, Bernard CC, Reid HH. A BAFF antagonist suppresses experimental autoimmune encephalomyelitis by targeting cell-mediated and humoral immune responses. Int Immunol. 2006;18:1473–1485. doi: 10.1093/intimm/dxl080. [DOI] [PubMed] [Google Scholar]