Abstract
Decision support systems have been used to promote the practice of evidence-based medicine. Computer-assisted diagnosis can serve as one element of evidence-based radiology. One area where such tools may provide benefit is analysis of vertebral compression fractures (VCFs), which can be a challenge in MRI interpretation. VCFs may be benign or malignant in etiology, and several MRI features may help to make this important distinction. We describe a web-based decision support system for discriminating benign from malignant VCFs as a prototype for a more general diagnostic decision support framework for radiologists. The system has three components: a feature checklist with an image gallery derived from proven reference cases, a prediction model, and a reporting mechanism. The website allows users to input the findings for a case to be interpreted using a structured feature checklist. The image gallery complements the checklist, for clarity and training purposes. The input from the checklist is then used to calculate the likelihood of malignancy by a logistic regression prediction model. Standardized report text is generated that summarizes pertinent positive and negative findings. This computer-assisted diagnosis system demonstrates the integration of three areas where diagnostic decision support can aid radiologists: first, in image interpretation, through feature checklists and illustrative image galleries; second, in feature-based prediction modeling; and third, in structured reporting. We present a diagnostic decision support tool that provides radiologists with evidence-based guidance for discriminating benign from malignant VCF. This model may be useful in other difficult-diagnosis situations and requires further clinical testing.
Key words: Decision support, computer-assisted diagnosis, compression fracture, magnetic resonance imaging, structured reporting
Background
Evidence-based medicine (EBM) has been defined as “the process of systematically finding, appraising and using contemporaneous research findings as the basis for clinical decisions”1. Over the past few decades, EBM has emerged in response to perceived variability and complexity in clinical practice, leading to an emphasis on the use of clinical research in routine medical practice2,3.At the same time, computer-based clinical decision support systems have continued to evolve in sophistication. Together, these trends have led to a range of systems designed to promote the application of research-based practice guidelines in areas such as management of chronic diseases and selection of antibiotics4,5.
While awareness of EBM is widespread in some specialties, it has received relatively less attention in radiology6,7. Barriers to a broader application of evidence-based radiology (EBR) include lack of time, unfamiliarity with how to translate published research results to clinical practice, and limited access to resources6, and while there have been technological developments in computer-aided radiology exam selection8–12 and artificial intelligence for image interpretation13–15, there continues to be an opportunity for further integration of evidence-based research within radiology decision support systems. Such integration has the potential to reduce the barriers to more widespread practice of EBR. In addition, the potential importance of EBM in radiology education has also been discussed16, and computer applications incorporating current knowledge may help to advance training in imaging as well.
In the realm of diagnostic decision support in radiology, approaches to computer-assisted image interpretation range from techniques in computer vision for image segmentation (i.e. computer-aided detection) to feature-based prediction modeling17. While the former may be considered more fundamental with regard to image understanding, the latter may be more practical in many situations, leveraging the radiologist’s skill in synthesizing feature detection and characterization. Feature-based prediction may utilize any of a number of modeling methods, including Bayesian networks, logistic regression, recursive partitioning, and neural networks13–15, in order to derive potential diagnoses. One specific problem amenable to feature-based prediction modeling and diagnostic decision support is analysis of vertebral compression fractures (VCFs) on magnetic resonance imaging (MRI).
Vertebral Compression Fractures
Metastases to the vertebrae are present in 5% to 10% of all patients with malignancy18. Malignant VCFs occur in approximately 10% to 15% of patients with skeletal metastasis19. Another common cause of VCFs is osteoporosis, a benign skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue, leading to enhanced bone fragility20. In the US, there are approximately 700,000 osteoporotic VCFs per year resulting in about 115,000 hospital admissions. The lifetime risk of an osteoporosis-related VCF is approximately 16% for women and 5% for men; the latter is likely an underestimate21. Acute/subacute VCFs are often associated with bone marrow edema, regardless of etiology. In addition, several other imaging findings may be present, and there may be significant overlap in the imaging appearance of benign (osteoporotic) and malignant (typically metastatic but sometimes myeloma-related) VCFs. Differentiating benign and malignant spinal compression fractures is a common problem confronting radiologists.
Structured Reporting
Efforts to standardize the format and content of radiology reports have been underway for over a decade, with the goal of improving the clarity and efficiency of communication in radiology22. This has provided part of the impetus for the RadLex project23, which is a controlled lexicon of radiological terms. In addition, the Radiology Reporting Committee of the Radiological Society of North America (RSNA) has recently described its work in identifying subspecialty-based best practices in radiology reporting24. Decision support systems based on imaging features are well positioned to leverage both controlled lexicons and structured reporting templates, and MRI feature analysis of VCFs integrates well with the objectives and techniques of standardized reporting.
Evidence-based prediction modeling can help a radiologist to integrate the range of findings present in a given case and may serve to clarify the certainty of a particular diagnosis. In order to achieve these goals in a clinically useful manner, speed and ease of use are of critical importance25. This work describes a web-based decision support system, using feature-based prediction modeling and a standardized reporting mechanism, for discriminating benign from malignant VCFs by MRI appearance.
Methods
Prediction Modeling
Feature-based prediction modeling for radiology interpretation is described in detail elsewhere15. Briefly, prediction model development includes feature identification, model selection, model derivation, followed by model validation. First, potentially relevant imaging features should be identified. In general, such imaging features may be dichotomous, non-dichotomous discrete, or continuous in nature. How any particular feature is treated may depend on the nature of the finding and the nature of the clinical question. For example, in the case of MRI features of VCFs, while vertebral body bone marrow edema might conceivably be graded on a continuous scale, such observations may be less reproducible compared to a discretization of this finding, with a dichotomous treatment (i.e., bone marrow edema either present or not) likely offering the greatest reproducibility. Given this, and because the clinical question with regard to VCFs is itself also dichotomous (i.e., benign or malignant cause of fracture), dichotomous treatment of variables was preferred in this work.
The nature of the imaging features identified also impacts the selection of a modeling method. Several methods have been used to construct prediction models, including logistic regression, neural networks, recursive partitioning, Bayesian networks, and case-based reasoning14,15. While certain of these options (e.g., neural networks, recursive partitioning) may be more well suited for discerning possible non-linearity in the relationships between variables, there may be an associated computational penalty and/or loss of transparency as to the meaning of the derived model. Logistic regression is a relatively simple method appropriate for modeling dichotomous outcomes and, given the dichotomous nature of the VCF outcome noted above, logistic regression was selected as the modeling method for this application.
After candidate features have been identified and logistic regression selected as the model of choice, individual features are tested for diagnostic significance using univariate analysis. Correlation among features will in general cause certain features to be deemed insignificant. That is, if multiple features are found to be correlated with one another, then they are not independent predictors of the given outcome and would not be expected to contribute separately to the model15. Once the final set of significant features has been determined, logistic regression is performed, leading to a set of weighting factors which allow for a closed-form calculation of the predicted outcome probability.
After model derivation is completed, model validation may be considered. This validation may include both narrow forms, whereby the derived model is tested against cases which are similar to those in the training set, and broad forms, in which the model is applied across diverse clinical settings, patient populations, and institutions.
Web-Based Implementation
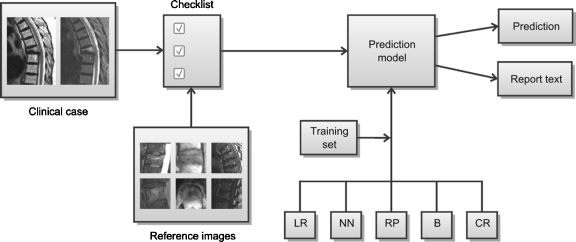
Platform-independent, cross-browser, web-based implementation of feature-based radiology prediction rules may be achieved using a combination of hypertext markup language (HTML) and JavaScript. In this work, HTML is used to provide a feature checklist, where each feature is accompanied by an annotated illustration for review. JavaScript is used to implement the probability calculation at the core of logistic regression modeling, which combines the features of a given case using the weighting factors described above. JavaScript is also used to generate a standardized report text based on the feature checklist input, describing pertinent positives and negatives in prose form. The block diagram in Figure 1 illustrates the functional organization of the system.
Fig 1.
Feature-based decision support systems for radiologic diagnosis include two primary components: a set of relevant imaging findings and a prediction model to aggregate these findings. Relevant findings in a given clinical case are summarized using a feature checklist. A gallery of reference images provides guidance in evaluating individual features. These features are used as input to a prediction model, which may be based on any of several modeling methods including logistic regression (LR), neural networks (NN), recursive partitioning (RP), Bayesian networks (B), and case-based reasoning (CR). Model parameters are derived using a set of training cases. The model may then be used to calculate predictions in clinical cases (e.g., the probability of a given outcome). In addition, the structured nature of the feature checklist lends itself to automatic generation of standardized report text.
Results
Using a combination of peer-reviewed literature, as well as other sources consisting of book chapters and local experts (i.e., practicing academic neuroradiologists and musculoskeletal radiologists), a total of 31 candidate MRI features were identified as possible contributors to a model for distinguishing between benign and malignant VCFs. Twenty-eight of these 31 features were treated as dichotomous. For the remaining three candidate features (i.e., pedicle involvement, enhancement pattern, and involvement of multiple vertebral levels), the available literature and expert opinion indicated that non-dichotomous discrete treatment would be most appropriate. For example, in the case of enhancement pattern, there is evidence that heterogeneous enhancement, as opposed to homogeneous appearance on post-contrast imaging, is suggestive of malignant VCF26,27. As a result, enhancement pattern was treated in this work as a non-dichotomous discrete variable with several possible values: heterogeneous, homogeneous, non-enhancing, or unknown with no contrast given.
The 31 candidate features were assessed in a series of 128 pathologically proven cases of benign and malignant VCF by three radiologists who were blinded to the diagnosis, using a checklist-based questionnaire. The diagnostic value of each feature was assessed using univariate analysis, showing nine of the 31 candidate features to be significant. Using these nine significant features, logistic regression was performed, leading to a series of weighting factors.
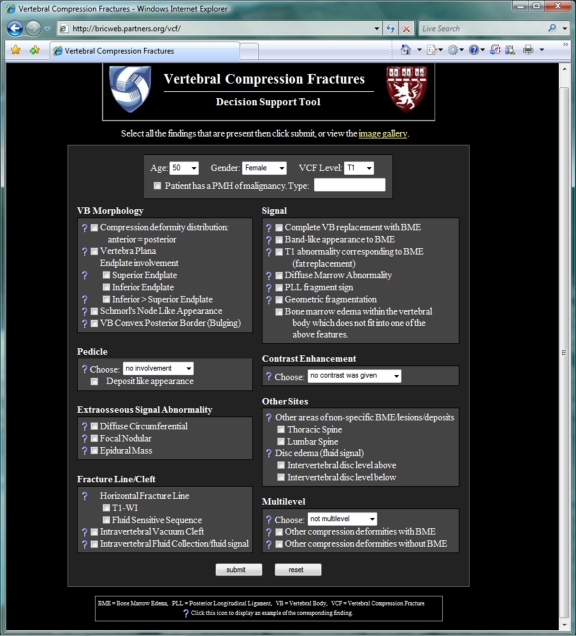
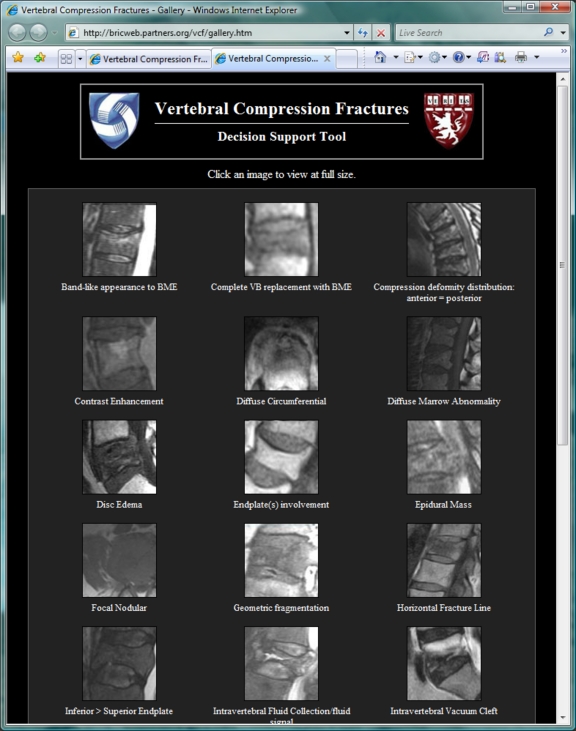
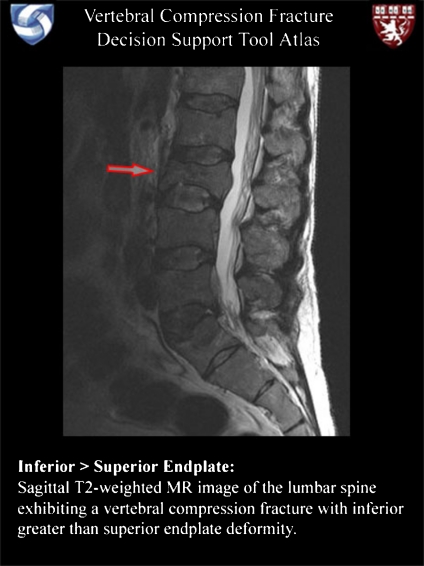
The web-based implementation of this prediction model for VCF analysis is available at http://bricweb.partners.org/vcf. The feature checklist of the top-level display (Fig. 2) consists of checkboxes for dichotomous features and pop-up menus for non-dichotomous discrete features. While only a subset of the initial 31 candidate features was deemed to be diagnostically significant, all 31 features are included in the checklist. Annotated illustrations for each feature may be browsed using a gallery of thumbnail images (Fig. 3), accessed with the “image gallery” link towards the top of the main page, or illustrations for each individual feature may be displayed using the question mark icons adjacent to each item in the checklist (Fig. 4). Each illustration has an associated caption describing the findings which constitute the given feature.
Fig 2.
The primary screen of the vertebral compression fracture decision support website presents a feature checklist to the user. The majority of these features are dichotomous in nature, shown as checkboxes. A few are non-dichotomous discrete variables, shown as pop-up menus. If a particular feature is unknown to the user, clicking the adjacent question mark will display an annotated illustration demonstrating that feature (Fig. 4).
Fig 3.
MRI features of vertebral compression fractures are illustrated using a series of images. These may be browsed in a gallery format, shown here, accessed using the “image gallery” link toward the top of the main page (Fig. 2). Each thumbnail in this gallery is labeled with a feature description. Clicking a particular thumbnail leads to a larger, annotated image with text-based description (Fig. 4).
Fig 4.
A detailed, annotated image or set of images is available for each of the MRI features listed in the checklist of the main page (Fig. 2). A combination of image marks and text-based explanations summarize the findings which constitute a given feature, promoting a uniform understanding of these features and providing a learning resource for trainees.
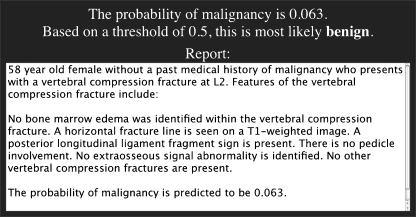
When the checklist has been completed, clicking the “submit” button towards the bottom of the main page triggers the JavaScript-based prediction model probability calculation, as well as the construction of a standardized report text based on a reporting template (Fig. 5). The prediction result is shown as a probability of malignancy given the constellation of MRI features present in the case. The standardized report text is presented for possible incorporation into the user’s reporting system, such as by a cut-and-paste operation when the reporting software is running on the same machine as the user’s web browser.
Fig 5.
Once the feature checklist has been completed, clicking the “submit” button towards the bottom of the main page triggers the prediction model probability calculation and template-based report text generation, both shown below the checklist items. These results are displayed respectively as a probability of malignancy and as a block of text available for cut-and-paste incorporation into the user’s reporting system.
Discussion
The combination of evidence-based practice and clinical decision support systems promises to substantially improve patient care28. Image interpretation guidance, feature-based prediction modeling, and standardized reporting may all contribute to this goal in the realm of radiology diagnostic decision support. For interpretation guidance, feature checklists serve to formalize and standardize the basis for reading an imaging examination. Accompanying image galleries, such as the one shown here, help to promote a uniform understanding of the relevant imaging findings. These illustrative images also serve as a learning resource, providing detailed yet easily accessible information which may be useful to trainees at the reading station. This type of feature-based reference image organization is based in part on prior work in image-oriented expert systems for decision support29. Furthermore, while the reference images included in the VCF analysis tool focus on pathological findings, anatomical illustrations are also an important area where these tools have the potential to assist learners.
Inclusion of all candidate features in the input checklist, rather than only the nine features significant in the prediction model, allows for a more complete description of the lesion present in the case, and inclusion of all features may still promote overall characterization in the report text. In a robust, broadly validated model, all features will not be needed for sufficient discrimination and could be omitted in future versions to make the system more parsimonious and probably more acceptable to the user. Only limited, narrow validation of the model presented here has been performed, and broader testing would be required before such a model could be deemed appropriate for routine clinical use.
The objectives of evidence-based decision support also integrate well with quantitative research methods, such as the prediction modeling technique used here. The VCF analysis tool provides an example of how the complexity of prediction modeling techniques may be encapsulated within a straightforward web-based interface designed to complement the radiologist’s clinical workflow. However, the basis of EBM requires practitioners to be able to evaluate the validity of specific research results, and users of evidence-based decision support tools must engage in continual critical appraisal of the research underlying any such system. At the same time, use of such systems over time may also contribute to the validity of the models used. As pathological proof is obtained in unknown cases, these could be used to augment the initial training set (consisting of 128 cases in this system, as described above), with cumulative refinement of the model.
Decision support systems should provide actionable results30, and another area for potential improvement of the VCF analysis tool relates to the interpretation of the probabilities produced by the prediction model. While results characterized by very high and very low probability are relatively easy to apply clinically, cases of intermediate probabilities may be more difficult for the radiologist to use in providing actionable recommendations. Management guidelines for cases of intermediate probability, such as recommendations for short-term follow-up imaging, suggested follow-up intervals, when to consider biopsy, and so on, may be helpful. Development of such guidelines would depend on further research and could be added to the application in order to provide a more comprehensive decision support tool. However, such systems remain tools to support rather than replace trained radiologists, who need to integrate imaging information with all available clinical, laboratory, pathological, and other data in clinical decision making31.
Future directions for this work include developing similar systems for other common imaging domains, such as routine spine MRI where there is documented substantial variability of interpretation for intervertebral disk and degenerative findings32,33, as well as for emerging domains where many radiologists may not have much experience or knowledge, such as MR neurography. In addition, integration with knowledge management systems34 may serve to advance the educational benefits of these tools. The broad, long-term goal is to create a generalized model and application framework for image interpretation decision support systems that could be a platform for a variety of interpretation tasks.
Conclusion
The practice of evidence-based medicine within radiology is growing, and decision support tools provide an important mechanism by which to facilitate further advancement of evidence-based radiology, with a goal of formalizing and standardizing image interpretation and results communication. The web-based tool presented here for distinguishing benign from malignant vertebral compression fractures by MRI appearance integrates image interpretation guidance, feature-based prediction modeling, and structured reporting.
Acknowledgements
The authors thank Dr. Ramin Khorasani, Dr. Francis Cook, Dr. Lucila Ohno-Machado, and Dr. Patrick Jeanmenne for their assistance. JAC acknowledges prior General Electric Radiology Research Academic Fellowship (GERRAF) support. KCW acknowledges the support of RSNA Research and Education Foundation Fellowship Training Grant #FT0904, as well as the support of the Walter and Mary Ciceric Research Award.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s10278-011-9359-0
References
- 1.Rosenberg W, Donald A. Evidence based medicine: an approach to clinical problem-solving. BMJ. 1995;310:1122–1126. doi: 10.1136/bmj.310.6987.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evidence-Based Medicine Working Group Evidence-based medicine: a new approach to teaching the practice of medicine. JAMA. 1992;268:2420–2425. doi: 10.1001/jama.268.17.2420. [DOI] [PubMed] [Google Scholar]
- 3.Elstein AS. On the origins and development of evidence-based medicine and medical decision making. Inflamm Res. 2004;53:S184–S189. doi: 10.1007/s00011-004-0357-2. [DOI] [PubMed] [Google Scholar]
- 4.Hunt DL, Haynes RB, Hayward RSA, Pim MA, Horsman J. Patient-specific evidence-based care recommendations for diabetes mellitus: development and initial clinic experience with a computerized decision support system. Int J Med Inform. 1998;51:127–135. doi: 10.1016/S1386-5056(98)00110-5. [DOI] [PubMed] [Google Scholar]
- 5.Sintchenko V, Coiera E, Gilbert G. Decision support systems for antibiotic prescribing. Curr Opin Infect Dis. 2008;21:573–579. doi: 10.1097/QCO.0b013e3283118932. [DOI] [PubMed] [Google Scholar]
- 6.The Evidence-Based Radiology Working Group Evidence-based radiology: a new approach to the practice of radiology. Radiology. 2001;220:566–575. doi: 10.1148/radiol.2203001465. [DOI] [PubMed] [Google Scholar]
- 7.Medina LS, Blackmore CC. Evidence-based radiology: review and dissemination. Radiology. 2007;244:331–336. doi: 10.1148/radiol.2442051766. [DOI] [PubMed] [Google Scholar]
- 8.Greenes RA. Computer-aided diagnostic strategy selection. Radiol Clin North Am. 1986;24:105–120. [PubMed] [Google Scholar]
- 9.Haddawy P, Jacobson J, Kahn CE. BANTER: A Bayesian network tutoring shell. Artif Intell Med. 1997;10:177–200. doi: 10.1016/S0933-3657(96)00374-0. [DOI] [PubMed] [Google Scholar]
- 10.Khorasani R. Role of information technology in improving the quality of care in radiology: an overview. J Am Coll Radiol. 2005;2:1035–1036. doi: 10.1016/j.jacr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Reed MH. Clinical decision rules in radiology. Acad Radiol. 2006;13:562–565. doi: 10.1016/j.acra.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 12.Mendelson RM, Bairstow PJ. Imaging pathways: will they be well trodden or less traveled? J Am Coll Radiol. 2009;6:160–166. doi: 10.1016/j.jacr.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Seltzer SE, Getty DJ, Pickett RM, Swets JA, Sica G, Brown J, Saini S, Mattrey R, Harmon B, Francis IR, Chezmar J, Schnall MO, Siegelman ES, Ballerini R, Bhat S. Multimodality diagnosis of liver tumors: feature analysis with CT, liver-specific and contrast-enhanced MR, and a computer model. Acad Radiol. 2002;9:256–269. doi: 10.1016/S1076-6332(03)80368-9. [DOI] [PubMed] [Google Scholar]
- 14.Burnside ES. Bayesian networks: computer-assisted diagnosis support in radiology. Acad Radiol. 2005;12:422–430. doi: 10.1016/j.acra.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Carrino JA, Ohno-Machado L. Development of radiology prediction models using feature analysis. Acad Radiol. 2005;12:415–421. doi: 10.1016/j.acra.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Wood BP. What’s the evidence? Radiology. 1999;213:635–637. doi: 10.1148/radiology.213.3.r99dc48635. [DOI] [PubMed] [Google Scholar]
- 17.Erickson BJ, Bartholmai B. Computer-aided detection and diagnosis at the start of the third millennium. J Digit Imaging. 2002;15:59–68. doi: 10.1007/s10278-002-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter BA, Shields AF, Olson DO. Magnetic resonance imaging of bone marrow disorders. Radiol Clin North Am. 1986;24:269–289. [PubMed] [Google Scholar]
- 19.Sherry HS, Levy RN, Siffert RS. Metastatic disease of bone in orthopedic surgery. Clin Orthop Relat Res. 1982;169:44–52. [PubMed] [Google Scholar]
- 20.Notelovitz M. Osteoporosis: prevention, diagnosis, and management. 5. West Islip: Professional Communications; 2008. [Google Scholar]
- 21.Riggs BL, Melton LJ., III The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17:505S–511S. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- 22.Langlotz CP. Structured radiology reporting: are we there yet? Radiology. 2009;253:23–25. doi: 10.1148/radiol.2531091088. [DOI] [PubMed] [Google Scholar]
- 23.Radiological Society of North America. RadLex. Available at http://www.radlex.org. Accessed 8 December 2009
- 24.Kahn CE, Langlotz CP, Burnside ES, Carrino JA, Channin DS, Hovsepian DM, Rubin DL. Toward best practices in radiology reporting. Radiology. 2009;252:852–856. doi: 10.1148/radiol.2523081992. [DOI] [PubMed] [Google Scholar]
- 25.Bates DW, Kuperman GJ, Wang S, Gandhi T, Kittler A, Volk L, Spurr C, Khorasani R, Tanasijevic M, Middleton B. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10:523–530. doi: 10.1197/jamia.M1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuenod CA, Laredo J-D, Chevret S, Hamze B, Naouri J-F, Chapaux X, Bondeville J-M, Tubiana J-M. Acute vertebral collapse due to osteoporosis or malignancy: appearance on unenhanced and gadolinium-enhanced MR images. Radiology. 1996;199:541–549. doi: 10.1148/radiology.199.2.8668809. [DOI] [PubMed] [Google Scholar]
- 27.Uetani M, Hashmi R, Hayashi K. Malignant and benign compression fractures: differentiation and diagnostic pitfalls on MRI. Clin Radiol. 2004;59:124–131. doi: 10.1016/j.crad.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Sim I, Gorman P, Greenes RA, Haynes RB, Kaplan B, Lehmann H, Tang PC. Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc. 2001;8:527–534. doi: 10.1136/jamia.2001.0080527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swett HA, Fisher PR, Cohn AI, Miller PL, Mutalik PG. Expert system-controlled image display. Radiology. 1989;172:487–493. doi: 10.1148/radiology.172.2.2664871. [DOI] [PubMed] [Google Scholar]
- 30.Khorasani R. Clinical decision support in radiology: what is it, why do we need it, and what key features make it effective? J Am Coll Radiol. 2006;3:142–143. doi: 10.1016/j.jacr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Shortliffe EH. Computer programs to support clinical decision making. JAMA. 1987;258:61–66. doi: 10.1001/jama.258.1.61. [DOI] [PubMed] [Google Scholar]
- 32.Lurie JD, Tosteson AN, Tosteson TD, et al. Reliability of readings of magnetic resonance imaging features of lumbar spinal stenosis. Spine. 2008;33:1605–1610. doi: 10.1097/BRS.0b013e3181791af3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrino JA, Lurie JD, Tosteson AN, et al. Lumbar spine: reliability of MR imaging findings. Radiology. 2009;250:161–170. doi: 10.1148/radiol.2493071999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang KC, Filice RW, Eng J. DexNote: a learner-centric tool for radiology knowledge tracking. Am J Roentgenol. 2009;193:W118–W121. doi: 10.2214/AJR.08.1451. [DOI] [PubMed] [Google Scholar]