Abstract
Recent research has demonstrated that patients with Alzheimer’s disease (AD) show deficits in semantic processing when compared to cognitively healthy individuals. This difference is thought to be attributed to losses in higher cortical systems that are predominantly associated with executive functioning. The first aim of the study will be to determine if differences in semantic clustering can accurately differentiate patients with amnestic mild cognitive impairment (aMCI) from cognitively normal (CN) individuals. The second aim will be to determine the extent to which semantic processing might be associated with executive functions. Data from 202 (134 CN, 68 aMCI) participants were analyzed to quantify differences in in semantic clustering ratios on the HVLT-R. Study participants ages ranged from 51 to 87 with education ranging from 6 to 20 years. ANCOVA revealed statistically significant differences on semantic clustering ratios (p<.001). Moderate correlations between semantic clustering Category Fluency Test (r = .45) were also found. Statistically significant group differences were also present on Trails-B and WAIS-R Digit Symbol performance (p<.001). Overall, these data indicate that deficits in semantic clustering are present in aMCI patients.
Introduction
Deficits in semantic processing have been demonstrated in patients with Alzheimer’s disease (AD) using a variety of neuropsychological tests and other novel methods (Aum Chan, & Chiu, 2003). This study, and others, consistently show that AD patients perform significantly lower on tasks that require semantic processing, relative to cognitively healthy individuals. Duong, Whitehead, Hanratty, and Chertkow (2006) cite several studies that demonstrate clear breakdowns in semantic processing networks in AD patients. In addition, Lam, Ho, Lui, & Tam (2006) show that decreases in semantic fluency, as measured by a category fluency task, are indicative of AD. Given these findings, it is reasonable to believe that individuals with amnestic-mild cognitive impairment (aMCI) might also show deficits in semantic processing.
As a diagnostic entity, aMCI was first characterized as a syndrome consisting decreased memory performance at or below 1.5 standard deviations (SD) on age and education adjusted normative values on a verbal memory test with the inclusion of subjective memory complaints by the affected individual (Petersen, Smith, Waring, Ivnik, Tangalos, & Kokmen, 1999). However, the diagnostic criteria for MCI have been refined to differentiate between amnestic and non-amnestic (nMCI) with the latter showing performance at or below 1.5 SD on a test or test(s) in one or more domains other than memory. Both entities can be further classified as single- or multiple- domain MCI depending upon the number of cognitive domains that demonstrate test performance(s) at or below 1.5 SD (Petersen and Negash, 2008).
Given that aMCI has been established as prodromal AD (Petersen et al., 1999; Manly, Tang, Schupf, Stern, Vonsattel, & Mayeaux, 2008), identifying aMCI early in the disease process may lead to better clinical outcomes. Given the increasing interest in aMCI as a therapeutic target and not just a diagnostic entity, utilizing neuropsychological measures with additional discriminatory power might help individuals at risk for developing AD receive beneficial treatment prior to disease onset. Given the established semantic processing deficits in AD patients, determining whether these deficits also occur in aMCI patients through the use of semantic clustering ratios may provide additional diagnostic value to the current criteria for aMCI.
Semantic clustering refers to the process by which an individual recalls semantically related words, consecutively, that were contained in a larger word list. This process can be seen individuals who are given the Hopkins Verbal Learning Test-Revised (HVLT-R) which utilizes a list of 12 words which are divided into three semantic categories (jewels, animals, and dwellings). In this case, one semantic cluster refers to the consecutive recall of two words from one category (i.e., horse and tiger). Semantic clustering ratios are often used to quantify semantic processing and are calculated by dividing the number of semantic clusters by the total number of words recalled from a word list.
The semantic clustering process is thought to be meditated by a system consisting of the pre-frontal cortex and the medial temporal lobe (Becker & Lim, 2003). In this model, emphasis is placed on the pre-frontal cortex as this area has been previously associated with the utilization of encoding and recall strategies. The use of these strategies thus facilitates recall of information from the medial temporal lobes. A study conducted by Baker, Sanders, Maccotta, and Buckner (2001) used fMRI to determine which brain regions might be responsible for both semantic and non-semantic processing and found that fronto-temporal activity was associated with both semantic and non-semantic processing during a verbal learning task. This study also found that semantic processing was superior to structured processing as measured by a word recognition task.
The results of Baker et al. (2001) also demonstrated that both left and right hemisphere regions were activated during semantic processing, however the majority of activity was concentrated in left hemisphere. A more recent study by Moulin, Laine, Rinne, Kaasinen, Sipliä, Hiltunen, and Kangasmäki (2007) used PET imaging to quantify cortical processing differences between patients with mild cognitive impairment (MCI) and individuals who are cognitively normal (CN). In this study, cerebral blood flow tended to increase during sequential learning trials of a word list in CN participants. However, MCI participants did not show this same blood flow increase, although increases in occipital activation were noted in this group across the learning trials. These observed differences are thought to reflect a significant difference in stimuli processing between CN and MCI participants, but also a decrease in the utilization of semantic processing systems that are thought to be mediated by left fronto-temporal regions. Others have also observed strong associations between semantic processing and pre-frontal regions (Savage, Deckersbach, Heckers, Wagner, Schacter, Alpert, Fischman, & Rauch, 2001; Demb, Desmond, Wagner, Vaidya, Glover, & Gabrielli, 1995).
The implication of these imaging studies suggest that semantic processing may be related to or mediated by the executive system given the localization of increased brain activity in the left prefrontal regions. Tremont, Halpert, Javorsky, and Stern (2000) found that individuals classified as having significant executive dysfunction had significantly lower performance on many components of the California Verbal Learning Test (CVLT). Several of these components also showed very modest, but statistically significant correlations to Trails-B performance. However, semantic clustering was not one of the components to show a significant correlation to Trails-B. Although this study does demonstrate a strong relationship between executive dysfunction and decreased verbal learning, one of the main conclusions of this study is that executive function is not strongly associated with semantic clustering.
A study conducted by Gaines, Shapiro, Alt, and Benedict (2006) used the HVLT-R to characterize semantic clustering indexes among CN, vascular dementia (VaD), and AD groups. In the CN group there were modest, but statistically significant correlations between HVLT-R components and Trails-B which were also found by Tremont et al. (2000). However, the Gaines et al. (2006) study found that semantic clustering ratios for the Total Learning and Delayed Recall components had almost no linear relationship with Trails-B. In spite of this, one of the more interesting findings of the study was that semantic clustering ratios in the CN group increased between the three learning trials and delayed recall trial. This was referred to as a “learning to learn index” and suggests that semantic clustering is reinforced during the consolidation process in cognitively healthy individuals. The “learning to learn index” occurs when the semantic clustering ratio increases from the initial learning trials and the delayed recall trial of a verbal memory test that utilizes word lists that contain semantically related items (e.g., cow, horse; diamond, sapphire). This increase in semantic clustering probably occurs as result of semantic networks aiding in the consolidation of information that has strong semantic relationships, thus facilitating recall. As expected, the VaD and AD groups performed significantly lower on the HVLT-R and also had lower ratios of semantic clustering than the CN group.
Gaines et al. (2006) point out that their sample size was relatively small and as a result, the ability to detect significant relationships between semantic clustering and executive function is hampered. However, this study provides strong evidence that semantic clustering can clearly differentiate CN, VaD, and AD groups. Given the evidence put forth by previous studies, how these findings manifest in MCI patients becomes a very important question. The intent of this study is investigate whether or not semantic clustering on the HVLT-R can differentiate CN and MCI patients and whether executive functioning plays a significant role in the mediation or control of the semantic clustering process.
Method
Sample
Data from 202 (134 CN, 68 aMCI) participants in the Florida Alzheimer’s Disease Research Center were used in the analysis. The inclusion and exclusion criteria were relatively liberal, however individuals with a history of major psychiatric and/or neurologic disorders were excluded from the study. The study recruited participants age 50 and above, however most individuals were age 65 and above. Demographic characteristics of the study sample are displayed in Table 1. All participants completed a comprehensive evaluation including: full clinical history, neurologic examination, informant-based interview, clinical laboratory tests, magnetic resonance imaging (MRI) of the brain, and a full neuropsychological battery. Consensus diagnoses with multiple clinicians were performed on all participants utilizing results from the assessments listed previously.. The aMCI group included participants with single and multiple-domain aMCI. The aMCI diagnosis was made when an individual’s verbal memory test score was at or below 1.5 SD when corrected for age and education. The same criteria were applied to other domains in order to differentiate single and multiple domain aMCI cases. CN participants were diagnosed as such based on an informant interview in which no decline in cognition was reported. Furthermore, these participants did not fall 1.5 SD below age- and education-corrected means on any cognitive test and also received a global score of zero on the Clinical Dementia Rating (CDR) (Morris, McKeel, Fulling, Torack, & Berg, 1988). Individuals with history of stroke or other cerebrovascular event were excluded from the analysis.
Table 1.
Demographic Characteristics and MMSE Scores by Group
| CN | aMCI | Total | Effect Size | p-value | |
|---|---|---|---|---|---|
| N | 134 | 68 | 202 | --- | --- |
| Male (%) | 35.07 | 60.87 | 43.84 | χ2 = 15.25 | <.0001 |
| Female (%) | 64.93 | 39.13 | 56.16 | χ2 = 15.25 | <.0001 |
| Age | 71.57 (6.59) | 74.30 (5.19) | 72.63 (6.21) | F = 10.60 | .001 |
| Education | 14.63 (2.55) | 13.78 (2.85) | 14.30 (2.69) | F = 5.34 | .022 |
| MMSE | 29.19 (1.05) | 27.30 (2.33) | 28.46 (1.90) | F = 67.49 | <.0001 |
Mean (sd)
Neuropsychological Tests
Results from the HVLT-R (Brandt & Benedict, 2001) were used to measure differences in immediate and delayed recall memory, but also to quantify differences in semantic clustering ratios in both types of recall as well. The HVLT-R is a verbal memory test consisting 3 trials in which 12 words are read aloud to an individual. After each trial, the individual is asked to recall as many words as they can remember. 20 to 25 minutes after the completion of the third trial, the individual is again asked to recall as many of the words as they can remember. The validity and reliability of the HVLT-R has been previously established (Benedict, Schretlen, Groninger, & Brandt, 1998; Brandt & Benedict, 2001).
Trails-B (Reitan & Wolfson, 1993), WAIS-R Digit Symbol (Wechsler, 1987), COWAT-FAS (Lezak, 1995), and the Category Fluency Test (Animals, Vegetables, Fruits) were used as measures of executive function (Lezak, 1995). Trails-B is a test that requires the individual to draw a line connecting circled numbers and letters in an alternating fashion (1-A-2-B-3-C, etc.). The WAIS-R Digit Symbol test requires the individual to fill in an empty box below a number with a symbol that is matched to the number in an array shown at the top of the page. The COWAT-FAS test requires the individual to name as many words as they can that begin with a given letter, F, A, and S. 60 seconds is allotted for each letter. Individuals can not use proper names, numbers, and can not use words with different tenses or endings once the root word has been given. The Category Fluency test requires that individual to name as many items as possible in a given category (Animals, Fruits, and Vegetables). 60 seconds is allotted for each category. In addition, the MMSE (Folstein, Folstein, & McHugh, 1975) was used as a measure of global cognitive function. The MMSE consists of 30 items which test orientation, short-term memory, visuospatial function and other areas of cognitive function.
All tests were administered according to standard criteria (Lezak, 1995). The tests described above were administered within a larger battery of tests that took approximately 2 hours for each participant to complete and were completed in one session.
Semantic Clustering Ratio Calculation
For the purposes of this study, semantic clustering will refer to the number of times that words from the same semantic category are recalled consecutively. The method for determining semantic clustering was the same as that used by Gaines et al. (2006) which states that if a patient were to recall the words “sapphire”, “emerald”, and “pearl” consecutively, this would constitute 2 semantic clusters as 2 semantically related words recalled consecutively are equal to 1 cluster. Semantic clustering for both Total Recall and Delayed Recall were calculated. In order to account for large differences in Total and Delayed Recall between groups, semantic clustering ratios were derived. The method by which semantic clustering ratios were calculated is similar to the one used by Gaines et al. (2006). This is derived by summing the number of semantic clusters for all 3 learning trials and dividing this by the Total Recall score (the total number of words correctly recalled for all 3 learning trials). A semantic clustering ratio for the Delayed Recall trial was calculated in the same manner.
Statistical Analyses
In order to examine differences in clustering between the NC and MCI groups, the data were analyzed in using the ANCOVA procedure, with clustering as the dependent variable, patient group (CN, MCI) as the between subjects variable, and age, gender, years of education and global cognitive performance (MMSE) as covariates. In addition to the ANCOVA, the extent to which the clustering index provides additional discriminatory power and diagnostic value was analyzed through a logistic regression model with patient group (CN/MCI) as the outcome. The use of the logistic model goes beyond the linear assumptions of the ANCOVA by providing a more accurate assessment of a variable’s discriminatory power. Correlational analyses will also be performed for the neuropsychological variables.
Results
Demographic characteristics are shown in Table 1. In general, the aMCI group was somewhat older and slightly less educated. Chi-square analysis found a statistically significant difference in gender frequency between the CN and aMCI groups (p<0.001) with women comprising approximately 65% of the aMCI. ANOVA found that there were statistically significant differences for age, (p = 0.004) and MMSE (p<0.001) with education approaching significance (p = 0.06) with the aMCI participants being greater in age and having lower education and MMSE scores when compared to the CN participants. Table 2 displays neuropsychological test performance for both groups. Statistically significant differences were present on all tests between the two groups. Differences in semantic clustering ratios are displayed graphically in Figure 1.
Table 2.
ANCOVA Results by Group
| CN | aMCI | F-value | |
|---|---|---|---|
| HVLT-R Total Recall | 25.49 (4.53) | 18.62 (4.29) | 36.05 |
| HVLT-R Delayed Recall | 9.15 (2.18) | 5.72 (2.44) | 29.36 |
| HVLT-R Total Recall SCR | 0.45 (0.17) | 0.33 (0.14) | 11.52 |
| HVLT-R Delayed Recall SCR | 0.53 (0.20) | 0.39 (0.18) | 9.40 |
| Trails-B | 82.96 (32.58) | 132.13 (55.19) | 16.69 |
| WAIS-R Digit Symbol | 47.70 (8.60) | 37.53 (7.91) | 19.97 |
| COWAT-FAS | 40.40 (10.33) | 27.76 (10.98) | 16.91 |
| Category Fluency | 47.67 (10.77) | 36.34 (7.22) | 20.26 |
Mean (sd)
SCR - semantic clustering ratio
All are significant at the p<.0001 level; df (5, 197)
Figure 1.
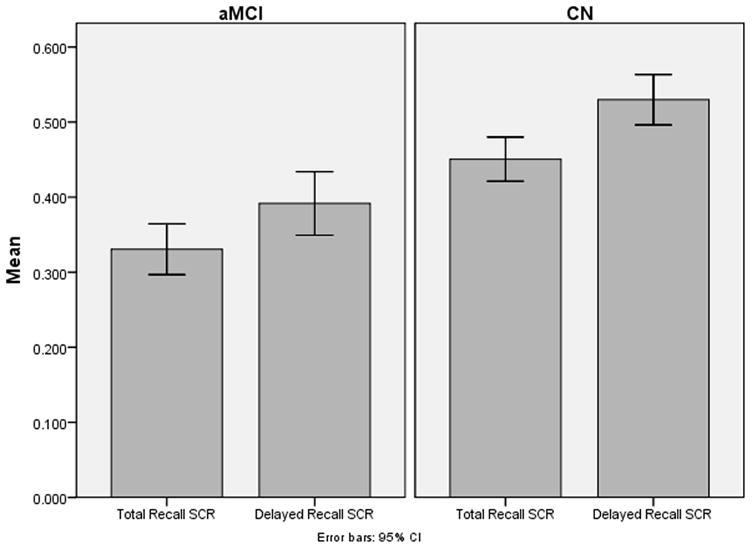
Semantic Clustering Ratios for aMCI and CN Groups
Table 3 displays the correlation coffecients between measures of executive functions and the HVLT-R variables. All values shown were significant at the p<.001 level. Overall, Trails-B and the WAIS-R Digit Symbol were not highly correlated with the semantic clustering ratios. COWAT-FAS and Category Fluency were moderately correlated with semantic clustering ratios. All measures of executive function showed strong correlations with Total and Delayed Recall. All values were statistically significant at or below the p = 0.01 level.
Table 3.
Correlation Values Between HVLT-R Measures and Executive Function Tests
| Trails-B | Digit Symbol | Category | FAS | |
|---|---|---|---|---|
| Total Recall SCR | −.23 | .24 | .34 | .22 |
| Delayed Recall SCR | −.22 | .23 | .36 | .20 |
| HVLT Total Recall | −.45 | .43 | .59 | .45 |
| HVLT Delayed Recall | −.38 | .40 | .61 | .39 |
SCR - semantic clustering ratio
Results from the logistic regression analysis are displayed in Table 4. This analysis showed statistically significant effects for HVLT-R Delayed Recall, Trails-B, and COWAT-FAS. Semantic clustering ratios for both HVLT-R Total Recall and HVLT-R Delayed Recall and all other variables failed to show significant effects. It should be noted that all reported values are rounded to the second decimal, so the actual value for the lower bound of the Trails-B confidence interval is 1.002.
Table 4.
Logistic Regression Results for Demographic and Neuropsychological Variables
| Variable | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Age | 1.01 | (0.94, 1.09) | 0.76 |
| Education | 1.18 | (0.97, 1.45) | 0.10 |
| Gender | 0.88 | (0.34, 2.28) | 0.78 |
| HVLT-R Total Recall SCR | 3.28 | (0.06, 179.25) | 0.55 |
| HVLT-R Delayed Recall SCR | 5.32 | (0.16, 166.09) | 0.35 |
| HVLT-R Total Recall | 0.91 | (0.76, 1.08) | 0.27 |
| HVLT-R Delayed Recall | 0.62 | (0.43, 0.89) | 0.01 |
| Trails-B | 1.01 | (1.00, 1.03) | 0.02 |
| WAIS-R Digit Symbol | 0.96 | (0.89, 1.02) | 0.16 |
| Category Fluency | 0.98 | (0.92, 1.05) | 0.55 |
| COWAT-FAS | 0.92 | (0.87, 0.97) | 0.001 |
SCR - semantic clustering ratio
Discussion
The results of the study demonstrate that deficits in semantic processing are present in patients with MCI which is shown by their decreased semantic clustering ratios and lower performance on the Category Fluency test when compared to CN individuals. To date, this appears to be one of the first studies to demonstrate these deficits in aMCI patients as previous studies have focused primarily on other clinical groups (Gaines et al., 2006; Tremont et al., 2000). In spite of decreased semantic clustering ratios in the aMCI group, this measure showed a marginal increase between Total Recall and Delayed Recall. This effect was also found in the CN group, but was far more pronounced. These results are consistent with the “learning to learn index” first proposed by Gaines et al. (2006) which suggests that the consolidation process enhances the semantic clustering process for recalling information at a later time.
What is interesting is that this effect is still present in aMCI patients, but is attenuated. When taken with their overall performance on the HVLT-R, the semantic clustering performance of aMCI patients demonstrate that systems other than memory can be affected in this clinical group. Although the semantic clustering deficits in the aMCI group clearly differentiate them from the CN group, the extent to which semantic clustering might is associated with executive functions is unclear. The results of this study show that traditional measures of executive function, such as Trails-B and WAIS-R Digit Symbol, do not show a strong linear relationship to semantic clustering. Previous studies have investigated this hypothesis and have arrived at the same conclusion as this study.
However, these same measures were highly correlated with HVLT-R Total Recall and Delayed Recall supporting the notion that executive functions play a significant role in the encoding process and general functioning of short-term memory. However, Category Fluency and the COWAT-FAS tests correlated weakly with semantic clustering ratios. This is somewhat surprising given that both the Category Fluency and FAS tests utilize varying semantic resources and could be expected to have at least a moderate relationship with semantic clustering. Given these findings it appears that executive functions are not highly associated with semantic clustering.
A study by Griffith, den Holander, Okonkwo, Evanochko, Harrell, Zamrini, Brockington, and Marson (2007) utilized magnetic resonance spectroscopy (MRS) to localize the neuroanatomic basis of executive function impairment in aMCI patients. Results of the neuropsychological testing found that differences between the aMCI and CN group on Category Fluency and WAIS-III Digit Symbol were nearly identical to those found in the current study. The MRS findings demonstrated that metabolic changes in the posterior cingulate gyrus had the strongest relationship to executive functioning. However, the authors point out that regional specificity of executive function control and mediation is difficult to determine given that multiple frontal regions also play significant roles in this domain. In addition, Treykov et al. (2007) state that “the precise anatomic correlates of executive functions are still a matter of debate”. From these findings, it seems that discerning the relationship between semantic clustering and executive functions is difficult given the complexity of neural networks that are likely to be involved in this relationship.
In terms of predicting disease status, the semantic clustering ratio is not a good predictor of aMCI as demonstrated by the logistic regression analysis. There are a variety of reasons as to why this might be the case, but it is very likely that a certain proportion of CN individuals may not fully utilize semantic clustering as a learning strategy in list-learning tasks. Furthermore, this study demonstrated that aMCI participants still utilized semantic clustering to some extent so it is possible that a high degree of overlap in semantic clustering performance is present between some CN and aMCI individuals.
Although tests of executive function may not be predictive of semantic processing deficits, the overall performance of aMCI patients on these measures warrants further discussion. Several recent studies have shown that aMCI patients do show deficits in domains other than memory. Kramer, Nelson, Johnson, Yaffe, Glenn, Rosen, and Miller (2006) demonstrate that deficits in executive functioning were present in a small group of aMCI patients. Specifically, these patients showed lower performance on Design Fluency, Category Fluency, and the Stroop Color/Word tasks. It should be noted that Kramer et al. (2006) used a cutoff of 1.0 SD on their measures of executive functions while the present study used a 1.5 SD cutoff.
A recent study by Small, Gagnon, and Robinson (2007) also supports the idea that executive function deficits are present in aMCI patients despite the fact that current diagnostic criteria do not include impaired performance in this area. Since executive functions have been shown to play a large role in the short-term memory process, it is possible that executive function tests might yield some degree of clinical utility in assessing those with aMCI.
One of the major weaknesses of this study is its relatively homogenous ethnic composition as the study sample was predominantly White-Anglo. As a result, the ability to generalize the study results to other ethnic groups is hampered. In addition, the aMCI participants were significantly older and, on average, were less educated than their CN counterparts. However, an attempt to correct for these factors was made with the use of ANCOVA. This study may also be hampered by the overall age of the study sample as decreases in overall cognitive performance, encoding strategy use, and processing resources have been noted in the older adult population (Jacobs, Rakitin, Zubin, Ventura, & Stern, 2001).
Although this study did not show that decreased semantic clustering ratios are predictive of aMCI, it did demonstrate that semantic processing deficits are present in this population. However, it is possible that longitudinal decreases in semantic clustering ratios might be predictive of cognitive status and could act as a cognitive marker for disease progression. Specifically, changes in semantic clustering ratios as measured by serial assessment over time might be more predictive of disease status rather than one assessment done at a single time-point.
Acknowledgments
The first author would like to thank Christine Belden, Psy.D. for her thoughtful review of this manuscript.
Funding for this study was supported by the National Institute of Health for the Florida Alzheimer’s Disease Research Center (1P50AG025711-01).
Contributor Information
Michael Malek-Ahmadi, University of South Florida Byrd Alzheimer’s Institute.
Ashok Raj, University of South Florida Byrd Alzheimer’s Institute.
Brent J. Small, University of South Florida School of Aging Studies
References
- Au A, Chan AS, Chiu H. Verbal learning in Alzheimer’s dementia. Journal of the International Neuropsychological Society. 2003;9:363–375. doi: 10.1017/S1355617703930025. [DOI] [PubMed] [Google Scholar]
- Baker JT, Sanders AL, Maccotta L, Buckner RL. Neural correlates of verbal memory encoding during semantic and structural processing tasks. Neuro-Report. 2001;12:1251–1256. doi: 10.1097/00001756-200105080-00039. [DOI] [PubMed] [Google Scholar]
- Balthazar MLF, Martinelli JE, Cendes F, Damasceno BP. Lexical semantic memory in amnestic mild cognitive impairment and mild Alzheimer’s disease. Arqivos de Neuropsiquiatri. 2007;65(3A):619–622. doi: 10.1590/s0004-282x2007000400014. [DOI] [PubMed] [Google Scholar]
- Barde LHF, Thompson-Schill SL. Models of functional organization of the lateral prefrontal cortex in verbal working memory: Evidence in favor of the process model. Journal of Cognitive Neuroscience. 2002;14(7):1054–1063. doi: 10.1162/089892902320474508. [DOI] [PubMed] [Google Scholar]
- Becker S, Lim J. A computational model of prefrontal control in free recall: Strategic memory use in the California Verbal Learning Task. Journal of Cognitive Neuroscience. 2003;15(6):821–832. doi: 10.1162/089892903322370744. [DOI] [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test—Revised: Normative data and analysis of inter-form and test–retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Brandt J, Benedict RHB. Professional manual. Lutz, FL: Psychological Assessment Resources; 2001. Hopkins Verbal Learning Test–Revised. [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15(9):5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong A, Whitehead V, Hanratty K, Chertkow H. The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia. 2006;44(10):1928–1935. doi: 10.1016/j.neuropsychologia.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;1975(12):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gaines JJ, Shapiro A, Alt M, Benedict RB. Semantic clustering indexes for the Hopkins Verbal Learning Test-Revised: initial exploration in elder control and dementia groups. Applied Neuropsychology. 2006;13(4):213–222. doi: 10.1207/s15324826an1304_2. [DOI] [PubMed] [Google Scholar]
- Griffith HR, den Hollander JA, Okonkwo O, Evanochko WT, Harrell LE, Zamrini EY, Borckington JC, Marson DC. Executive function is associated with brain proton magnetic resonance spectroscopy in amnestic mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2007;29(6):599–609. doi: 10.1080/13803390600826595. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Rakitin BC, Zubin NR, Ventura PR, Stern Y. Cognitive correlates of mnemonics usage and verbal recall memory in old age. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 2001;14(1):15–22. [PubMed] [Google Scholar]
- Jones S, Luakka EJ, Backman L. Differential verbal fluency deficits in the preclinical stages of Alzheimer’s disease and vascular dementia. Cortex. 2006;42(3):347–355. doi: 10.1016/s0010-9452(08)70361-7. [DOI] [PubMed] [Google Scholar]
- Karrasch M, Laine M, Rinne JO, Rapinoja P, Sinvera E, Krause CM. Brain oscillatory responses to an auditory-verbal working memory task in mild cognitive impairment and Alzheimer’s disease. International Journal of Psychophysiology. 2006;59:168–178. doi: 10.1016/j.ijpsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Du A, Schuff N, Hollnagel C, Weiner MW, Miller BL, Kaplan DC. Dissociations in hippocampal and frontal contributions to episodic memory performance. Neuropsychology. 2005;19(6):799–805. doi: 10.1037/0894-4105.19.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Nelson A, Johnson JK, Yaffe K, Glenn S, Rosen HJ, Miller BL. Multiple cognitive deficits in amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2006;22:306–311. doi: 10.1159/000095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam LC, Ho P, Lui VW, Tam CW. Reduced semantic fluency as an additional screening tool for subjects with questionable dementia. Dementia and Geriatric Cognitive Disorders. 2006;22(2):159–164. doi: 10.1159/000094543. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychologial assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JPG, Mayeaux R. Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Jr, Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer’s disease. Annals of Neurology. 1988;24(1):17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- Moulin CJA, Laine M, Rinne JO, Kassinen V, Sipila H, Hiltunen J, Kangasmaki A. Brain function during multi-trial learning in mild cognitive impairment: A PET activation study. Brain Research. 2007;1136:132–141. doi: 10.1016/j.brainres.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Murphy KJ, Rich JB, Troyer AK. Verbal fluency patterns in amnestic mild cognitive impairment are characteristic of Alzheimer’s type dementia. Journal of the International Neuropsychological Society. 2006;12(4):570–574. doi: 10.1017/s1355617706060590. [DOI] [PubMed] [Google Scholar]
- Nutter-Upham KE, Saykin AJ, Rabin LA, Roth RM, Wishart HA, Pare N, Flashman AL. Verbal fluency in amnestic MCI and older adults with cognitive complaints. Archives of Clinical Neuropsychology. 2008;23:229–241. doi: 10.1016/j.acn.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Negash S. Mild cognitive impairment: An overview. CNS Spectrums. 2008;13(1):45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2. South Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- Sandrini M, Cappa SF, Rossi S, Rossinni PM, Miniussi C. The role of prefrontal cortex in verbal episodic memory: rTMS evidence. Journal of Cognitive Neuroscience. 2003;15(6):855–861. doi: 10.1162/089892903322370771. [DOI] [PubMed] [Google Scholar]
- Savage CR, Deckersbach T, Heckers S, Wagner AD, Schacter DL, Alpert NM, Fischman AJ, Rauch SL. Prefrontal regions supporting spontaneous and direct application of verbal learning strategies: Evidence from PET. Brain. 2001;124:219–231. doi: 10.1093/brain/124.1.219. [DOI] [PubMed] [Google Scholar]
- Small BJ, Gagnon E, Robinson B. Early identification of cognitive deficits: Preclinical Alzheimer’s disease and mild cognitive impairment. Geriatrics. 2007;62(4):19–23. [PubMed] [Google Scholar]
- Traykov L, Raoux N, Latour F, Gallo L, Hanon O, Baudic S, Bayle C, Wenisch E, Remy P, Rigaud A. Executive functions deficit in mild cognitive impairment. Cognitive & Behavioral Neurology. 2007;20:219–224. doi: 10.1097/WNN.0b013e31815e6254. [DOI] [PubMed] [Google Scholar]
- Tremont G, Halpert S, Javorsky DJ, Stern RA. Differential impact of executive function on verbal learning list and story recall. The Clinical Neuropsychologist. 2000;14(3):295–302. doi: 10.1076/1385-4046(200008)14:3;1-P;FT295. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Zhang Y, Han B, Verhaeghen P, Nilsson LG. Executive functioning in older adults with mild cognitive impairment: MCI has effects on planning, but not on inhibition. Aging, Neuropsychology, and Cognition. 2007;14:557–570. doi: 10.1080/13825580600788118. [DOI] [PubMed] [Google Scholar]


