Abstract
Background
Previous studies reported a higher risk of cognitive decline and dementia among individuals with impaired lung function. However, many did not adjust for important confounders or did not include women and nonwhites.
Methods
We studied 10,975 men and women aged 47–70 (23% African-Americans), enrolled in the Atherosclerosis Risk in Communities Study. Pulmonary function tests and a cognitive assessment, including the Delayed Word Recall, the Digit Symbol Substitution, and the World Fluency Tests, were done in 1990–92. Repeated cognitive assessments were performed in 1996–98 for the entire cohort, and in 1993–95 and 2004–06 in 904 eligible individuals. Dementia hospitalization was ascertained through 2005.
Results
In analysis adjusted for lifestyles, APOE genotype, and cardiovascular risk factors, impaired lung function was associated with worse cognitive function at baseline. No association was found between lung function and cognitive decline over time. Impaired lung function at baseline was associated with higher risk of dementia hospitalization during follow-up, particularly among younger individuals. The hazard ratios (95% confidence intervals) of dementia hospitalization were 1.6 (0.9, 2.8) and 2.1 (1.2, 3.7) comparing the lowest to the highest quartile of forced expiratory volume in 1 second and forced vital capacity, respectively. Presence of a restrictive ventilatory pattern, but not of an obstructive pattern, was associated with reduced cognitive scores and higher dementia risk.
Conclusion
Reduced lung function was associated with worse performance in cognitive assessments and with an increased risk of dementia hospitalization. Future research should determine whether maintaining optimal pulmonary health might prevent cognitive impairment and dementia.
Keywords: Lung function, cognitive decline, dementia, prospective studies
INTRODUCTION
With the ageing of the U.S. population, cognitive impairment and dementia will grow as a public health problem, requiring a better understanding of its etiology and impact [1, 2]. Previous epidemiologic research has shown that a host of environmental (non-genetic) factors, such as lifestyles and cardiovascular risk factors, are associated with the incidence of dementia and cognitive impairment in the general population [3, 4]. In addition, several reports have suggested a relationship between decreased lung function and cognitive decline [5–8], or dementia [9–11]. Lower lung function could be associated with dementia risk and cognitive decline through different mechanisms, such as chronic hypoxia or the development of a pro-inflammatory state [12–14].
Even though previous studies were prospective, some of them did not adequately control for smoking, apolipoprotein E (APOE) genotype, and other potential confounders, or did not include women and African-Americans. Furthermore, whether obstructive or restrictive ventilatory patterns are differently associated with cognitive function and dementia has not been assessed. Therefore, the objective of this study was to determine, in a large population-based cohort, whether lung function, assessed as forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), was associated with cognitive decline and the incidence of dementia. Additionally, we explored whether this association differed by age, gender, race, and APOE genotype.
MATERIALS AND METHODS
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective study of the natural history and etiology of atherosclerotic diseases in four US communities. Detailed methods have been presented elsewhere [15]. Briefly, the ARIC Study recruited 15,792 men and women aged 45–64 years in Forsyth County, North Carolina; Jackson, Mississippi; selected suburbs of Minneapolis, Minnesota; and Washington County, Maryland. ARIC participants were examined at baseline (1987–1989) and three more times approximately every 3 years (last exam 1996–1998). Response rates among survivors for the successive examinations were 93%, 86% and 80%. Additionally, annual phone follow-up calls are made to all surviving cohort members (93% response rate on average). The study was approved by Institutional Review Boards at participating institutions and participants provided written informed consent in each exam.
The second visit in the ARIC study, conducted from 1990 to 1992, included a cognitive assessment. For the purpose of the present analysis, we restricted our sample to ARIC participants who attended the second examination, were white from Minnesota, Washington County and Forsyth County communities, or African–American from Jackson and Forsyth County, and consented to the use of genetic data (n=14,231). This visit (“visit 2” in the overall ARIC study) constitutes the baseline visit for the current report. We excluded individuals who had a history of stroke, transient ischemic attack, or coronary artery disease (n=1359), had missing cognitive function assessed at baseline (n=220), did not have pulmonary function measures (n=417), did not have APOE genotype information (n=571) or had missing information in any marker of socioeconomic status (n=856) or any other covariate (n=409). Overall, 10,975 ARIC participants were eligible. Of these, 8820 (80%) participants had a second cognitive assessment at visit 4, conducted in 1996–1998. Additional cognitive assessments were conducted in a sample of participants from two ARIC communities, Forsyth County and Jackson, in 1993–1995 (n=2136), and in 2004–2006 (n=1134).
Assessment of lung function
Lung function was measured at ARIC visit 2 by trained and certified technicians via the forced vital capacity (FVC) maneuver, in which the maximal volume of air is exhaled during a forced expiration starting from a position of full inspiration and ending at complete expiration [16]. Forced expiratory volume in 1 second (FEV1) is the volume of gas exhaled in the first second of expiration, and FVC is the total volume of gas exhaled. Collins Survey II water-seal spirometers (Collins Medical, Inc., Braintree, Massachusetts) driven by IBM PC/XT computers and under the control of Pulmo-Screen software (PDS Healthcare Products, Inc., Louisville, Colorado) were used to assist the technicians with quality control, calculation of pulmonary function variables, and compilation of results for transmission to the ARIC Pulmonary Function Reading Center. Quality control was carefully monitored throughout the study. Participants performed the FVC maneuver until there were two error-free reproducible maneuvers (FEV1 and FVC within 5 percent) out of three acceptable maneuvers, with the maneuvers repeated up to eight times if necessary [16].
Cognitive assessment
A detailed description of the cognitive assessment has been published [17]. The assessment included the delayed word recall (DWR) test, the digit symbol substitution (DSS) test of the Wechsler Adult Intelligence Scale–Revised (WAIS-R), and the first-letter word fluency (WF) test. Trained interviewers in a standardized order administered the tests during one session in a quiet room. Interviewer performance was monitored by tape recording, and samples of the testing sessions were reviewed to confirm that there were no systematic differences between the different interviewers who administered the tests. The same test versions were used in all visits.
The DWR is a test of verbal learning and recent memory that requires the participant to recall 10 common nouns following a 5-minute interval [18]. In order to produce elaborative processing during the encoding stage of learning, individuals were required to construct sentences incorporating the words presented. During the delay interval, the DSS test was given. After 5 minutes, free recall of the words was sought.
The DSS of the WAIS-R, a test of executive function, is a paper-and-pencil task entailing timed translation of numbers to symbols using a key given at the top of the test page [19]. The test was scored as the number of correct translations completed within 90 seconds.
The WF test assesses psychomotor speed, requiring the participant to generate as many words as possible, but not proper names or places, beginning with a particular letter of the alphabet within 60 seconds [20]. Three separate 1-minute trial periods were used for each of the letters F, A, and S.
Other baseline measurements
Information on medical history, as well as socioeconomic and lifestyle factors such as education, income, smoking and physical activity, was obtained by trained interviewers in all study visits. Smoking status was characterized as pack-years of smoking and as current, former, or never smoking. Never smokers were defined as persons who had not smoked more than 400 cigarettes in their lifetime. Physical activity was assessed using the previously validated Baecke questionnaire [21]. Body mass index (weight, in kilograms/height2, in meters) was calculated from measurements taken with participants standing in scrub suits and without shoes. Sitting blood pressure was measured three times using a random-zero sphygmomanometer, and the average of the last two readings was used. Blood specimens were drawn and processed following a standardized protocol [22]. Vital exhaustion, which measures excessive fatigue, demoralization, and irritability, and is correlated with depressive symptoms [23], was measured using the 21-item Maastricht vital exhaustion questionnaire [24].
Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or self-reported use of antihypertensive medication. Diabetes mellitus was defined as a fasting glucose level ≥126 mg/dl (7.0 mmol/liter), a nonfasting glucose level of >200 mg/dl (11.1 mg/dl), a self-reported physician diagnosis, or pharmacologic hypoglycemic treatment.
Genotyping of the APOE polymorphisms was performed using the TaqMan assay (Applied Biosystems, Foster City, CA) with variants at codons 130 and 176 (formerly 112 and 158) assayed separately. The data from these two codons were combined to generate the six APOE genotypes. The ABI 7900 and Sequence Detection System software (Applied Biosystems) was used for allele detection and genotype calling [25]. Genotypes were classified as:ε2/ε2+ε2/ε3, ε3/ε3 (reference), ε2/ε4+ε3/ε4, ε4/ε4.
Dementia hospitalization
Incident dementia was identified through hospital discharge codes obtained from ARIC participants hospitalizations through 31 December 2005. Hospitalizations in ARIC are identified by participant or proxy report in the annual follow-up and by surveillance of local hospital discharge lists. The ICD-9 codes used to define dementia referred to Alzheimer disease (331.0), vascular dementia (290.4) or any other code that could have been used for dementia of other etiology (290.0, 290.1, 290.2, 290.3, 290.9, 294.1, 294.2, 294.8, 294.9, 331.1, 331.2, 331.8, 331.9). The first occurrence of any of these codes was considered the event time. For analyses of incident dementia, we excluded ARIC participants with low scores (below 5th race and gender-specific percentile) in any of the cognitive tests at exam 2. Therefore, we assumed cases to be new (incident) events. In a previous publication, we have shown a strong association of APOE genotype and scores in cognitive tests with dementia hospitalization, which indirectly supports the validity of this endpoint [4].
Statistical analysis
In statistical models, pulmonary function measurements (FEV1, FVC, FEV1/FVC ratio) were considered as gender-specific quartiles and as continuous variables. Additionally, we further classified individuals in four ventilatory patterns according to their FEV1/FVC ratio and percentage of predicted FVC (FVC%): normal pattern (FEV1/FVC≥70%, FVC%≥80%), restrictive pattern (FEV1/FVC≥70%, FVC%<80%), obstructive pattern (FEV1/FVC<70%, FVC%≥80%), and mixed pattern (FEV1/FVC<70%, FVC%<80%) [26]. We first estimated the cross-sectional association of pulmonary function tests with cognitive test scores obtained at visit 2 using multivariable linear regression models. A first model tested the association of each pulmonary function test with DWR, DSS and WF scores separately, adjusting for age, gender, and race. An additional model adjusted for potential confounders of the association, including study center, smoking (current, past, never), pack-years of smoking (continuous), systolic blood pressure (continuous), body mass index (continuous), total cholesterol (continuous), HDL cholesterol (continuous), APOE genotype (ε2/ε2+ε2/ε3, ε3/ε3, ε2/ε4+ε3/ε4, ε4/ε4), use of antihypertensive medication (yes/no), diabetes (yes/no), use of lipid lowering medication (yes/no), education (six categories), occupation (nine categories), income (ordinal, with eight levels), and sports-related physical activity (continuous). Finally, we ran models restricted to non-smokers and adjusting for the mentioned variables. In all analyses, pulmonary function tests were categorized in gender-specific quartiles. Linear trends were tested including pulmonary function test results as continuous variables in the models. Sensitivity analyses were conducted additionally adjusting for height and using percent of age, gender, race and height predicted lung function instead of measured lung function.
The association of lung function with change in cognitive scores between visit 2 and visit 4 was also estimated with multivariable linear regression, with the difference in cognitive test between visit 4 and visit 2 as the main outcome variable. Models were similar to those used for the cross-sectional analysis. In addition, we explored 16-year change in cognitive score tests in the selected sample of participants who had additional cognitive assessments in 1993–1995 and 2004–2006 (n=904) using a linear model for repeated measures (PROC MIXED in SAS). Models included the variables considered in previous analyses, as well as time of follow-up and an interaction term between exposures and time.
Finally, we estimated adjusted hazard ratios (HR) of dementia hospitalization and their 95% confidence intervals (CI) by lung function tests at visit 2 using Cox proportional hazard models, with time from visit 2 to dementia hospitalization as the dependent variable. We estimated adjusted survival curves using a SAS macro developed by Zhang et al [27].
To assess whether associations differed by age, gender, race or APOE genotype, we conducted stratified analysis and tested interactions including multiplicative terms between the stratifying variables and the pulmonary function tests.
RESULTS
Tables 1 and 2 report baseline characteristics of the 10,975 ARIC women and men included in the study according to quartiles of FEV1 at visit 2. Individuals with worse lung function were more likely to be older, African-American, smokers, less educated, diabetic, hypertensive, and more likely to have higher body mass index and the APOEε4 allele. A similar pattern was observed for FVC (data not shown).
Table 1.
Selected characteristics of study participants by quartiles of forced expiratory volume in 1 second (FEV1), women, ARIC Study, 1990–1992. Numbers correspond to means (standard deviation) or percentages.
| FEV1 | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| N | 1551 | 1581 | 1562 | 1545 |
| FEV1, range, L | <2.0 | 2.0–2.3 | 2.4–2.7 | >2.7 |
| mean (SD), L | 1.7 (0.3) | 2.2 (0.1) | 2.5 (0.1) | 2.9 (0.2) |
| FVC, L | 2.4 (0.4) | 2.9 (0.3) | 3.3 (0.3) | 3.8 (0.4) |
| Age, years | 59.1 (5.5) | 57.2 (5.6) | 55.8 (5.4) | 54.1 (4.8) |
| Race, % African-Americans | 40.3 | 27.7 | 21.5 | 13.4 |
| Education | ||||
| Less than high school | 32.0 | 23.3 | 16.0 | 10.8 |
| High school completed | 41.8 | 46.5 | 48.3 | 46.4 |
| College degree | 26.2 | 30.3 | 35.7 | 42.8 |
| Current smoker, % | 33.2 | 21.3 | 14.8 | 12.3 |
| Pack-years | 15.6 (20.7) | 9.9 (15.9) | 6.7 (12.1) | 5.9 (11.2) |
| Physical activity, sports index | 2.2 (0.7) | 2.3 (0.7) | 2.4 (0.7) | 2.4 (0.8) |
| Body mass index, kg/m2 | 29.1 (6.9) | 28.6 (6.1) | 27.8 (5.8) | 26.9 (5.2) |
| Diabetes, % | 20.2 | 15.7 | 10.7 | 6.6 |
| Hypertension, % | 46.2 | 36.8 | 31.3 | 20.8 |
| Systolic blood pressure, mmHg | 126.0 (20.1) | 121.4 (18.7) | 118.0 (17.4) | 115.5 (16.6) |
| Total cholesterol, mmol/L | 5.6 (1.1) | 5.6 (1.0) | 5.5 (1.0) | 5.4 (1.0) |
| HDL cholesterol, mmol/L | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 1.5 (0.4) |
| APOE ε4+, % | 32.4 | 30.7 | 30.8 | 28.3 |
| Delayed word recall | 6.5 (1.6) | 6.8 (1.4) | 7.0 (1.4) | 7.3 (1.3) |
| Digit substitution symbols | 40.6 (14.4) | 44.5 (13.8) | 49.3 (13.3) | 53.2 (12.9) |
| Word fluency | 30.8 (12.5) | 34.1 (12.1) | 34.8 (12.2) | 36.8 (11.7) |
Table 2.
Selected characteristics of study participants by quartiles of forced expiratory volume in 1 second (FEV1), men, ARIC Study, 1990–1992. Numbers correspond to means (standard deviation) or percentages.
| FEV1 | ||||
|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |
| N | 1185 | 1189 | 1168 | 1194 |
| FEV1, range, L | <2.8 | 2.8–3.3 | 3.4–3.7 | >3.7 |
| mean (SD), L | 2.3 (0.5) | 3.0 (0.1) | 3.5 (0.1) | 4.1 (0.4) |
| FVC, L | 3.5 (0.6) | 4.2 (0.4) | 4.6 (0.4) | 5.4 (0.5) |
| Age, years | 59.7 (5.4) | 57.7 (5.7) | 56.5 (5.5) | 54.2 (5.0) |
| Race, % African-Americans | 29.9 | 24.2 | 13.0 | 6.9 |
| Education | ||||
| Less than high school | 29.2 | 22.2 | 13.5 | 8.3 |
| High school completed | 36.5 | 36.9 | 39.2 | 35.8 |
| College degree | 34.3 | 40.9 | 47.3 | 56.0 |
| Current smoker, % | 37.1 | 25.5 | 17.6 | 12.2 |
| Pack-years | 31.2 (26.8) | 22.0 (23.9) | 16.5 (19.2) | 12.5 (16.7) |
| Physical activity, sports index | 2.4 (0.8) | 2.6 (0.8) | 2.6 (0.8) | 2.8 (0.8) |
| Body mass index, kg/m2 | 27.6 (4.8) | 28.1 (4.6) | 27.9 (3.9) | 27.2 (3.5) |
| Diabetes, % | 18.3 | 17.4 | 10.4 | 7.5 |
| Hypertension, % | 43.9 | 37.5 | 28.1 | 21.4 |
| Systolic blood pressure, mmHg | 126.6 (19.4) | 123.9 (17.4) | 120.3 (16.6) | 117.6 (14.3) |
| Total cholesterol, mmol/L | 5.3 (1.0) | 5.3 (0.9) | 5.3 (1.0) | 5.2 (1.0) |
| HDL cholesterol, mmol/L | 1.1 (0.4) | 1.1 (0.3) | 1.1 (0.3) | 1.1 (0.3) |
| APOE ε4+, % | 32.9 | 31.9 | 29.1 | 27.2 |
| Delayed word recall | 6.0 (1.6) | 6.3 (1.5) | 6.5 (1.5) | 6.7 (1.4) |
| Digit substitution symbols | 37.6 (13.5) | 39.7 (13.4) | 45.3 (11.8) | 48.9 (11.0) |
| Word fluency | 30.1 (13.0) | 32.0 (12.5) | 34.0 (11.9) | 36.1 (12.6) |
In cross-sectional analysis, individuals with lower FEV1 and FVC at visit 2 scored lower in the cognitive tests, even after adjustment for multiple confounders (table 3). In multivariable analysis, DWR, DSS and WF among those in the lowest quartile of FEV1 were 0.15 (95% 0.07, 0.24), 1.6 (95% 1.1, 2.2) and 1.2 (95% CI 0.5, 1.8) points lower, respectively, than among those in the upper quartile. Results were similar for FVC or for the sample restricted to nonsmokers (table 3), after additionally adjusting for height or vital exhaustion, or using percent of predicted lung function as the main exposure (data not shown). No clear associations with cognitive tests were found for the FEV1/FVC ratio. Compared to individuals with a normal ventilatory pattern, those with restrictive or mixed patterns had lower scores in cognitive tests, while no association was found between obstructive ventilatory pattern and cognitive tests (table 4).
Table 3.
Difference in cognitive score by gender-specific quartiles of FEV1, FVC and FEV1/FVC at visit 2, ARIC Study, 1990–1992.
| FEV1 | Q1 | Q2 | Q3 | Q4 | P for trend |
|---|---|---|---|---|---|
| DWR | |||||
| Model 1 | −0.32 (−0.40, −0.24) | −0.15 (−0.23, −0.08) | −0.10 (−0.17, −0.02) | Ref | <0.0001 |
| Model 2 | −0.15 (−0.24, −0.07) | −0.05 (−0.13, 0.03) | −0.06 (−0.14, 0.01) | Ref | 0.0005 |
| Model 3 | −0.18 (−0.32, −0.04) | −0.03 (−0.14, 0.09) | −0.10 (−0.21, 0.01) | Ref | 0.03 |
| DSS | |||||
| Model 1 | −4.81 (−5.44, −4.18) | −2.82 (−3.42, −2.23) | −1.44 (−2.02, −0.85) | Ref | <0.0001 |
| Model 2 | −1.63 (−2.20, −1.07) | −0.80 (−1.31, −0.28) | −0.59 (−1.09, −0.10) | Ref | <0.0001 |
| Model 3 | −2.09 (−3.01, −1.17) | −0.69 (−1.48, 0.09) | −0.69 (−1.43, 0.04) | Ref | <0.0001 |
| WF | |||||
| Model 1 | −3.58 (−4.27, −2.89) | −1.92 (−2.58, −1.26) | −1.25 (−1.89, −0.61) | Ref | <0.0001 |
| Model 2 | −1.15 (−1.80, −0.50) | −0.22 (−0.81, 0.37) | −0.46 (−1.02, 0.11) | Ref | 0.0003 |
| Model 3 | −0.98 (−2.00, 0.03) | 0.33 (−0.54, 1.20) | −0.54 (−1.36, 0.27) | Ref | 0.02 |
| FVC | Q1 | Q2 | Q3 | Q4 | P for trend |
| DWR | |||||
| Model 1 | −0.32 (−0.40, −0.24) | −0.15 (−0.23, −0.07) | −0.07 (−0.15, 0.00) | Ref | <0.0001 |
| Model 2 | −0.15 (−0.23, −0.06) | −0.05 (−0.13, 0.03) | −0.02 (−0.10, 0.05) | Ref | 0.0001 |
| Model 3 | −0.18 (−0.31, −0.04) | −0.06 (−0.18, 0.06) | −0.01 (−0.12, 0.10) | Ref | 0.01 |
| DSS | |||||
| Model 1 | −5.01 (−5.65, −4.37) | −2.84 (−3.43, −2.24) | −1.64 (−2.22, −1.06) | Ref | <0.0001 |
| Model 2 | −1.71 (−2.28, −1.14) | −0.94 (−1.45, −0.42) | −0.61 (−1.11, −0.12) | Ref | <0.0001 |
| Model 3 | −2.32 (−3.22, −1.42) | −1.11 (−1.92, −0.30) | −0.88 (−1.64, −0.12) | Ref | <0.0001 |
| WF | |||||
| Model 1 | −3.91 (−4.62, −3.21) | −2.27 (−2.93, −1.61) | −1.24 (−1.88, −0.60) | Ref | <0.0001 |
| Model 2 | −1.11 (−1.76, −0.45) | −0.59 (−1.19, 0.00) | −0.32 (−0.89, 0.24) | Ref | 0.0002 |
| Model 3 | −1.12 (−2.12, −0.12) | −0.19 (−1.08, 0.71) | −0.19 (−1.03, 0.65) | Ref | 0.01 |
| FEV1/FVC | Q1 | Q2 | Q3 | Q4 | P for trend |
| DWR | |||||
| Model 1 | −0.02 (−0.10, 0.06) | 0.06 (−0.01, 0.14) | 0.06 (−0.01, 0.14) | Ref | 0.19 |
| Model 2 | 0.03 (−0.05, 0.11) | 0.06 (−0.02, 0.14) | 0.07 (−0.01, 0.14) | Ref | 0.68 |
| Model 3 | 0.06 (−0.07, 0.19) | 0.01 (−0.10, 0.12) | 0.08 (−0.03, 0.18) | Ref | 0.54 |
| DSS | |||||
| Model 1 | −1.22 (−1.82, −0.61) | 0.31 (−0.29, 0.90) | 0.18 (−0.41, 0.76) | Ref | <0.0001 |
| Model 2 | −0.42 (−0.96, 0.12) | 0.14 (−0.36, 0.65) | 0.13 (−0.37, 0.62) | Ref | 0.03 |
| Model 3 | 0.03 (−0.86, 0.93) | 0.26 (−0.50, 1.01) | 0.83 (0.12, 1.54) | Ref | 0.69 |
| WF | |||||
| Model 1 | −0.17 (−0.83, 0.49) | 0.63 (−0.02, 1.29) | −0.09 (−0.73, 0.55) | Ref | 0.25 |
| Model 2 | −0.03 (−0.65, 0.59) | 0.29 (−0.29, 0.87) | −0.14 (−0.71, 0.42) | Ref | 0.85 |
| Model 3 | −0.21 (−1.19, 0.78) | 0.12 (−0.71, 0.96) | 0.00 (−0.78, 0.79) | Ref | 0.71 |
DWR: Delayed Word Recall test; DSS: Digit Symbol Substitution test; WF: Word Fluency test
Model 1: General linear model adjusted for age, gender, and race.
Model 2: As model 1, adjusted additionally for study center, cigarette smoking, pack-years of smoking, systolic blood pressure, body mass index, total cholesterol, HDL cholesterol, APOE genotype, use of hypertension medications, diabetes, use of lipid lowering medications, education, occupation, income and sports-related physical activity.
Model 3: As model 2, but restricted to non-smokers in visit 2
Table 4.
Difference in cognitive score by patterns of ventilatory function at visit 2, ARIC Study, 1990–1992
| Normal pattern | Obstructive pattern | Restrictive pattern | Mixed pattern | |
|---|---|---|---|---|
| N | 7969 | 1912 | 642 | 452 |
| DWR | ||||
| Model 1 | Ref | −0.06 (−0.14, 0.01) | −0.27 (−0.39, −0.16) | −0.22 (−0.36, −0.09) |
| Model 2 | Ref | −0.02 (−0.09, 0.06) | −0.17 (−0.28, −0.06) | −0.07 (−0.21, 0.07) |
| Model 3 | Ref | 0.05 (−0.09, 0.20) | −0.19 (−0.37, −0.01) | −0.19 (−0.54, 0.17) |
| DSS | ||||
| Model 1 | Ref | −1.30 (−1.86, −0.74) | −2.58 (−3.47, −1.69) | −4.58 (−5.63, −3.54) |
| Model 2 | Ref | −0.53 (−1.03, −0.04) | −0.71 (−1.47, 0.05) | −1.63 (−2.55, −0.72) |
| Model 3 | Ref | −0.27 (−1.22, 0.68) | −1.82 (−3.04, −0.61) | −1.64 (−4.01, 0.73) |
| WF | ||||
| Model 1 | Ref | −0.14 (−0.76, 0.47) | −2.48 (−3.46, −1.50) | −2.76 (−3.91, −1.60) |
| Model 2 | Ref | 0.08 (−0.48, 0.64) | −1.16 (−2.02, −0.29) | −1.00 (−2.04, 0.05) |
| Model 3 | Ref | 0.74 (−0.31, 1.78) | −1.47 (−2.81, −0.13) | −2.91 (−5.52, −0.30) |
DWR: Delayed Word Recall test; DSS: Digit Symbol Substitution test; WF: Word Fluency test
Model 1: General linear model adjusted for age, gender, and race.
Model 2: As model 1, adjusted additionally for study center, cigarette smoking, pack-years of smoking, systolic blood pressure, body mass index, total cholesterol, HDL cholesterol, APOE genotype, use of hypertension medications, diabetes, use of lipid lowering medications, education, occupation, income and sports-related physical activity.
Model 3: As model 2, but restricted to non-smokers in visit 2
The average decline in cognitive test scores between ARIC visit 2 and 4 was 0.14 (95% CI 0.11–0.17) for DWR, 2.5 (95% CI 2.4–2.7) for DSS, and 0.47 (95% CI 0.31–0.64) for WF. Neither FEV1 nor FVC at ARIC visit 2 were associated with change in cognitive score between both visits (supplement table S1). Among 904 ARIC participants who had two additional cognitive assessments (the last one in 2004–2006), the average decline per 10 years (and 95% CIs) was 0.53 (0.45, 0.61) for DWR, 3.1 (2.8, 3.4) for DSS, and 1.3 (1.0, 1.6) for WF. Overall, individuals included in this analysis had slightly better pulmonary function and cognitive scores than the entire cohort. For example, age, gender and race-adjusted mean FVC was 3.7 L vs. 3.6 L in those included and not included (p<0.001), and mean DWR was 6.7 vs. 6.4 in each group (p<0.001). As in the analysis considering only two cognitive assessments, pulmonary function tests were not associated with changes in cognitive score over time (supplement table S2).
We also studied the association of lung function with incidence of dementia hospitalization among 9837 ARIC participants. During a median follow-up of 14.1 years, 205 cases of dementia hospitalization were identified. Both FEV1 and FVC were associated with this outcome. The HR (95% CI) of dementia hospitalization among those in the lowest quartile of FEV1 compared to those in the highest one was 1.6 (0.9, 2.3). The corresponding figure for FVC was 2.1 (1.2, 3.7) (table 5). The FEV1/FVC ratio was not associated with the incidence of dementia hospitalization. Consistently, dementia risk was higher among individuals presenting a restrictive ventilatory pattern (HR 1.6, 95% CI 1.0–2.6, in an age, gender, and race-adjusted analysis, and 1.4, 95% CI 0.9–2.3 in a multivariable model), but not among those with obstructive or mixed ventilatory patterns (multivariable HR 1.0, 95% CI 0.7–1.4 and 1.3, 95% CI 0.7–2.3, respectively), compared to normal.
Table 5.
Hazard ratios (95% confidence intervals) of dementia hospitalization by quartiles of FEV1, FVC and FEV1/FVC ratio at visit 2, ARIC, 1990–2005
| FEV1 | Q1 | Q2 | Q3 | Q4 | P for trend |
|---|---|---|---|---|---|
| N. cases | 87 | 54 | 46 | 18 | |
| Person-years | 29736 | 33288 | 34434 | 35765 | |
| Model 1 | 2.46 (1.44, 4.19) | 1.79 (1.04, 3.09) | 1.89 (1.09, 3.27) | 1 (ref) | <0.0001 |
| Model 2 | 1.59 (0.91, 2.78) | 1.40 (0.81, 2.44) | 1.68 (0.97, 2.92) | 1 (ref) | 0.03 |
| Model 3 | 1.83 (0.71, 4.69) | 1.95 (0.80, 4.72) | 1.88 (0.78, 4.54) | 1 (ref) | 0.21 |
| FVC | Q1 | Q2 | Q3 | Q4 | P for trend |
| N. cases | 88 | 54 | 47 | 16 | |
| Person-years | 30120 | 32861 | 33949 | 36294 | |
| Model 1 | 2.95 (1.68, 5.18) | 2.22 (1.26, 3.92) | 2.33 (1.32, 4.12) | 1 (ref) | <0.0001 |
| Model 2 | 2.08 (1.16, 3.72) | 1.83 (1.03, 3.26) | 2.09 (1.18, 3.70) | 1 (ref) | 0.006 |
| Model 3 | 2.19 (0.82, 5.86) | 1.99 (0.77, 5.12) | 2.46 (0.97, 6.20) | 1 (ref) | 0.20 |
| FEV1/FVC | Q1 | Q2 | Q3 | Q4 | P for trend |
| N. cases | 60 | 44 | 47 | 54 | |
| Person-years | 31850 | 33698 | 33904 | 33771 | |
| Model 1 | 1.06 (0.72, 1.56) | 0.80 (0.53, 1.21) | 0.93 (0.63, 1.38) | 1 (ref) | 0.07 |
| Model 2 | 0.84 (0.55, 1.28) | 0.78 (0.52, 1.19) | 0.97 (0.65, 1.45) | 1 (ref) | 0.75 |
| Model 3 | 1.09 (0.52, 2.30) | 0.83 (0.41, 1.68) | 1.60 (0.88, 2.90) | 1 (ref) | 0.56 |
Model 1: Cox proportional hazards model adjusted for age, gender, and race.
Model 2: As model 1, adjusted additionally for study center, cigarette smoking, pack-years of smoking, systolic blood pressure, body mass index, total cholesterol, HDL cholesterol, APOE genotype, use of hypertension medications, diabetes, use of lipid lowering medications, education, occupation, income and sports-related physical activity.
Model 3: As model 2, but restricted to non-smokers in visit 2
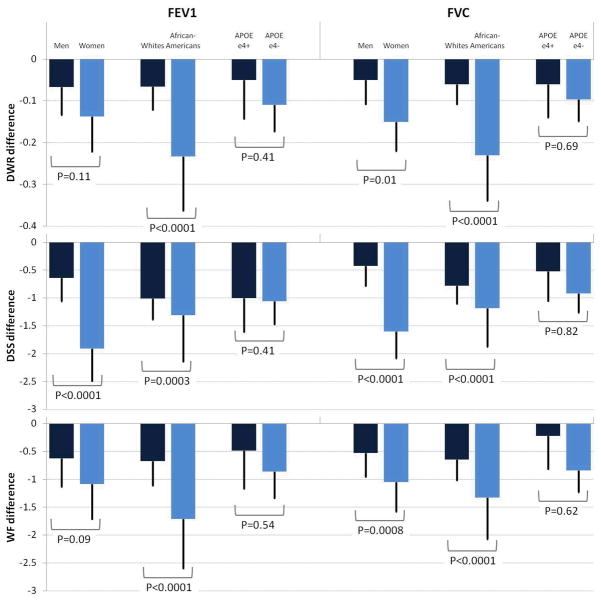
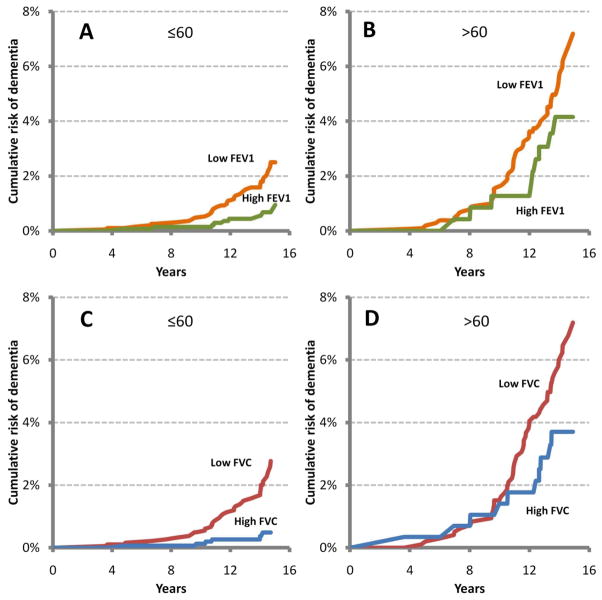
Associations were similar in individuals with or without the APOEε4 allele. However, gender and race modified the association of lung function with cognitive scores in the cross-sectional analysis. Worse lung function was more strongly associated with lower scores in the cognitive tests in women and African-Americans, compared to men and whites, respectively (figure 1 and supplement table S3). However, no differences by gender, race, or APOE genotype were found for the associations of lung function with dementia hospitalization or cognitive score change (data not shown). Finally, age did not modify the association of lung function with cognitive function in cross-sectional or longitudinal analysis, but in younger individuals (≤60 at baseline) worse lung function was more strongly related with the incidence of dementia hospitalization than in the older group (figure 2). HR (95% CI) of dementia comparing extreme quartiles of FEV1 was 1.7 (0.8, 4.0) in those 60 or younger, and 1.3(0.6, 2.8) in older participants (p for interaction=0.07), while the corresponding figures for FVC were 2.5 (1.0–6.6) for younger and 1.6 (0.8–3.2) for older participants (p for interaction=0.02)
Figure 1.
Cognitive score difference by 1 liter increase in FEV1 or FVC at visit 2, ARIC, 1990–1992. Analysis stratified by gender, race, and APOE genotype.
Figure 2.
Cumulative risk of dementia hospitalization by FEV1 and FVC and by age (≤60 or >60). Low and high FVC or FEV1 defined as extreme quartiles (low: quartile 1, high: quartile 4). Results are based on survival curves from a Cox model, adjusting for age, gender, race, cigarette smoking, and APOE genotype, using the methodology proposed by Zhang et al.[27] Panel A: low vs. high FEV1 in ≤60 years old. Panel B: low vs. high FEV1 in >60 years old. Panel C: low vs. high FVC in ≤60 years old. Panel D: low vs. high FVC in >60 years old.
DISCUSSION
In this large population-based study, we found that individuals with reduced FEV1 or FVC performed worse in three different cognitive tests and had an increased risk of being hospitalized with a diagnosis of dementia. Lung function, however, was not associated with cognitive score change over time. Cross-sectional associations were stronger in women and African-Americans than in men and whites, respectively. However, this interaction was not present in the longitudinal analysis. Worse lung function was a stronger predictor of dementia hospitalization in younger than older individuals. Finally, presence of a restrictive ventilatory pattern, but not of an obstructive pattern, was associated with reduced cognitive scores and higher dementia risk. Our results are mostly consistent with those from previous publications, which showed higher risk of dementia and cognitive impairment in individuals with lower lung function [5–10]. We extend these results to a large biracial population.
The present analysis has significant strengths. We have included a large population, with a comprehensive assessment of potential confounders of the association between lung function and cognitive impairment or dementia, including APOE genotype. Major limitations, however, include the limited time elapsed between cognitive assessments in the entire cohort and the low sensitivity of the dementia diagnosis. Even though in a previous publication we showed that cognitive impairment and APOE genotype were strong predictors of dementia hospitalization in the ARIC Study [4], dementia hospitalization is characteristically insensitive to mild dementia. Moreover, information from hospitalization discharge codes is not adequate to differentiate between Alzheimer type dementia and vascular dementia. In addition, considering the strong associations of lung function with education, smoking, diabetes and other variables known to affect cognitive test performance, we are concerned that our models may not have entirely eliminated potential residual confounding of the cross-sectional associations by socioeconomic variables or lifestyles. Reverse causation in the cross-sectional analysis might account for some results if those with worse cognitive function are more likely to underperform in the pulmonary function tests. Finally, the longitudinal cognitive assessment included a sample somewhat healthier than the entire cohort, which could cause selection bias or make difficult to find associations if these are restricted to sicker individuals.
In addition to the highlighted methodological limitations, different pathogenic mechanisms could explain the association of lung function with cognitive performance and risk of dementia. First, chronic hypoxia, a potential consequence of low lung function, might lead to neurodegeneration [12, 14]. Unfortunately, we did not have information on the extent of hypoxia. Second, reduced lung function might facilitate development of ischemic brain injury. In fact, previous prospective studies have found that individuals with low lung function or reduced arterial oxygen saturation are more likely to develop white matter lesions and lacunar infarcts [28, 29]. Third, impaired lung function has been associated with diabetes, subclinical atherosclerosis, and an increased risk of cardiovascular outcomes [30–32]. In turn, diabetes and cardiovascular disease might cause cognitive impairment and increase the risk of dementia [3, 4, 11, 33]. Finally, worse lung function might cause cognitive impairment and dementia through the development of a pro-inflammatory state. High levels of C-reactive protein, elevated in individuals with reduced lung function [34], have been associated with higher risk of dementia [35].
We found that a restrictive ventilatory pattern, characterized by decreased FVC but normal FEV1/FVC ratio, was associated with worse cognitive function and higher risk of dementia compared with a normal pattern. No association was found between an obstructive ventilatory pattern and cognitive function or dementia. No previous studies have evaluated whether different abnormal ventilatory patterns affect dementia or cognitive function. Mechanisms underlying these results are unclear. Cerebrovascular disease, known to cause cognitive decline and dementia, could be more frequent among individuals with restrictive ventilatory pattern [26, 36]. A restrictive ventilatory pattern, but not an obstructive pattern, has been also associated with the incidence of diabetes in the ARIC study and other populations [30, 37]; diabetes might lead to cognitive dysfunction and dementia [3, 4].
Contrary to a previous publication [10], we did not find an interaction between lung function and APOE genotype. Differences in the study population and in the method of dementia ascertainment, or random variability could be responsible for the inconsistency. In the ARIC Study, however, lung function was a better predictor of dementia hospitalization in younger than older participants. Analogous results have been reported previously for other potential risk factors for dementia, such as smoking and other cardiovascular risk factors [4, 38]. Similarly, the association of lung function with scores in cognitive tests was stronger in women than in men, parallel to results from other studies showing a stronger association of APOE genotype with dementia or cognitive impairment among women [39, 40]. No previous studies, however, have found race to be an effect modifier of risk factors for cognitive impairment or dementia.
In conclusion, we found that low lung function is associated with worse performance in cognitive assessments and with an increased risk of dementia hospitalization. Our results highlight the need to conduct intervention studies to determine whether maintaining optimal pulmonary health might prevent cognitive decline and dementia later in life.
Supplementary Material
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 2.Borenstein Graves A. Alzheimer’s disease and vascular dementia. In: Nelson LM, Tanner CM, Van Den Eeden SK, McGuire V, editors. Neuroepidemiology: from principles to practice. New York: Oxford University Press; 2004. pp. 102–130. [Google Scholar]
- 3.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 4.Alonso A, Mosley TH, Gottesman R, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalization associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) Study. J Neurol Neurosurg Psychiatr. 2009;80:1194–1201. doi: 10.1136/jnnp.2009.176818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur Studies of Successful Aging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 6.Emery CF, Pedersen NL, Svartengren M, McClearn GE. Longitudinal and genetic effects in the relationship between pulmonary function and cognitive performance. J Gerontol B Psychol Sci Soc Sci. 1998;53:311–317. doi: 10.1093/geronb/53b.5.p311. [DOI] [PubMed] [Google Scholar]
- 7.Chyou P-H, White LR, Yano K, et al. Pulmonary function measures as predictors and correlates of cognitive functioning in later life. Am J Epidemiol. 1996;143:750–756. doi: 10.1093/oxfordjournals.aje.a008812. [DOI] [PubMed] [Google Scholar]
- 8.Richards M, Strachan D, Hardy R, Kuh D, Wadsworth M. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosomatic Medicine. 2005;67:602–608. doi: 10.1097/01.psy.0000170337.51848.68. [DOI] [PubMed] [Google Scholar]
- 9.Guo X, Waern M, Sjogren K, et al. Midlife respiratory function and incidence of Alzheimer’s disease: A 29-year longitudinal study in women. Neurobiol Aging. 2007;28:343–350. doi: 10.1016/j.neurobiolaging.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Giltay EJ, Nissinen A, Giampaoli S, Kromhout D. Apolipoprotein E genotype modifies the association between midlife lung function and cognitive function in old age. Dement Geriatr Cogn Disord. 2009;28:433–441. doi: 10.1159/000255600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso A, Jacobs DR, Jr, Menotti A, et al. Cardiovascular risk factors and dementia mortality: 40 years of follow-up in the Seven Countries Study. J Neurol Sci. 2009;280:79–83. doi: 10.1016/j.jns.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Peers C, Dallas ML, Boycott HE, Scragg JL, Pearson HA, Boyle JP. Hypoxia and neurodegeneration. Ann N Y Acad Sci. 2009;1177:169–177. doi: 10.1111/j.1749-6632.2009.05026.x. [DOI] [PubMed] [Google Scholar]
- 13.Engstrom G, Lind P, Hedblad B, et al. Lung function and cardiovascular risk: relationship with inflammation-sensitive plasma proteins. Circulation. 2002;106:2555–2560. doi: 10.1161/01.cir.0000037220.00065.0d. [DOI] [PubMed] [Google Scholar]
- 14.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35:913–922. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 15.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 16.Atherosclerosis Risk in Communities Study Manual 4: Pulmonary Function. Chapel Hill, NC: National Heart, Lung, and Blood Institute of the National Institutes of Health, Collaborative Studies Coordinating Center, University of North Carolina; 1987. [Google Scholar]
- 17.Cerhan JR, Folsom AR, Mortimer JA, et al. Correlates of cognitive function in middle-aged adults. Gerontology. 1998;44:95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 18.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–145. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. The Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corp; 1981. [Google Scholar]
- 20.Lezak MD. Neuropsychological assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- 21.Baecke JAH, Burema J, Fritjers JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 22.Atherosclerosis Risk in Communities Study Manual 7: Blood Collection. Chapel Hill, NC: National Heart, Lung, and Blood Institute of the National Institutes of Health, Collaborative Studies Coordinating Center, University of North Carolina; 1987. [Google Scholar]
- 23.McGowan L, Dickens C, Percival C, Douglas J, Tomenson B, Creed F. The relationship between vital exhaustion, depression and comorbid illnesses in patients following first myocardial infarction. Journal of Psychosomatic Research. 2004;57:183–188. doi: 10.1016/S0022-3999(03)00610-X. [DOI] [PubMed] [Google Scholar]
- 24.Appels A, Höppener P, Mulder P. A questionnaire to assess premonitory symptoms of myocardial infarction. Int J Cardiol. 1987;17:15–24. doi: 10.1016/0167-5273(87)90029-5. [DOI] [PubMed] [Google Scholar]
- 25.Volcik KA, Barkley RA, Hutchinson RG, et al. Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC Study participants. Am J Epidemiol. 2006;164:342–348. doi: 10.1093/aje/kwj202. [DOI] [PubMed] [Google Scholar]
- 26.Scarlata S, Pedone C, Fimognari FL, Bellia V, Forastiere F, Incalzi RA. Restrictive pulmonary dysfunction at spirometry and mortality in the elderly. Respiratory Medicine. 2008;102:1349–1354. doi: 10.1016/j.rmed.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Loberiza FR, Klein JP, Zhang M-J. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 28.van Dijk EJ, Vermeer SE, de Groot JC, et al. Arterial oxygen saturation, COPD, and cerebral small vessel disease. J Neurol Neurosurg Psychiatr. 2004;75:733–736. doi: 10.1136/jnnp.2003.022012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo X, Pantoni L, Simoni M, et al. Midlife respiratory function related to white matter lesions and lacunar infarcts in late life. The Prospective Population Study of Women in Gothenburg, Sweden. Stroke. 2006;37:1658–1662. doi: 10.1161/01.STR.0000226403.00963.af. [DOI] [PubMed] [Google Scholar]
- 30.Yeh H-C, Punjabi NM, Wang N-Y, Pankow JS, Duncan BB, Brancati FL. Vital capacity as a predictor of incident type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabet Care. 2005;28:1472–1479. doi: 10.2337/diacare.28.6.1472. [DOI] [PubMed] [Google Scholar]
- 31.Schroeder EB, Welch VL, Couper D, et al. Lung function and incident coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2003;158:1171–1181. doi: 10.1093/aje/kwg276. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder EB, Welch VL, Evans GW, Heiss G. Impaired lung function and subclinical atherosclerosis: the ARIC study. Atherosclerosis. 2005;180:367–373. doi: 10.1016/j.atherosclerosis.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study Cohort. J Am Geriatr Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 34.Jiang R, Burke GL, Enright PL, et al. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008;168:602–610. doi: 10.1093/aje/kwn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia aging study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 36.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal analysis. Thorax. 2010;65:499–504. doi: 10.1136/thx.2009.126052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wannamethee SG, Shaper AG, Rumley A, et al. Lung function and risk of type 2 diabetes and fatal and nonfatal major coronary heart disease events: possible associations with inflammation. Diabet Care. 2010;33:1990–1996. doi: 10.2337/dc10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernán MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19:448–450. doi: 10.1097/EDE.0b013e31816bbe14. [DOI] [PubMed] [Google Scholar]
- 39.Bretsky PM, Buckwalter JG, Seeman TE, et al. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Bartrés-Faz D, Junqué C, Moral P, López-Alomar A, Sánchez-Aldeguer J, Clemente IC. Apolipoprotein E gender effects on cognitive performance in age-associated memory impairment. J Neuropsychiatry Clin Neurosci. 2002;14:80–83. doi: 10.1176/jnp.14.1.80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.