Abstract
Externalizing is a broad construct that reflects propensity toward a variety of impulse control problems, including antisocial personality disorder and substance use disorders. Two event-related potential responses known to be reduced among individuals high in externalizing proneness are the P300, which reflects post-perceptual processing of a stimulus, and the error-related negativity (ERN), which indexes performance monitoring based on endogenous representations. The current study employed a simulated gambling task to examine the relationship between externalizing proneness and the feedback-related negativity (FRN), a brain response that indexes performance monitoring related to exogenous cues, which is thought to be highly related to the ERN. Time-frequency (TF) analysis was used to disentangle the FRN from the accompanying P300 response to feedback cues by parsing the overall feedback-locked potential into distinctive theta (4–7 Hz) and delta (< 3 Hz) TF components. Whereas delta-P300 amplitude was reduced among individuals high in externalizing proneness, theta-FRN response was unrelated to externalizing. These findings suggest that, in contrast with previously reported deficits in endogenously-based performance monitoring (as indexed by the ERN), individuals high in externalizing tendencies show intact monitoring of exogenous cues (as indexed by the FRN). The results also contribute to a growing body of evidence indicating that the P300 is attenuated across a broad range of task conditions in high-externalizing individuals.
Keywords: externalizing, disinhibition, performance monitoring, feedback-related, negativity, event-related potential, time-frequency
Impulse control problems of differing types, including child and adult antisocial behavior and abuse of alcohol and other drugs, exhibit high rates of comorbidity in the population, leading to suggestions that these disorders may be etiologically related (for early proposals of this sort, see: Achenbach & Edelbrock, 1978; Jessor & Jessor, 1977). Recently, researchers have documented an underlying dimension of proneness toward disorders of this type, labeled “externalizing,” that is associated also with personality traits of impulsivity, aggression, and sensation seeking (Krueger, 1999; Krueger, McGue, & Iacono, 2001; Krueger et al., 2002). Variation in general proneness to externalizing problems and traits has been shown to be highly heritable (>80%; Krueger et al., 2002). From this standpoint, the dimension of externalizing proneness represents an important target for neurobiological research on psychopathology, and studies have begun to examine brain-processing deviations associated with variations in externalizing proneness. These studies have demonstrated inverse relationships between levels of externalizing tendencies and amplitude of two brain event-related potential (ERP) components: the error-related negativity, or ERN (a negative polarity response that occurs following performance errors on speeded behavioral tasks; Hall, Bernat, & Patrick, 2007), and the P300 (a positive polarity response that occurs to task-relevant stimuli; Patrick et al., 2006).
Understanding the the relationship between diminished ERN response and externalizing proneness is an important priority because the ERN appears to reflect an underlying process of high functional relevance to externalizing disorders—namely, a reduced ability to recognize errors in performance and to adjust behavior accordingly. The current study contributes to this objective by examining externalizing proneness in relation to another brain-based measure of performance monitoring, believed to be related to the ERN—the feedback-related negativity (FRN, or f-ERN; Gehring & Willoughby, 2002; Holroyd & Coles, 2002; Miltner, Braun, & Coles, 1997). The FRN is a negative-polarity ERP component that occurs following the presentation of explicit feedback signaling poor performance or loss outcomes.1 The FRN has been posited to reflect an underlying neural process similar to the ERN (i.e., a common performance-monitoring process that relies heavily on engagement of the anterior cingulate cortex; Gehring & Willoughby, 2002; Holroyd & Coles, 2002; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003). However, whereas the ERN reflects an endogenous (internally-cued) error detection or action monitoring process, the FRN reflects the processing of external performance cues. As discussed below, these commonalities and distinctions make the FRN a potentially useful measure for further examining and clarifying underlying performance monitoring deficits associated with externalizing proneness.
In addition to testing for a relationship between externalizing proneness and the FRN, the current study also examined whether P300 amplitude would be reduced in the choice-feedback paradigm in which the FRN is measured. Prior work has consistently demonstrated reduced P300 amplitude in individuals with impulse control problems including alcohol dependence (Polich, Pollock, & Bloom, 1994) and antisocial personality disorder (e.g., Costa et al., 2000), along with disinhibitory personality traits (e.g., Justus, Finn, & Steinmetz, 2001). Recently, Patrick et al. (2006) established a link between reduced P300 and general externalizing proneness, operationalized as the overlap in symptoms among differing DSM disorders (conduct disorder, adult antisocial behavior, alcohol dependence, and drug dependence). These prior studies have focused on P300 response to simple target stimuli (requiring a response) in a visual oddball task, the procedure most commonly used in the P300 literature. The performance monitoring literature has shown that the P300 can also be measured following the presentation of feedback stimuli, and this P300 response appears functionally distinct from the FRN that follows the same stimuli (Frank, Woroch, & Curran, 2005; Yeung & Sanfey, 2004). Thus, we sought to determine whether the oddball P300 reduction associated with externalizing proneness generalizes to feedback stimuli. Evaluating this relationship across differing contexts is important for gaining understanding of the generality of P300-related processing deficits in individuals high in externalizing proneness.
Time-Frequency Decomposition
An important challenge in measuring both FRN and P300 responses to stimuli within a common feedback task is that these two ERP components overlap partially in time, complicating standard time-domain methods of response quantification. To better isolate these distinctive feedback-locked components, we employed time-frequency (TF) analysis, an emerging tool in the psychophysiological literature that provides for separation of ERP components that overlap in time but have differing spectral (frequency) characteristics. Prior work has shown that the FRN and P300 in fact operate at different frequencies, and that they can be separated using TF approaches. Specifically, the P300 is composed largely of activity in the delta (< 3 Hz) range (Ba ar-Eroglu, Ba ar, Demiralp, & Schürmann, 1992; Ba ar-Eroglu, Demiralp, Schürmann, & Ba ar, 2001; Bernat, Malone, Williams, Patrick, & Iacono, 2007; Demiralp, Ademoglu, Istefanopulos, Ba ar-Eroglu, & Ba ar, 2001; Gilmore, Malone, Bernat, & Iacono, 2009), whereas the FRN (like the ERN) is composed more predominantly of activity in the theta (4–7 Hz) range (Gehring & Willoughby, 2004). In the current study, a recently developed TF decomposition method (Bernat, Williams, & Gehring, 2005) was used to isolate theta and delta components of the ERP response to explicit performance feedback. This method has been used previously to characterize both theta activity related to the ERN (Bernat et al., 2005; Hall et al., 2007) and delta activity underlying the P300 response (Bernat et al., 2007; Gilmore et al., 2009). To illustrate the utility of the TF approach for isolating these distinctive brain responses, the Results section includes a direct comparison of time-domain and TF approaches to the quantification of FRN and P300 responses in the current dataset.
Current Study
The current study was conducted with two specific aims in mind: (a) to evaluate whether the FRN exhibits reduced amplitude as a function of higher externalizing tendencies, and (b) to assess for accompanying reductions in amplitude of the P300 response to feedback stimuli in the same task. As stated earlier, these aims were intended to shed light on two broader questions about deficits in brain reactivity related to externalizing proneness: (1) Is increased externalizing proneness associated with generalized deficits in performance monitoring, affecting registration of external feedback (reflected by the FRN) as well as self-recognition of errors (reflected by the ERN)?; and (2) To what extent do P300 response deficits, demonstrated for target stimuli in oddball tasks in prior work, generalize to stimuli of other types in a non-oddball task?
Method
Participants
Participants were 166 undergraduate students recruited from introductory psychology classes at the University of Minnesota who received either monetary compensation or course credit. Eighteen of these were excluded from analyses: eight because of incomplete questionnaire data, three due to equipment problems during collection, four due to excessive artifacts, and two who discontinued prior to the completion of testing. Thus, the final study sample consisted of 149 participants (58 male; age, M=20.57, SD=3.70).2 A subset of these (N = 89) overlapped with the sample tested in the ERN study by Hall et al. (2007), with the remainder (N = 60) selected using the same sampling strategy as in Hall et al. Individuals scoring in the lowest and highest quartiles of the distribution of scores on an abbreviated version of the Externalizing Spectrum Inventory (ESI; see below) were over-sampled in the selection process to enhance the representation of individuals extreme (low and high) in externalizing proneness. Of the 149 participants comprising the final sample, 57 scored as High and 40 scored as Low, with the remainder falling within the middle 50% of scores on the ESI.
Measures
Participants completed a 100-item version of the ESI, a self-report measure that was developed to assess a broad range of behavioral and personality characteristics associated with externalizing psychopathology (Krueger et al., 2007). The 100-item version (ESI-100) used here was the same as that used by Hall et al. (2007); scores on the ESI-100 correlate very highly (r = .98) with scores from the full 415-item ESI. As evidence of the construct validity of the ESI-100 in the current sample, Table 1 presents correlations between ESI-100 scores and scores on other self-report measures with conceptual or empirical links to externalizing psychopathology, namely: the Alcohol Dependence Scale (H. A. Skinner & Allen, 1982); the Short Drug Abuse Screening Test (A. Skinner, 1982); the Socialization scale (Gough, 1960); the Behavior Report on Rule Breaking, a measure of adolescent and adult antisocial behaviors composed of items from several other published measures (Clark & Tifft, 1966; Hindelang, Hirschi, & Weis, 1981; Nye & Short, 1957); and broad factors of the Multidimensional Personality Questionnaire-Brief Form (MPQ-BF; Patrick, Curtin, & Tellegen, 2002).
Table 1.
Correlations of Scores on the 100-Item Externalizing Spectrum Inventory (ESI) with Differing Criterion Measures
| Criterion Measure | n | r with ESI |
|---|---|---|
| Alcohol Dependence Scale | 146 | .56* |
| Short Drug Abuse Screening Test | 144 | .60* |
| Socialization scale | 113 | −.58* |
| Behavior Report on Rule Breaking | ||
| Total | 114 | .80* |
| Adult | 114 | .73* |
| Adolescent | 114 | .74* |
| MPQ | ||
| Positive Emotionality Factor | 134 | −.07 |
| Negative Emotionality Factor | 134 | .66* |
| Constraint Factor | 134 | −.50* |
Note: MPQ = Multidimensional Personality Questionnaire
p < .001
Procedure
Testing was conducted in a dimly lit, sound-attenuated room. Experimental stimuli were presented centrally on a 21-inch Dell high-definition CRT color monitor, at a viewing distance of 100 cm, using E-Prime version 1.1 software (Psychology Software Tools, Inc.). Behavioral responses were made using the PST Serial Response Box from the same company.
The experimental task was a modified version of Gehring and Willoughby’s (2002) gambling task in which the participant chose between two monetary options on each trial and then received feedback indicating whether the choice resulted in winning or losing money on that trial. The modification was that feedback was presented 100 ms after the button press to have the feedback occur more immediately following the choice. The target stimuli consisted of two adjacent squares, each enclosing a number (5 or 25) representing a monetary value (in cents). The target stimulus remained on the screen until a choice was made between the square on the left and the one on the right, after which a blank screen appeared for 100 ms, followed by a feedback stimulus that indicated the outcome of the participant’s decision. That is, the chosen box turned either red or green to signify either a win or a loss (with red or green as the winning color counterbalanced across participants), and the unchosen box turned the other color (either green or red) to indicate what the outcome of the trial would have been had that box been chosen. The feedback stimulus appeared for 1000 ms, followed by a blank screen for 1500 ms preceding the onset of the next trial. Replicating the design used by Gehring and Willoughby (2002), all four possible combinations of 5 and 25 (i.e., 5-5, 5-25, 25-5, and 25-25) were evenly crossed with the four possible win/loss outcomes (win-win, win-loss, loss-win, loss-loss), resulting in 16 trial types; thus, while the participant’s choice produced a designated outcome on each trial, signaled by the feedback, outcomes on future trials were not predictable from outcomes associated with prior choices (analogous to a roulette wheel or slot machine). Two sets of these 16 trial types, ordered randomly, were included in each block. Upon completion of a block, participants received feedback about their win/loss ratio within that block. Participants completed 12 blocks of 32 trials.
Electroencephalographic Recording
Participants in the study were tested in two waves. Participants in the first wave (N = 42) were tested using a 64-channel Neuroscan, Inc. Synamps amplifier, and those in the second wave (N = 125) were tested using a 64-channel Neuroscan Synamps2 amplifier. In each phase, EEG activity was recorded using 64-channel Quick-caps containing sintered Ag-AgCl electrodes positioned in accordance with the International 10–20 System (Jasper, 1958). Activity was recorded from a greater number of scalp sites in Wave 2, but only electrodes in common across the two waves were included in the analyses reported here. Additionally, problems with the FP1 and FP2 scalp sites in Wave 1 necessitated dropping these sites from both waves. Thus, 51 electrodes are included in the reported data, as follows: AF3, AF4, F7, F5, F3, F1, Fz, F2, F4, F6, F8, FT7, FC3, FC1, FCz, FC2, FC4, FT8, T7, C5, C3, C1, Cz, C2, C4, C6, T8, TP7, CP3, CP1, CPz, CP2, CP4, TP8, P7, P5, P3, P1, Pz, P2, P4, P6, P8, PO5, PO3, POz, PO4, PO6, O1, Oz, O2. Ocular activity was monitored using electrodes positioned on the outer canthus of each eye (Horizontal EOG) as well as above and below the left eye (Vertical EOG). Impedances were kept below 10 kΩ. All EEG signals were referenced to CPz and digitized on-line at 1000 Hz. The signals were then epoched off-line from 1000 ms before to 2000 ms after feedback onset, and re-referenced to averaged mastoid activity. Trial-level EEG data were corrected for ocular and movement artifacts using an algorithm developed by Semlitsch, Anderer, Schuster, & Presslich, (1986), as implemented in the Neuroscan Edit software, version 4.3. As a final step, the processed data were downsampled off-line to 128 Hz using the Matlab (Mathworks, Inc.) resample function to handle anti-aliasing filtering before downsampling.
Data Preprocessing
The data were averaged across trials within monetary condition (Gain versus Loss trials), and epochs were baseline-corrected for the 150 ms preceding feedback stimulus presentation. A careful visual inspection of the data was undertaken to identify and exclude movement and other artifacts, in particular, to minimize their impact on the time-frequency PCA decomposition (detailed below). Toward this end, several exclusionary criteria were applied. First, to exclude ocular artifacts remaining after ocular correction, trials on which activity at frontal electrode sites F1 or F2 exceeded 75 μV within a 1500 ms post-stimulus window (relative to median activity within a 750 ms window immediately preceding the stimulus) were excluded from further processing. Then, within each trial, individual electrode sites at which activity exceeded ± 75 μV in either the pre- (−750 to 0) or post-stimulus (0 to 1500) time regions (relative to one another) were also omitted from analysis. Applying these criteria, 9.9% of trials were excluded. Additionally, across all subjects and electrodes, 24 subject-electrodes (out of 8517) became disconnected at some point during the procedure. Missing data for these leads were replaced with the average activity of their nearest-neighbors. Gain and Loss condition averages were computed for each participant. These averages served as the starting point for all analyses detailed in this report.
Data Reduction
Time-domain components: FRN and P300
The time-domain (TD) FRN component was defined as the maximum negative deflection in the ERP waveform occurring between 203.13 and 328.13 ms post stimulus onset; the P300 was defined as the maximum positive deflection occurring between 250 and 601.56 ms post stimulus onset (with ms corresponding to bins of 128 Hz re-sampled signal). Electrode sites FCz and Cz were most proximal topographically to the center of FRN and P300 Gain-Loss condition differences, respectively, and were thus employed in the TD statistical analyses reported below.
Time-frequency components: theta and delta
Time-frequency (TF) analysis is a technique that can be used to quantify the time-varying spectral properties of ERP signals. This approach allows separation of activity that has either a unique time-course or rate of oscillation (frequency). Principal components analysis (PCA) of time-frequency transforms of the ERPs (see Bernat, et al., 2005) was applied in order to disaggregate FRN and P300 components. To enhance separation of theta and delta activity relevant to the FRN and P300 (as suggested by previous work; Bernat et al., 2005, 2007; Gilmore et al., 2009; Hall et al., 2007), brain response activity in the window of −1000 to +2000 ms relative to feedback stimulus onset was filtered in two distinct ways before applying the TF-PCA: 1) using consecutive 3 and 9 Hz high- and low-pass 3rd order Butterworth filters (respectively), to isolate theta-band activity, and 2) using a 3 Hz lowpass 3rd order Butterworth filter, to isolate delta-band activity. These theta- and delta-filtered signals were then each transformed into time-frequency energy distributions (surfaces) using the binomial reduced interference distribution (RID) variant of Cohen’s class of time-frequency transforms (for details, see Bernat et al., 2005). Next, the TF-PCA was applied to an area corresponding to the zero-to-750 ms time range and 0-to-10 Hz frequency range, separately for theta- and delta-filtered TF distributions. The variance accounted for by the first principal component (PC) in each analysis (theta band: 54.95%; delta band: 78.89%) substantially exceeded that accounted for by the next PC (theta band: 12.80%; delta band: 7.89%), indicating that retention of a single PC was justifiable in each case. These TF-based theta and delta PCs (depicted in Figure 1) served as the primary dependent variables in the analyses of brain reactivity to feedback stimuli reported below. As with the time-domain FRN and P300 measures, electrodes FCz and Cz, respectively, were most proximal topographically to the maximum of the theta and delta Gain-Loss condition differences (see Figure 2). Data from these electrode sites were thus employed in the statistical analyses of TF component scores reported below.
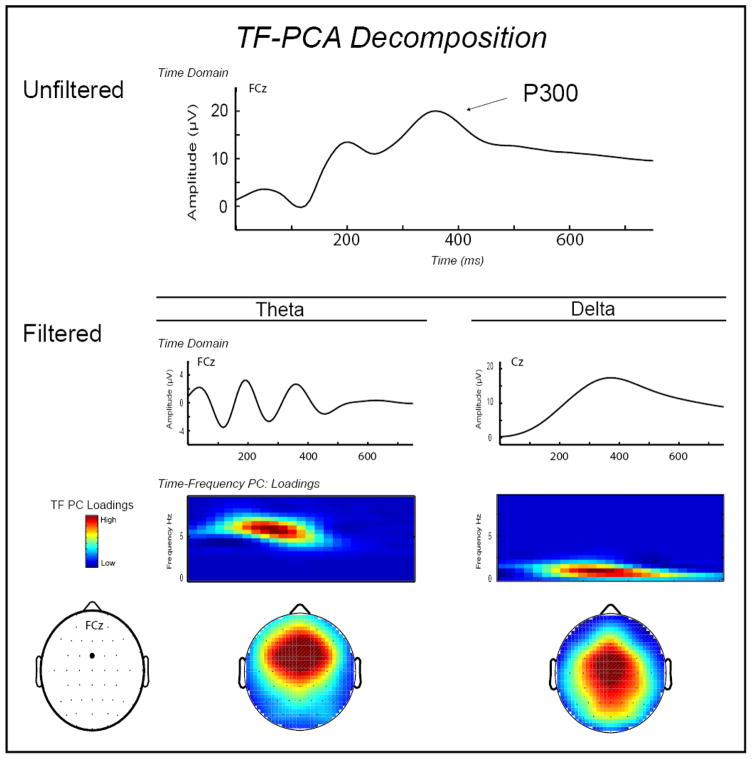
Figure 1.
Results from a time-frequency (TF) decomposition of average ERP activity for Gain and Loss trials combined. Waveform plot, top level: Average unfiltered ERP activity at FCz for all trials. Waveform plots, second level: Average time-domain ERP activity on all trials, frequency-filtered (3rd order Butterworth) to capture activity in the theta (3–9 Hz bandpass) range corresponding to FRN response (FCz; left plot) and activity in the delta (3 Hz lowpass) range corresponding to the P300 response (Cz; right plot). Color surface plots, third level: Time-frequency representation of the theta-FRN and delta-P300 principal component scores following feedback onset on Loss and Gain trials combined. Topographical maps, bottom level: Scalp topography distributions for the mean of the TF-PCA energy for the theta-FRN (left map) and delta-P300 (right map) components. From the topographic maps, it can be seen that the theta-FRN activity is maximal fronto-centrally (at FCz), whereas the delta-P300 activity is maximal more centrally (at Cz), consistent with interpretation of these components as measures of “FRN” and “P300,” respectively.
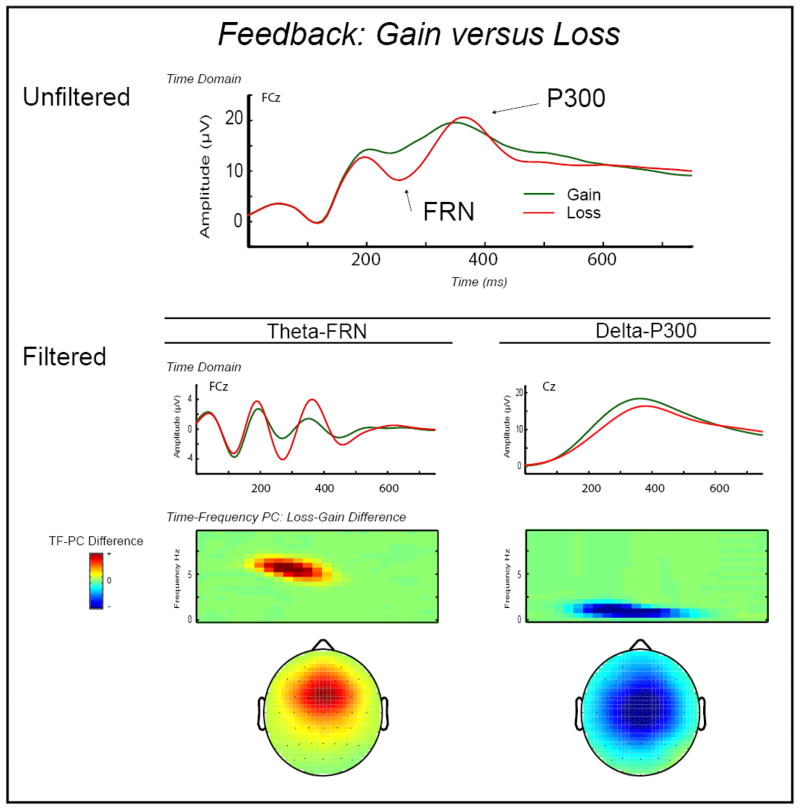
Figure 2.
Time-domain and time-frequency representations of FRN and P300 differences for Loss versus Gain trials. Top line plot: Average response-locked ERP waveforms at FCz, depicting the expected negativity for Loss versus Gain trials associated with the FRN as well as the time-domain P300. Waveform plots, second level: Average time-domain ERP activity for Loss and Gain trials separately, frequency-filtered to capture activity in the theta (3–9 Hz) range corresponding to FRN response (FCz; left plot) and activity in the delta (3 Hz) range corresponding to the P300 response (Cz; right plot). These plots demonstrate that theta and delta show opposing effects for loss compared with gain feedback such that theta is stronger for loss versus gain whereas delta is stronger for gain versus loss. Color surface plots, third level: Loss-Gain difference scores for the principal component loadings on theta-FRN (left map) and delta-P300 (right map), derived from a TF decomposition of average EEG activity following Loss and Gain trials. Topographical maps, bottom level: Scalp topography distributions for the mean condition difference (Loss-Gain) of TF-PCA loadings for theta-FRN (left map) and delta-P300 (right map). Similar to the time-domain FRN and P300, electrodes FCz and Cz, respectively, were most proximal topographically to the maximum theta and delta Gain-Loss differences. However, compared to the highly correlated time-domain FRN and P300, the Gain-Loss difference scores for theta and delta were uncorrelated. The implication is that these theta and delta TF measures index separate processes that differentiate between Loss and Gain feedback outcomes.
Data Analysis
Analyses of behavioral response data are first reported, followed by analyses of the brain response data. For completeness, analyses of TD FRN and P300 measures are presented briefly after analyses of the TF measures. For each measure, an initial 3-way repeated-measures general linear model (GLM) was conducted, with Frequency Band (delta, theta) and Feedback condition (Gain, Loss) included as within-subjects factors, and continuous scores on the ESI-100 included as a between-subjects factor. Follow-up 2-way repeated-measures GLM analyses assessed effects of Externalizing scores and Feedback condition (Gain, Loss) separately for theta and delta PC measures. Simple effects tests and correlations are also presented to clarify the direction and relative magnitude of effects.
Results
Behavioral Results: Externalizing Proneness Related Differences in Risk-Taking Behavior
Behavioral analyses evaluated the extent to which choices indicative of risk-taking evidenced relations with externalizing proneness. Following prior work, risk-taking was operationalized as the proportion of high number (25) choices in response to 25-5 or 5-25 number pairings (cf. Gehring & Willoughby, 2002). For the sample as a whole, the mean proportion of risky choices was .60 (SD = .14; range = .27 to 1.00); across participants, the proportion of risky choices correlated positively with scores on the ESI-100, r = .21, p = .01, such that individuals higher in externalizing proneness made more risky choices. However, as noted below (see Footnote 4), heightened risk-taking did not mediate observed relations between externalizing proneness and ERP response.
Brain Responses to Gain versus Loss Feedback: Comparison of Effects for Time Domain and Time-Frequency Measures
Statistical comparisons between time-domain (TD) and time-frequency (TF) signal representations of the data revealed that the theta and delta TF-PCA measures together accounted for a majority of the variance in both the time-domain FRN and P300 measures. These analyses indicated that the TD measures represented a mixture of TF theta and delta activity, and that the TF theta and delta measures provided largely independent, and more parsimonious, indices of ERP activity to the primary Loss and Gain feedback outcomes, respectively.3 As illustrated by the top two line plots of Figure 2, this mixture of TF activity can be understood in terms of the changing phase of the more rapid theta oscillation (i.e., alternating positive and negative polarity). Specifically, there is an earlier negative polarity peak in the theta oscillation (maximal around 275 ms) corresponding to the FRN, followed by a subsequent positive deflection that reaches its maximum near the same time at which the P300 response reaches its peak (i.e., around 375 ms). In contrast, the delta activity component corresponding to the P300 consists of a unidirectional slow-wave that contributes positive amplitude to the time domain signal in both the FRN and P300 windows. Because activity at different frequencies within a common temporal window contributes additively to the aggregate TD signal, this differing polarity produces salient distorting effects on time domain FRN and P300 measures derived from the unfiltered, aggregate ERP signal. Within the FRN window (203–328 ms; in which the polarity of the theta oscillation is predominantly negative), increased theta activity for Loss trials translates into enhanced negative TD signal amplitude whereas increased delta activity for Gain trials translates into enhanced positive signal amplitude, yielding an exaggerated net Gain-Loss difference (t[148] = 18.02) in comparison with either TF component measure alone (ts[148] = −11.76 and 9.28 for theta and delta, respectively). On the other hand, within the P300 window (250–602 ms; in which the polarity of theta is predominantly positive), theta increases for Loss and delta increases for Gain both translate into increased positive TD signal amplitude, yielding a negligible net Gain-Loss difference (t[148] = 1.22, ns; see Figure 2). The TF energy measures do not suffer from this complication, because all increases in energy are represented in unipolar fashion—as increased positive numbers (i.e., no polarity).
Effects of Externalizing Proneness on Brain Responses to Performance Feedback
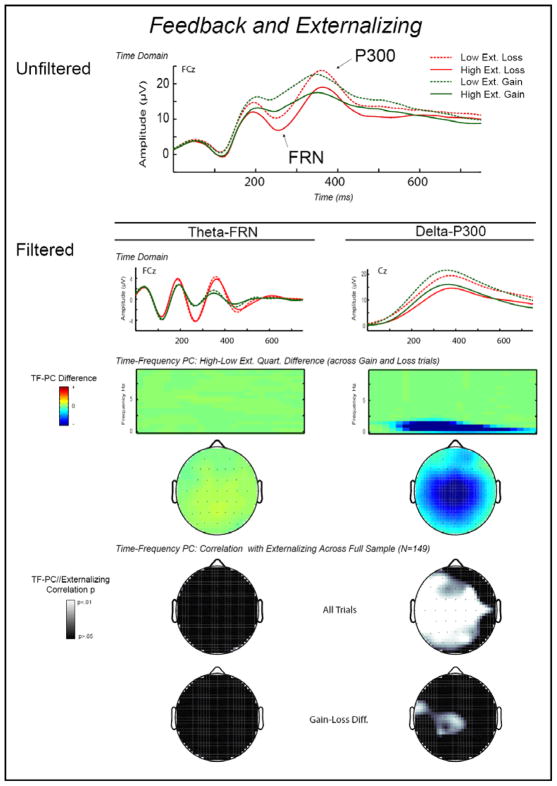
Figure 3 presents ERP response data for gain versus loss trials as a function of scores on the ESI-100, in terms of TD peak scores and TF component scores. The topmost line plot depicts unfiltered TD waveforms for gain and loss trials for participants falling within the top and bottom quartiles of the distribution of scores on the ESI-100. Here, a broad amplitude reduction is evident for individuals in the top quartile relative to those in the bottom quartile. In this unfiltered data, however, it is unclear whether this overall amplitude reduction reflects differences in theta-FRN or delta-P300. The basis of the overall group effect becomes apparent in the two adjacent line plots below this, which display theta- and delta-filtered TD signal averages, and in the color surface plots following these, which depict results for the theta and delta TF-PCs. Specifically, it is clear that the group difference in ERP response to feedback is confined to the delta-P300 component, with no significant difference evident for the theta-FRN component. Notably, both low and high externalizing groups show robust amplification of theta oscillatory activity following loss feedback relative to gain feedback. In fact, the groups are so similar in this component of responding that corresponding waveforms for gain and loss trials nearly overlap (Figure 3, left filtered line plot). In contrast, the delta-P300 waveforms for low and high externalizing groups clearly diverge (see Figure 3, right filtered line plot). The statistical topographical maps depicting correlations between continuous Externalizing (ESI-100) scores and theta and delta TF component scores at varying scalp sites (Figure 3, bottom section) corroborate this visual impression—significant effects are observed broadly for delta-P300, but not at all for theta-FRN. Specifically, higher externalizing proneness is associated with reduced delta-P300 response to both gain and loss feedback. Evidence of a significant Externalizing × Gain/Loss trial interaction was found (i.e., significant correlations between ESI-100 scores and gain-minus-loss difference scores were evident at some scalp sites), reflecting somewhat lesser modulation of delta-P300 response following gain feedback as compared to loss feedback among individuals higher in externalizing proneness. The following series of analyses further support and clarify these visual impressions and basic statistical effects.
Figure 3.
Time-domain and time-frequency representations of FRN and P300 to Loss and Gain feedback, depicted separately for subgroups of high (N = 57) and low (N = 40) externalizing participants as defined by scores on a 100-item version of the Externalizing Inventory (Krueger, Markon, Patrick, Benning, & Kramer, 2007). High and low externalizing groups were formed by oversampling from the top and bottom 25% of scorers in an undergraduate screening pool. Waveform plot, top level: Average unfiltered ERP activity following Loss and Gain feedback for these high and low externalizing subgroups. Here, a broad amplitude reduction is evident for individuals in the high relative to those in the low externalizing group. In this unfiltered data, however, it is unclear whether this overall amplitude reduction reflects differences in theta-FRN or delta-P300. Waveform plots, second level: Average time-domain ERP activity following Loss and Gain feedback stimuli for these extreme subgroups, frequency-filtered (3rd order Butterworth) to capture activity in the theta (3–9 Hz bandpass) range corresponding to FRN response (left plot) and activity in the delta (3 Hz lowpass) range corresponding to the P300 response (right plot). Color surface plots, third level: Time-frequency representation of TF-PCA principal component scores reflecting the theta-FRN and delta-P300 activity from the ERP signal, derived from a TF decomposition of average EEG activity following Loss and Gain trials. Statistical maps, bottom level: Scalp topography distributions, for the overall study sample (N = 149) that included these extreme subgroups, of p-values from correlations between externalizing scores and scores on the theta-FRN and delta-P300 TF-PCA components for: (1) all trials combined, (2) Gain trials, (3) Loss trials, and (4) Gain-Loss difference scores. These topographic statistical maps demonstrate that the association between externalizing and theta-FRN is pervasively nonsignificant, whereas the delta-P300 activity is significantly reduced for high externalizing individuals for the conditions reported, in particular when considering the average response or Loss and Gain trials separately. Thus, the reduced EEG activity in the unfiltered time-domain waveform at the top is attributable to reductions in the delta but not the theta frequency band.
Omnibus analysis: Effects of feedback condition and externalizing proneness on TF component scores
Main effects were observed for Band, F(1,147) = 103.08, p < .001, Feedback, F(1,147) = 7.02, p < .009, and Externalizing, F(1,147) = 7.97, p < .007. The main effect for Band reflected the greater energy generally evident at lower frequencies (e.g., delta) in biological signals such as ERPs. The Feedback main effect was superseded by a Feedback × Band interaction, F(1,147) = 91.91, p < .001, representing the opposing direction of the gain/loss difference for theta relative to delta described earlier in the Data Reduction section. The main effect of Externalizing was also moderated by significant Band × Externalizing and Feedback × Externalizing interactions, Fs(1,147) = 9.80 and 4.99, respectively, p < .002 and p < .027. The robust Band × Externalizing interaction corroborated the major inference derived from the data in Figure 3—namely, that delta-P300 was broadly reduced for individuals higher in externalizing proneness, whereas theta-FRN exhibited no measurable relationship with externalizing proneness. The Externalizing × Feedback interaction effect, although significant, was modest in relation to the Externalizing × Band effect. In addition, some evidence of a 3-way (Band × Frequency × Externalizing) interaction was found, F(1,147) = 3.63, p < .059. Based on these considerations, effects of externalizing proneness and feedback condition were further examined in separate analyses for the theta and delta TF-PCs.
Two-way analyses examining effects for theta and delta TF-PCs separately
These analyses, presented in Table 2, clearly demonstrate that only the magnitude of delta-P300 response is significantly related to externalizing proneness; affiliated theta effects are entirely nonsignificant.4 For the delta-P300 component, the main effect of Externalizing reflects the general reduction in delta response to feedback across gain and loss trials. The Feedback × Externalizing interaction is also significant, albeit smaller, indicating a modest incremental reduction in amplitude for individuals high in externalizing proneness for Gain trials as compared to Loss trials.
Table 2.
Results of Two-Way Repeated Measures GLMs Examining Effects of Externalizing Scores and Feedback Condition (Gain, Loss) on TF Theta and Delta Component Scores
| df | Theta-FRN | Delta-P300 | |
|---|---|---|---|
| Feedback | 1, 147 | 54.21*** | 53.63*** |
| Externalizing | 1, 147 | 0.01 | 9.07** |
| Feedback × Ext. | 1, 147 | 0.01 | 5.75* |
Note: Externalizing scores refer to scores on the 100-item version of the Externalizing Spectrum Inventory.
p<.05
p<.01
p<.001
Externalizing proneness and brain response: Comparison of effects for TD versus TF measures
It is informative to compare the markedly different effects of externalizing proneness on the two aforementioned TF component measures (theta-FRN, delta-P300) with effects for more traditional TD FRN and P300 measures. When the analyses depicted in Table 2 were repeated using TD FRN and P300 scores in place of the corresponding TF component scores, significant main effects of Externalizing were found for both the TD FRN variable and the TD P300 variable. The TD P300 showed the expected amplitude reduction as a function of higher externalizing proneness. In the case of TD FRN, higher externalizing proneness was associated with an apparent augmentation of the negative-polarity FRN (i.e., an effect opposite to the decrement in response-ERN amplitude reported by Hall et al., 2007). Based on the aforementioned overlap between the negative-going theta component of the feedback response and the positive-going delta component within the time window of the FRN, we hypothesized that the apparent enhancement of TD FRN for individuals high in externalizing proneness reflected diminished delta activity within this window (i.e., lesser positive contribution to signal amplitude) rather than enhanced theta activity (i.e., heightened negative contribution to signal amplitude).
To evaluate this hypothesis, regression analyses were performed in which scores on the ESI-100 served as the criterion variable, and TD and TF component scores served as predictors. In an initial regression model, TD FRN and P300 component scores were entered as predictors of ESI-100 scores. The overall model was significant, F(1,147) = 6.90, p < .01, but neither TD variable contributed uniquely to prediction, indicating that a single overlapping process accounted for amplitude reductions in both TD components. To test whether (as hypothesized) this single process was captured by TF delta, a further hierarchical regression analysis was conducted in which ESI-100 scores again served as the criterion variable, but brain response predictors were entered sequentially, with TF delta entered first, and TD FRN and P300 entered second and third, respectively. The goal was to evaluate whether FRN and P300 contributed at all uniquely to the prediction of externalizing proneness beyond TF delta, or if instead their associations with ESI-100 scores were attributable to TF delta. In the first step of the model, TF delta evidenced a significant association with Externalizing scores, F(1,147) = 9.07, p < .003. Neither the TD FRN nor the TD P300 yielded any significant increment in R2 when entered in steps 2 and 3, indicating that these components did not contribute uniquely to prediction beyond TF-delta. The results of this analysis confirm that the TF delta component captures all of the variance in the time-domain measures associated with externalizing proneness, and accounts for the apparent augmentation of TD FRN as well as the observed reduction in TD P300 response.
Externalizing proneness and performance monitoring: Dissociating effects for feedback-ERN versus response-ERN
The findings for the FRN in the current study differ dramatically from those reported by Hall et al. (2007) for the response-ERN. Whereas participants high in externalizing proneness showed markedly reduced response-ERN following performance errors in the Hall et al. investigation, higher ESI-100 scores were associated with no discernable reduction in theta activity reflecting the FRN following loss feedback—despite the fact that the test sample for the current study was markedly larger (N = 149) and incorporated all but three participants from the Hall et al. study. To further address the dissociation in effects for the two studies, we decided it would be informative to directly compare results for the theta-FRN and response-ERN in the subset of participants in the current study (n = 89) who also participated in the Hall et al. study. Participants in this subsample consisted of: 35 high ESI-100 scorers (12 male), 27 intermediate scorers (13 male), and 27 low scorers (8 male).
To directly compare activity associated with the loss-feedback FRN and incorrect-response ERN in this participant sample, a repeated-measures GLM analysis was conducted in which TF-theta component scores for loss trials (measured in the current study) were included along with TD ERN peak scores (measured in the Hall et al. study) as a within-subjects (FRN/ERN) factor, and continuous scores on the ESI-100 were included as a between-subjects factor. The FRN/ERN × Externalizing interaction was significant, F(1,87) = 9.91, p < .002, qualifying lower-order main effects. Follow-up simple effects GLMs separately for the FRN and ERN indicated that this interaction was attributable to a significant relationship of ERN amplitude with Externalizing scores, F(1,87) = 9.88, p < .002, compared with a null relationship for the FRN, F(1,87) < 1. (A comparably robust FRN/ERN × Externalizing interaction was evident when data for subgroups of individuals low and high in externalizing proneness were employed in the analysis in place of continuous scores for all participants, F[1,60] = 9.32, p < .003.) To further ensure comparability of measures across the two experiments, we repeated the GLM using TF-theta component scores corresponding to the ERN in place of TD ERN peak scores, in conjunction with TF-theta scores for loss trials from the current study; Hall et al. (2007) reported that the theta component captured most of the variance related to externalizing proneness in the response-ERN. This analysis likewise produced a significant FRN/ERN × Externalizing interaction, for both continuous ESI-100 scores (F[1,87] = 5.64, p < .02) and low versus high group comparisons (F[1,60] = 7.92, p <.007), with follow-up tests confirming a robust association with externalizing proneness for the ERN-theta component only.
Discussion
In the current study, we examined brain responses to feedback stimuli in a gambling task in order to: (1) evaluate the relationship between externalizing proneness and FRN response, and (2) replicate the finding of an association between externalizing proneness and reduced P300 amplitude within a new task paradigm, distinct from oddball tasks used in most P300 studies to date. Identifying the relationship between the FRN and externalizing proneness was important to determining whether the ERN amplitude reductions associated with externalizing proneness (reflecting deficits in monitoring on the basis of internal representations) generalize to the FRN (i.e., monitoring on the basis of exogenous cues). Exploring the relationship between externalizing proneness and P300 amplitude within an alternative, feedback-stimulus paradigm was important for evaluating the generality of the association between P300 and externalizing proneness.
To achieve these aims, we needed to overcome the problem of component overlap for the FRN and P300 within the time domain. The approach we used was time-frequency analysis, a method that considers the differing spectral characteristics of overlapping brain potential components in order to separate them. This technique proved to have interesting implications for the time-domain FRN and P300 measures. The two time-frequency components of the feedback response, theta-FRN and delta-P300, were found to reflect relatively independent processes that were differentially sensitive to the primary Gain and Loss components of feedback (with theta-FRN increased for Loss, and delta-P300 increased for Gain). In contrast, the time domain FRN and P300 components represented somewhat complex mixtures of theta and delta activity, consistent with the idea that these processes overlap substantially in time.
One implication of disentangling this overlap is better measurement of the time course of each of these processes. First, the loss-sensitive theta activity following feedback extended well beyond the conventional FRN time window, into the P300 window, reaching its maximum around 400 ms (cf. Luu, Tucker, and Makeig, 2004). Similarly, delta activity associated with P300 was found to extend earlier in time, occurring during the conventional time-domain FRN window. This also indicated that externalizing-related delta-P300 amplitude reductions in the current study were not isolated to the conventional P300 time-window, extending earlier in time. Interestingly, a recent study using the TF-PCA approach to more effectively index the time-course of delta activity underlying externalizing-related P300 amplitude reductions in a standard oddball task suggested a similar early time course (Gilmore et al., 2009).
The current study also permits some inferences to be made about the observed theta-FRN activity relative to the ERN. First, the dissociation between the FRN and ERN in relation to externalizing proneness supports the view that these are not identical processes, a point that has been debated recently in the field. Furthermore, insofar as both the ERN and FRN are thought to have similar primary sources in the ACC (e.g. Dehaene et al., 1994; Holroyd et al., 2004), the current findings suggest that the self-monitoring deficits associated with externalizing proneness do not reflect a simple global impairment in the functioning of the anterior cingulate cortex (ACC). At the same time, to the extent that the ERN and FRN are presumed to reflect a highly similar cognitive-monitoring process, it is surprising that we did not find a negative relationship between theta-FRN amplitude and externalizing proneness similar to that which has been reported for the ERN.
Nonetheless, the totality of the data from the current study renders it unlikely that a lack of engagement in the task or insufficient statistical power accounted for the absence of the expected association. First, the fact that the difference in theta-FRN amplitude between the gain and loss trials was large and commensurate with that reported in prior work demonstrates that participants as a whole in the current study responded appropriately to losses and were thus engaged in the task. Second, Hall et al. (2007) did find a robust relationship between externalizing proneness and the ERN in a markedly reduced subset of the participants tested in the current study task (i.e., 89 versus 149 participants)—indicating that power to detect a difference in a putatively related brain response should have been adequate in the current study. Consistent with this perspective, we were successful in detecting an association between externalizing proneness and reduced delta-P300 response in the current study, despite the lack of any association for theta-FRN. Finally, when we directly compared findings for ERN and FRN responding in participants from the Hall et al. (2007) who completed both types of tasks, we found a significant interaction between brain component (ERN vs. FRN) and externalizing scores—with follow-up tests revealing a significant association for ERN in this sample, but not for FRN. Together, these findings suggest that externalizing proneness is marked by deficits in monitoring of performance on the basis of endogenous representations, as reflected in the ERN, but not exogenous cues, as reflected in the FRN.
The current study also replicated previously-reported findings of reduced P300 in relation to externalizing problems of various types. Replication of this finding here is noteworthy considering how the P300 response was elicited in the current study and how we quantified externalizing proneness. First, whereas prior studies documenting this relationship have measured P300 in relation to target stimuli in an oddball task (e.g. Gilmore et al., 2009; Patrick et al., 2006), P300 in the current study was recorded in relation to non-oddball, feedback stimuli. Second, in contrast with prior studies, in which externalizing has been operationalized in terms of specific impulse control disorders (e.g., Iacono et al., 2002) or as a composite of DSM symptom variables (i.e., conduct disorder, adult antisocial behavior, and alcohol, drug, and nicotine dependence; Patrick et al., 2006), we quantified externalizing proneness using a specially-designed questionnaire inventory (Krueger et al., 2007). Thus, the current work provides evidence that the finding of reduced P300 amplitude in high-externalizing individuals generalizes to multiple task conditions and methods of measuring this domain of psychopathology. By further exploring the P300-externalizing association across varying task procedures in future work, we stand to gain a clearer understanding of what brain processing differences underlie this well-documented correlate of externalizing proneness.
In this regard, a further notable point is that reductions in delta-P300 as a function of externalizing proneness were evident for both gain and loss feedback, indicating a global reduction in P300 response rather than an effect localized to one type of feedback or the other. However, along with a main effect for externalizing proneness, a small but significant Externalizing × Gain/Loss interaction was evident, indicating that amplitude reductions were slightly larger for responses to gain feedback. One possible explanation is that this simply reflects greater variance in response for Gain as compared to Loss trials (i.e., because delta-P300 responses were greater to Gain than Loss), affording greater opportunity to detect an effect of externalizing proneness in this condition. Another possibility is that the motivational impact of the gain feedback was diminished for individuals higher in externalizing proneness. Although the direction of this finding contrasts with the notion of high externalizing individuals as hypersensitive to reward, it is notable that the reward stimulus in the current context (i.e., gain feedback cue) was highly symbolic. Thus it may be that individuals high in externalizing proneness are hypersensitive to immediate tangible reward, but diminished in their reactivity to distal, symbolic cues for reward. Future work could directly investigate this question by manipulating reward levels or context, and assessing variation in delta-P300 relative to externalizing proneness.
Taken together, the current results indicate that individuals high in externalizing proneness process external performance feedback normally in terms of post-stimulus theta-FRN activity, generally associated with performance monitoring. Importantly, this suggests that participants across the range of externalizing proneness were similarly engaged in processing the feedback at this level. However, the reduction in delta-P300 response, continuing somewhat later in the post-feedback interval, indicates that there is an aspect of sustained feedback processing (i.e. continuing after the theta-FRN, and associated with P300) that is abnormal in individuals high in externalizing proneness. Further, this P300 amplitude reduction appears to be more general than specific – occurring robustly to both Gain and Loss feedback stimuli in this simulated gambling task, as well as in standard oddball tasks. This suggests that such observed P300 amplitude reductions may not be related to specific cognitive functions often associated with target P300 in oddball tasks, and that some more general process may be involved.
Limitations and Future Directions
Some limitations of the current study must be acknowledged. One pertains to the approach that was used to decompose the feedback-related ERP into distinctive FRN and P300 components (i.e., initial frequency-filtering, followed by PCA decomposition of the TF data). We used this approach because of prior data linking these two components to particular frequency bands, and because our primary objective was to separate these components in order to evaluate each in relation to externalizing proneness. However, in future work, it may be of interest to undertake more detailed analyses employing unfiltered time-frequency data or filtered data reflecting a greater number of components. A second point is that the task procedures commonly used to investigate the ERN and FRN differ in numerous ways, so it is unclear to what extent their contrasting relations with externalizing proneness reflect a fundamental distinction between the ERN and FRN (i.e., the brain’s response to self-identified performance errors versus the response to negative external feedback) or a product of differing performance conditions in the tasks (flanker versus gambling) within which they are recorded. Evaluating the relationship between the FRN and externalizing proneness across other task conditions that better mirror those in which the ERN is typically investigated would be helpful in ruling out this possibility. For example, it would be of interest to see whether learning tasks (cf. Holroyd and Coles, 2002) in which outcomes inform choices on future trials would show the same result with regard to the FRN. Finally, the basis of the well-documented reduction in delta-P300 amplitude for individuals high in externalizing remains unclear. Future work could selectively investigate processes that may be related to externalizing-related delta-P300 amplitude reductions, or evaluate specific cognitive manipulations or training to assess whether they could ameliorate P300 amplitude deficits.
Acknowledgments
This work was supported by grants MH65137, MH080239 and MH089727 from the National Institute of Mental Health.
Footnotes
We use the term “FRN” for this component to distinguish it clearly from the ERN and to highlight that it follows a feedback stimulus rather than a response error. Other terms, including f-ERN and medial frontal negativity (MFN), have also been used for this component. Our choice of terminology does not reflect support for any particular theoretical stance on these measures.
Age and gender were assessed as potential mediators of observed brain response relationships with externalizing proneness. Age did not significantly correlate with scores on the ESI-100, and was thus not assessed further. Gender showed significant relations with ESI-100 scores, t(147)=3.11, p<.002, and with delta-P300 amplitude, F(1,145)=11.12, p<.001. However, when included as a factor in the GLM examining effects of Externalizing and Feedback condition on delta-P300, gender showed no interaction with Externalizing (F < 1), and effects related to Externalizing were unchanged. In sum, neither age nor gender appeared to moderate the delta-P300/Externalizing relationship.
Across all trials (both gain and loss) combined, theta and delta time-frequency (TF) measures evidenced a significant but modest association with one another, r = .325, indicating that although they share some variance, they are not simply yoked expressions of the same underlying process in the data. To clarify associations between these TF measures and time domain (TD) response measures, theta and delta TF component scores were entered together as predictors in regression models in which TD FRN and P300 alternatively served as the criterion variable. For the TD FRN measure, the theta and delta TF components together accounted for a majority of the variance (R2 = .55), with each contributing uniquely to prediction (theta, t[147] = −2.76, p < .008; delta: t[147] = 13.15, p < .001). The stronger relationship of delta than theta to the TD FRN underscores the problem of overlapping processes in the TD measures: Although the FRN itself has been localized to the theta range, the theta oscillation corresponding to the FRN (reflecting registration of performance feedback specifically) occurs against the background of a slower (delta) oscillation that reflects general processing and assimilation of perceptual input. In the case of the TD P300 measure, theta and delta together accounted for nearly all of the variance (R2 = .90), with each again contributing uniquely to prediction: ts(147) = 7.23 and 31.02, respectively, ps < .001. Taken together, these results support the view that time domain FRN and P300 measures can be understood as mixtures of TF theta and delta.
Considering data for gain and loss trials separately, theta and delta TF component scores each showed robust differentiation between outcomes of the two types, but in opposing directions: The magnitude of theta response was significantly larger for loss trials as compared to gain trials, t(148) = 12.24, whereas the magnitude of delta response was significantly larger for gain trials as compared to loss trials, t(148) = 8.74. However, the gain versus loss difference for theta was uncorrelated with the difference for delta (r = .11, ns), indicating that the two TF components tap independent processes related to the registration of feedback stimulus input. Regression analyses were again used to clarify associations between Gain-Loss difference effects for these TF measures and Gain-Loss differences for time domain (TD) response measures (cf. Gehring and Willoughby, 2002). Specifically, Gain-Loss difference scores for theta and delta TF components were entered together as predictors in regression models in which Gain-Loss difference scores for TD FRN and P300 alternatively served as the criterion variable. These analyses revealed that the Gain-Loss effect for each TD measure comprised a mixture of the Gain-Loss effects for the two TF components (theta and delta). For the TD FRN measure, TF theta and delta components together accounted for a majority of variance in the model (R2 = .55), with each contributing uniquely to prediction, ts(147) = −11.60 and 5.64, respectively, ps < .001. Notably, the commonly used FRN Gain-Loss difference-wave approach yielded a similar result (R2 = .61; (R2=.72; ttheta(147)= 12.32; tdelta(147) = 7.43, all these and following ps < .001), as did a peak-to-peak measure between P2 and the FRN (R2=.72; ttheta = 12.48; tdelta = 3.42) and P3 and the FRN (R2=.72; ttheta(147)= 7.37; tdelta(147) = 7.23). For Gain-Loss differences in the TD P300 measure, TF theta and delta components also accounted for a majority of variance in the model (R2 = .58), with each again contributing uniquely to prediction, ts(147) = 6.81 and 12.91, respectively. Thus, all assessed time domain measures represented a mixture of theta and delta time-frequency activity.
Given the behavioral finding of a positive relationship between proportion of risky choices in the task and level of externalizing proneness, we performed additional analyses to rule out the possibility that: (1) group differences in the number of trials contributing to each brain response average (arising from group differences in number of risky choices) might have accounted for effects of externalizing proneness on delta-P300 response, and (2) differences in risk-taking might have mediated the relationship between externalizing proneness and reduced delta-P300 response.
To evaluate the possibility of unequal numbers of trials explaining the primary findings, we performed follow-up analyses on a subset of trials for which participant choice was arbitrary (5-5 and 25-25 targets). For these trials, all participants received equivalent proportions of gain and loss feedback. Using a separate TF-PCA decomposition that included all 16 outcome types separately (in contrast with the original decomposition that collapsed across all gain and all loss trials), we extracted the theta-FRN and delta-P300 measures using only trials for which participant choice was irrelevant (i.e., all 5-5 and 25-25 target trials). Statistical analyses were consistent with those presented in the primary analysis that aggregated across all trials. The Externalizing × Gain/Loss GLM for delta-P300 yielded main effects for both Externalizing, F(1,147) = 8.77, p = .004, and Gain/Loss, F(1,147) = 27.66, p < .001, with no interaction, F(1,147) = 1.85, p = .176. In contrast, the Externalizing × Gain/Loss GLM for theta-FRN yielded no main effect of Externalizing and no Externalizing × Gain/Loss interaction.
Regarding the possible mediating role of risk taking in the association between externalizing and delta-P300 response, delta-P300 was indeed found to correlate significantly with risk taking for trials of both types (gain: r = −.22, p = .007; loss: r = −.19, p = .024). However, a regression analysis in which both ESI-100 scores and proportion of risky choices were entered as predictors of delta-P300 response (averaged across gain and loss trials) yielded unique predictive effects for both Externalizing (t = −2.77, p = .006) and risk taking (t = −2.17, p = .032). This indicates that elevated risk taking did not account for the observed negative association between externalizing proneness and delta-P300 response.
References
- Achenbach TM, Edelbrock CS. The classification of child psychopathology: A review and analysis of empirical efforts. Psychological Bulletin. 1978;85(6):1275–1301. [PubMed] [Google Scholar]
- Barratt ES, Stanford MS, Felhous AR, Kent TA. The effects of phenytoin on impulsive and premeditated aggression: A controlled study. Journal of Clinical Psychopharmacology. 1997;17:341–349. doi: 10.1097/00004714-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Ba ar-Eroglu C, Ba ar E, Demiralp T, Schürmann M. P300-response: Possible psychophysiological correlates in delta and theta frequency channels. A review. International Journal of Psychophysiology. 1992;13:161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Ba ar-Eroglu C, Demiralp T, Schürmann M, Ba ar E. Topological distribution of oddball ‘P300’ responses. International Journal of Psychophysiology. 2001;39:213–220. doi: 10.1016/s0167-8760(00)00142-2. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. International Journal of Psychophysiology. 2007;64:62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time-frequency energy using PCA. Clinical Neurophysiology. 2005;116:1314–1334. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. International Journal of Psychophysiology. 2007;64:62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinich MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Clark JP, Tifft LL. Polygraph and interview validation of self-reported deviant behavior. American Sociological Review. 1966;31:516–523. [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Conner S, Hesselbrock V, Rohrbaugh J, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biological Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topography. 2001a;13:251–267. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Istefanopulos Y, Ba ar-Eroglu C, Ba ar E. Wavelet analysis of oddball P300. International Journal of Psychophysiology. 2001b;39:221–227. doi: 10.1016/s0167-8760(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Dikman ZV, Allen JJB. Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology. 2000;37:43–54. [PubMed] [Google Scholar]
- Dolan M, Park I. The neuropsychology of antisocial personality disorder. Psychological Medicine. 2002;32:417–427. doi: 10.1017/s0033291702005378. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug and Alcohol Dependence. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naïve alcoholics. Drug Alcohol Depend. 2002;92(1–3):141–8. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Woroch BS, Curran T. Error-related negativity predicts reinforcement learning and conflict biases. Neuron. 2005;47:495–501. doi: 10.1016/j.neuron.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nature Neuroscience. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Are all medial frontal negativities created equal? Toward a richer empirical basis for theories of action monitoring. In: Ullsperger M, Falkenstein M, editors. Errors, Conflicts, and the Brain. Current Opinions on Performance Monitoring. Leipzig: Max Planck Institute of Cognitive Neuroscience; 2004. pp. 14–20. [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. doi: 10.1111/j.1469-8986.2009.00876.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough HG. Theory and measurement of socialization. Journal of Consulting Psychology. 1960;24:23–30. [Google Scholar]
- Goyer PF, Andreason PJ, Semple WE, Clayton AH. Positron-emission tomography and personality disorders. Neuropsychopharmacology. 1994;10:21–28. doi: 10.1038/npp.1994.3. [DOI] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychological Science. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindelang MJ, Hirschi T, Weis JG. Measuring delinquency. Beverly Hills, CA: Sage; 1981. [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error-processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MGH, Cohen JD. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nature Neuroscience. 2004;7:497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Jessor R, Jessor SL. Problem behavior and psychosocial development: A longitudinal study of youth. New York: Academic Press; 1977. [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300, disinhibited personality, and early-onset alcohol problems. Alcoholism: Clinical and Experimental Research. 2001;25:1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Kiel KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biological Psychiatry. 2000;48:210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning S, Kramer M. Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. Journal of Abnormal Psychology. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, McGue M, Iacono WG. The higher-order structure of common DSM mental disorders: Internalization, externalization, and their connections to personality. Personality and Individual Differences. 2001;30:1245–1259. [Google Scholar]
- Lipsey MW. The effect of treatment on juvenile delinquents: Results from meta-analysis. In: Loesel F, Bender D, editors. Psychology and law: International perspectives. Oxford, UK: Walter De Gruyter; 1992. pp. 131–143. [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115:1821–35. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9:788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measure of executive function. Clinical Psychology Review. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Müller SV, Möller J, Rodriguez-Fornells A, Münte TF. Brain potentials related to self-generated and external information used for performance monitoring. Clinical Neurophysiology. 2005;116:63–74. doi: 10.1016/j.clinph.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Bernat EM. Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. 2009 doi: 10.1111/j.1469-8986.2010.01047.x. Manuscript under review, revision invited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Buchsbaum MS, Hazlett EA, Goodman M, Koenigsberg HW, Lo J, Iskander L, Newmark R, Brand J, O’Flynn K, Siever LJ. Fluoxetine increases relative metabolic rate in prefrontal cortex in impulsive aggression. Psychopharmacology. 2004;176:451–458. doi: 10.1007/s00213-004-1913-8. [DOI] [PubMed] [Google Scholar]
- Nye FI, Short JF., Jr Scaling delinquent behavior. American Sociological Review. 1957;22:326–331. [Google Scholar]
- Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM. From markers to mechanisms: Using psychophysiological measures to elucidate basic processes underlying aggressive externalizing behavior. In: Hodgins S, Viding E, Plodowski A, editors. Persistent violent offenders: Neuroscience and rehabilitation. London: Oxford University Press; (in press) [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescewith nt males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO. Information processing, neuropsychological function, and the inherited predisposition to alcoholism. Neuropsychology Review. 1990;1:343–369. doi: 10.1007/BF01109029. [DOI] [PubMed] [Google Scholar]
- Raine A, Buchsbaum MS, Stanley J, Lottenberg S, Abel L, Stoddard J. Selective reductions in pre-frontal glucose metabolism in murderers. Biological Psychiatry. 1994;36:365–73. doi: 10.1016/0006-3223(94)91211-4. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Skinner A. The Drug Abuse Screening Test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Strehl U, Leins U, Goth G, Klinger C, Hinterberger T, Birbaumer N. Self-regulation of slow cortical potentials: A new treatment for children with attention-deficit/hyperactivity disorder. Pediatrics. 2006;118:e1530–e1540. doi: 10.1542/peds.2005-2478. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Alterman AI, Edwards KL. Vulnerability to alcoholism in men: A behavior-genetic perspective. Journal of Studies on Alcohol. 1985;46:329–356. doi: 10.15288/jsa.1985.46.329. [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Allen JJ. Theta EEG dynamics of the error-related negativity. Clin Neurophysiol. 2007;118(3):645–68. doi: 10.1016/j.clinph.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. The role of intact frontostriatal circuits in error processing. Journal of Cognitive Neuroscience. 2006;4:651–64. doi: 10.1162/jocn.2006.18.4.651. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tancredi LR, Grant C, Gillespie H, Valentine A, Mullani N, et al. Brain glucose metabolism in violent psychiatric patients: A preliminary study. Psychiatry Research: Neuroimaging. 1995;61:243–253. doi: 10.1016/0925-4927(95)02671-j. [DOI] [PubMed] [Google Scholar]
- Yeung N, Sanfey AG. Independent coding of reward magnitude and valence in the human brain. Journal of Neuroscience. 2004;24:6258–6264. doi: 10.1523/JNEUROSCI.4537-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]