Abstract
A variety of conditions other than acute myocardial infarction may cause ST-elevation. Our objective was to evaluate the impact of cardiac magnetic resonance (CMR) on differential diagnosis from a prospective series of patients with suspected ST-elevation myocardial infarction (STEMI) and completely normal coronary arteries. Among 1,145 patients with suspected STEMI, 49 patients had completely normal coronary arteries and entered a prospective registry. CMR was done within 24 h, if possible, and included function analyses, T2-weighted imaging (T2 ratio), T1-weighted imaging before and after gadolineum administration (global relative enhancement; gRE), and late gadolineum enhancement (LGE). All patients were asked for a follow-up CMR after approximately 3 months. The incidence of patients with suspected STEMI and normal coronary arteries was 4.3% and mean age was 45 ± 14 years (STEMI group 64 ± 13 years; P < 0.001). 55% had a recent history of infection. Cardiac biomarkers showed a moderate elevation on admission. There was a significant change from baseline to follow-up for LV end-diastolic volumes (EDV) (P < 0.001), LV mass (P < 0.05), mean T2 ratio (P < 0.05), and LGE volume (P < 0.05). Major diagnostic groups were myocarditis (29%), pericarditis (27%), and takotsubo cardiomyopathy (10%). 18% were regarded as non-diagnostic. The study showed an incidence of 4.3% of patients with suspected STEMI and completely normal coronary arteries. Early CMR was valuable in the evaluation of the differential diagnoses and to exclude myocardial abnormalities in patients with uncertain aetiology. Further studies are needed for the assessment of long-term outcome.
Keywords: Acute myocardial infarction, Normal coronary arteries, Cardiac magnetic resonance, Differential diagnosis
Introduction
Standard 12-lead electrocardiogram (ECG) is a key diagnostic tool to direct patients with suspected acute myocardial infarction to the angiography suite [1]. However, a variety of other serious conditions aside from acute myocardial infarction may also cause ST-elevation, including pericarditis, myocarditis, cardiomyopathy, cardiac contusion, congestive heart failure, and non-cardiac causes including pulmonary embolism, septicaemia, renal failure, and aortic dissection [2, 3]. Various series of such patients have been described, but the aetiology and pathogenesis of the condition is still a source of debate. Also, this cohort of patients has been shown to have a poor prognosis [4]. The combination of ECG and biomarkers has been used to determine the extent and location of myocardial injury; however, current diagnostic approaches may have limitations.
In order to establish the underlying cause for the ECG abnormality, CMR offers a potential opportunity due to its ability to identify myocardial oedema and inflammation, as well as scarring associated with myocardial infarction [5]. Due to the high spatial resolution and introduction of gadolinium contrast, CMR is being used for a growing number of clinical applications. A multisequential approach has been shown to be promising for the detection of non-ischemic conditions, such as myocarditis [6]. Thus, we aimed to evaluate the impact of CMR on differential diagnosis from a prospective series of patients with suspected STEMI and completely normal coronary arteries using a combination of available sequences [7].
Materials and methods
Our hospital is a tertiary care institution, which for the last 3 years approximately have admitted 800 STEMI patients per year. All patients 18–75 years old with chest pain <24 h, significant ST-elevation, new left bundle-branch block, and completely normal coronary arteries at angiography were included. Patients with visible lumen irregularities, spasm, or thrombus on standard coronary angiography, and/or prior percutaneous coronary intervention (PCI), were excluded (n = 2). However, provocative tests for the exclusion of coronary spasm were not performed. Included patients entered into a prospective registry that included a repeat 12-lead ECG, history, clinical examination, blood tests, chest x-ray, echocardiography, and CMR. ST-segment elevation was defined as J-point elevation >1 mm in leads II, III, aVF, I, aVL, and/or >2 mm in leads V1-6, with a total of minimum 2 mm in the inferior leads, and/or 4 mm in the anterior leads. History of angina was recorded according to the CCS classification. The blood tests included troponin-T, creatine kinase-myocardial band (CK-MB), probrain natriuretic peptide (proBNP), and C-reactive proteine (CRP). The CMR was performed as soon as the clinical situation allowed it, if possible within 24 h. Further investigations, such as computer tomography (CT), were done when needed to establish a diagnosis. All patients were asked for a follow-up, which included a repeat ECG, biomarkers, and CMR.
Patients were excluded from CMR if they had contraindications for CMR at study entry such as implanted pacemakers (n = 0) or severe claustrophobia (n = 3), or contrast media associated contraindications like renal insufficiency (n = 0). All patients signed an informed consent to enter the study. The study was approved by the Regional Ethics Committee and Review Board of our institution.
CMR imaging protocol
The CMR studies were performed by using a 1.5-Tesla unit (Intera CV, Philips Medical Systems, Best, The Netherlands). A standard five-channel phased-array cardiac coil was used, and a signal correction shimming technique was used to compensate for possible inhomogeneity of the field. If repositioning of the patient was needed, the entire sequence was repeated. For functional analysis ECG-gated steady state free precession (SSFP) cine images were acquired in the two-chamber and four-chamber views, as well as sequential 10 mm short-axis slices from the atrio-ventricular ring to the apex (TE 1.9 ms, TR 3.8 ms, flip angle 60°). T2 weighted breath-hold black-blood triple inversion images were acquired in the same short axis plane (TE 80 ms, TR 2 × R-R interval, TI 180 ms, slice thickness 10 mm, flip angle 180°, pixel size 0.96 × 0.96 mm). After an i.v. bolus of 0.15 mmol/kg gadolineum-diethylenetriaminepentaacetate (Gd-DTPA, Magnevist, Schering, Germany), a short axis T1 weighted gradient echo inversion-recovery (IR) early (global) relative enhancement sequence (gRE) (TR 1 × R-R interval, TE 20 ms, slice thickness 10 mm) was performed. Measurements started immediately after injection of Gd-DTPA (automated injector, Medrad, Indianola, USA) and lasted for 3–4 min (without any change of parameters in between). Finally, late gadolineum enhancement (LGE) images in the short and long axis views were acquired after 15 min, using a similar gradient echo IR sequence. Inversion times were adjusted to null normal myocardium using the Look-Locker sequence.
CMR analysis
Off-line analyses were performed on a dedicated workstation (ViewForum release 5.2, Philips Medical Systems, Best, The Netherlands). Ventricular volumes and function were measured for both ventricles using standard techniques. The endocardial and epicardial borders were drawn manually on each dynamic image. The LV ejection fraction (EF), end diastolic volume (EDV), systolic wall thickening (SWT), and myocardial mass were calculated from the short axis (SA) views. The right ventricle (RV) EF was calculated from the four-chamber view. Two blinded experienced readers (KHS, PH; both 14 years of experience) examined the T2, gRE, and LGE images, and differences in interpretation were resolved in an additional consensus reading.
Visualization of myocardial oedema was performed using the T2-weighted sequence by measuring the ratio of global myocardial signal intensity to skeletal muscle. Depending on the homogeneity, the skeletal muscle signal intensity (SI) was drawn on the same slice. The T2-ratio was calculated as SI myocardium/SI skeletal muscle, and according to our own validation a T2 ratio of 2.0 was used as cutoff value. The early gadolineum enhancement sequence was measured in a similar way, and regions of interest were copied from the pre-contrast to the post-contrast images. The gRE was calculated as previously described by Friedrich [8], and a cutoff value of 4 or higher was used. The LGE sequence was analyzed segment by segment (17 segments), and the total volume of enhancement was calculated (>2 standard deviations (SD) compared to a normal or non-enhancing segment) [9]. The LGE was described as either epimyocardial or centromyocardial, a location associated with myocarditis. The CMR was also analysed for the presence of concomitant pleural and pericardial effusion, and/or enhancement of the pericardial layers.
Control group
For validation of CMR sequences and determination of imaging specific cutoff values, 13 healthy volunteers (8 males, 5 females, 39 ± 12 years) with no current or past evidence of cardiovascular disorders underwent the CMR protocol. We also used the validation process to secure sufficient homogeneity with the use of the surface coil and signal intensity correction software.
Statistical analysis
Each categorical variable is expressed as number and percentage of patients. Demographical and clinical data are presented as mean with SD or proportions. Baseline characteristics were compared between subgroups using an independent sample t-test or Mann–Whitney test as appropriate. A Fischer’s exact or chi-square test for contingency tables was obtained to detect associations between categorical independent variables. When comparing continuous variables, a two-sided Student’s t test was used. P values less than 0.05 were considered statistically significant, and P values less than 0.001 highly significant. All statistical analysis was performed using GraphPad Prism for Macintosh, version 5.0 (GraphPad Software, La Jolla, CA, USA).
Results
During an 18 months period in 2007 and 2008, 1,145 patients were referred to our hospital with suspected STEMI. Without testing for coronary spasm, 49 patients had normal coronary arteries (4.3%) and all were willing to participate in the study. The baseline characteristics are summarized in Table 1. The mean age of the cohort was 45 ± 14 years, while the mean age in the STEMI group in the same period was 64 ± 13 years (P < 0.001). 12% of the patients were females, compared to 25% (n = 288) in the STEMI group (P < 0.001). The median time from coronary angiography to CMR was 20 ± 14 h. Follow-up was done in 86% of the patients after 100 ± 20 days and finished by the end of 2009. There was a low prevalence of cardiac risk factors, and only two patients had diabetes mellitus and three patients a prior history of angina (CCS class I–II). 19 (38.8%) patients were regular smokers and 12 (24.5%) patients had hypertension, in addition two patients were regular users of cannabis. 27 (55%) patients had a recent history of infections; most common was respiratory tract infections or flu-like symptoms, either as a direct viral infection or a post-viral immune-mediated reaction.
Table 1.
Baseline characteristics
| Characteristics | Value (n = 49) |
|---|---|
| Mean age, years | 45.0 ± 14.0 |
| Male sex (%) | 45 (91.8) |
| Family history of CVD (%) | 20 (40.8) |
| Angina (%) | 3 (6.1) |
| Diabetes (%) | 2 (4.1) |
| Hypertension (%) | 12 (24.5) |
| Smoking (%) | 19 (38.8) |
| BMI > 30 (%) | 3 (6.1) |
| Drug abuse (%) | 2 (4.1) |
| Recent infection | |
| Respiratory tract (%) | 14 (28.6) |
| Flu-like symptoms (%) | 10 (20.4) |
| Other (%) | 3 (6.1) |
| Electrocardiographic changes | |
| Anterior ST-elevation (%) | 23 (46.9) |
| Anterior and inferior ST-elevation (%) | 24 (49.0) |
| T-inversion (%) | 6 (12.2) |
| Mean anterior ST-elevation, mm | 6.3 ± 3.7 |
| Mean inferior ST-elevation, mm | 1.8 ± 2.5 |
| Biomarkers | |
| CK-MB (mg/1) | 22.5 ± 45.8 |
| Troponin-T (μg/1) | 0.48 ± 0.87 |
| proBNP (pmol/1) | 106.5 ± 272.9 |
| CRP (mg/1) | 53.4 ± 77.5 |
| Echocardiography (n = 43) | |
| Mean EF (%) | 57.5 ± 7.5% |
| EF < 50%, n (%) | 5 (11.6%) |
| Medication on admission | |
| ACE-1/AT-II blocker, n (%) | 7 (14.3) |
| Beta-blocker, n (%) | 5 (10.2) |
| Statins, n (%) | 9 (18.4) |
| None, n (%) | 28 (57.1) |
ECG on admission showed anterior ST-elevation in 47% of the patients, and combined anterior and inferior ST-elevation in 49% of the patients. No patients presented with inferior ST-elevation alone. ST-depression was seen in 10% of the patients, and T-inversions in 12%. All patients had QT and PQ times within normal range. All ECG changes normalized at follow-up, except from persistent T-inversions in 5% of the patients. Both cardiac biomarkers and CRP showed moderate elevation, however, all biomarkers normalized at follow-up (P < 0.001). 43 (88%) patients had an echocardiography done, and mean EF on admission was 57.5%. No patients required electrical cardioversion, and none had pre-existing renal impairment. Nine patients were on primary prevention therapy, with statins being the most common treatment.
Segmental analysis of the T2 and gRE sequences in volunteers showed <5% signal differentiation, we therefore decided to use the surface coil with signal correction software. CMR results are shown in Table 2. There was a significant change from baseline to follow-up for LV end-diastolic volumes (EDV) (156.2 ± 28.1 ml vs. 137.1 ± 25.4; P < 0.001), LV mass (131.2 g ± 26.2 vs. 117.4 ± 27.0; P < 0.05), mean T2 ratio (1.86 ± 0.26 vs. 1.71 ± 0.28; P < 0.05), and LGE volume (12.2 ± 11.4 g vs. 5.9 ± 19.9; P < 0.05). LGE was seen most frequently in segments 4, 5, 6, 11, 12, and 16. No significant change was associated with LV EF, LV SWT, RV EF, or gRE. Mean T2 ratio was also significantly higher at baseline versus the control group (P < 0.05). Pleural effusion (n = 4) and pericardial effusion (n = 5) seen on admission were normalized in all patients at follow-up. Mitral valve regurgitations (n = 2) and tricuspid valve regurgitation in one patient with reduced RV function on admission were also normalized at follow-up.
Table 2.
CMR findings at baseline and follow-up
| Baseline | Follow-up | P-value | |
|---|---|---|---|
| LV EF (%) | 58.2 ± 10.8 | 61.0 ± 7.9 | NS |
| LV EDV (ml) | 156.2 ± 28.1 | 137.1 ± 25.4 | <0.001 |
| LV SWT (%) | 73 ± 32 | 79 ± 27 | NS |
| LV mass (g) | 131.2 ± 26.2 | 117.4 ± 27.0 | <0.05 |
| RV EF (%) | 63.3 ± 9.6 | 64.4 ± 10.0 | NS |
| Elevated T2 ratio, n (%) | 17 (39.5) | 5 (13.5) | <0.001 |
| Mean T2 ratio | 1.86 ± 0.26* | 1.71 ± 0.28 | <0.05 |
| Elevated gRE, n (%) | 5 (11.6) | 4 (10.8) | |
| Mean gRE | 3.05 ± 1.25 | 2.79 ± 0.88 | NS |
| LGE, n (%) | 14 (32.6) | 5 (13.5) | <0.001 |
| Mean LGE volume (g) | 12.2 ± 11.4 | 5.9 ± 19.9 | <0.05 |
| Pleural effusion, n (%) | 4 (9.3) | 0 | <0.001 |
| Pericardial effusion, n (%) | 5 (11.6) | 0 | <0.001 |
* P < 0.05 vs. the control group
Differential diagnosis
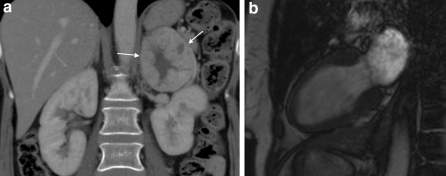
The patients were grouped according to their final diagnosis, using an integrated approach of clinical findings, ECG, biomarkers and CMR. Based on these findings, all patients were classified into 7 groups, which are listed in Table 3. 14 patients (29%) were classified as myocarditis (Fig. 1a–d). In addition to acute chest pain and ECG changes, these patients also had elevation of troponin-T and/or CK-MB, and “2 out of 3” abnormal sequences on CMR (T2, gRE, LGE) [9]. 5 patients had persistent LGE at follow-up. 13 patients (27%) were classified as pericarditis (Fig. 2a–d) [10]. In addition to chest pain (pericarditic), these patients also showed widespread (upward concave) ST-segment elevation, 85% showed elevation of CRP, and 39% had pericardial effusion, however, no patients in this subgroup showed elevation of cardiac biomarkers during the initial hospital stay. One patient showed additional hyperintense signal from the pericardium on T2-weighted images and hyperenhancement on the LGE images at follow-up. 5 patients (10%) were classified as Takotsubo cardiomyopathy (TTC) (Fig. 3a–d), also called stress cardiomyopathy or ventricular ballooning syndrome, and the diagnosis was based on the modified Mayo Clinic criteria [11]. No abnormal signal activity was documented on the LGE sequence. TTC was confirmed when there was a complete normalization of LV EF at follow-up. 2 patients (4%) with increased end-diastolic volumes and reduced systolic function, and without other CMR abnormalities, were categorized as dilated cardiomyopathy (DCM) (Fig. 4a–d) [12]. Nine patients (18%) with normal range volumes, no CMR abnormalities, normal clinical findings, and normal biomarkers both at discharge and follow-up, were considered non-diagnostic. However, 5 (10%) patients with a moderate troponin-T elevation (0.38 ± 0.32) as the only finding, and one patient with a phaeochromocythoma (Fig. 5a, b), were classified in separate groups. No patients showed any signs of myocardial infarction at CMR, neither at baseline nor at follow-up.
Table 3.
Differential diagnosis
| Diagnosis | n = 49 | Mean age (SD), years |
|---|---|---|
| Myocarditis (%) | 14 (28.6) | 36.5 ± 13.9 |
| Pericarditis | 13 (26.5) | 45.5 ± 14.7 |
| Takotsubo cardiomyopathy | 5 (10.2) | 63.5 ± 4.6 |
| DCM | 2 (4.1) | 36.6 ± 9.7 |
| Phaeochromocythoma | 1 (2.0) | 51.3 |
| Raised troponin | 5 (10.2) | 39.6 ± 16.2 |
| Non diagnostic | 9 (18.4) | 43.2 ± 8.7 |
| STEMI, same period | 64.0 ± 13.1* |
* P < 0.001 vs. the study group
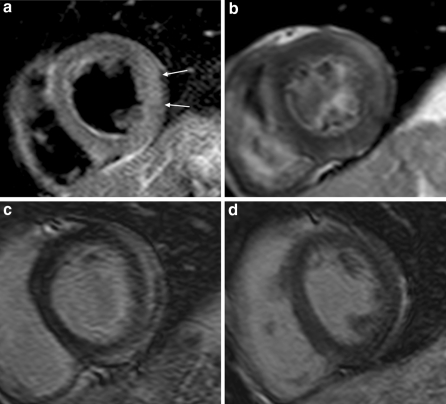
Fig. 1.
Short axis views of patient with myocarditis in the lateral wall. Images show oedema on the T2-weighted sequence (white arrows) (a), increased gRE (b) and epicardial LGE (c). Follow-up shows normalization of LGE (d)
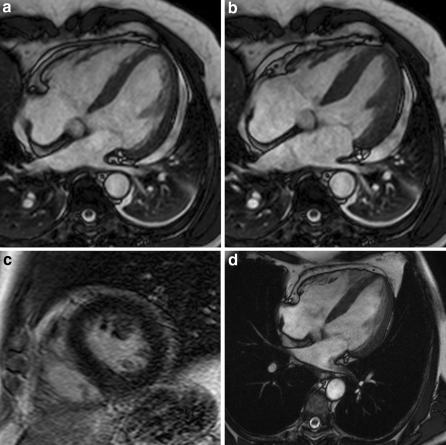
Fig. 2.
4-chamber views of patient with pericarditis. Functional images demonstrate normal ventricular function (a end-diastolic, b end-systolic) with additional pleural- and pericardial effusion, and a normal LGE sequence (c). Follow-up shows unchanged normal ventricular function with no effusion (d end-diastole)
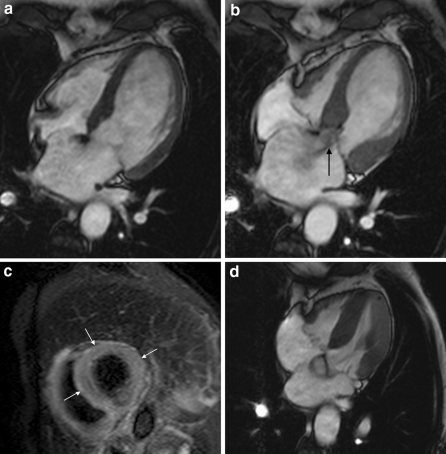
Fig. 3.
4-chamber views of patient with Takotsubo cardiomyopathy. Functional images demonstrate apical ballooning (a end-diastole, b end-systole), and mitral valve insufficiency (black arrow), in addition to apical myocardial oedema on the T2-weighted sequence (increased T2 ratio) (white arrows) (c). Follow-up shows complete normalization of ventricular dysfunction (d end-systole)
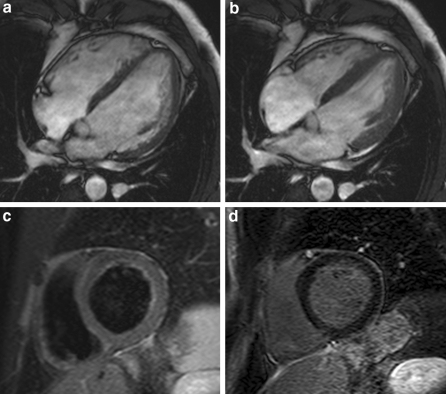
Fig. 4.
4-chamber views of patient with DCM. Functional images demonstrate increased LVEDV and reduced EF (a end-diastole, b end-systole), in addition to a normal T2-weighted (c) and LGE sequence (d)
Fig. 5.
Patient with phaeochromocytoma. Coronal CT image demonstrate tumor of the left adrenal gland (a) (white arrows). Functional images (long axis view) demonstrate apical ballooning (b end-systole) in the same patient
Some significant differences were seen between the groups. Compared to the control group, at baseline the LVEF was significantly lower in the myocarditis (P < 0.05) and the TTC groups (P < 0.05), the T2 ratio was higher in the myocarditis (P < 0.001) and TTC groups (P < 0.05), and the SWT was lower in the TTC group (P < 0.05). There were no significant differences regarding RV EF, LV EDV, LV mass, or gRE compared to the control group. Compared to reference values, CK-MB, troponin T, and proBNP were elevated in the myocarditis group (P < 0.05), and Troponin T was elevated in the TTC group (P < 0.05). CRP was elevated in the pericarditis (P < 0.05) and myocarditis groups (P < 0.05).
Other clinical investigations
Depending on the clinical presentation, biomarkers, and CMR, some patients were additionally investigated. All patients with persistent LGE at follow-up had an additional CMR approximately 12 months after the acute event that showed a complete normalization in all, except a mild lateral myocardial scarring in one patient. CT pulmonary angiography was performed in five patients with elevated D-dimer, but did not show evidence of embolism. One patient had an abdominal CT performed, which confirmed the suspicion of an adrenal tumor, and the patient was successfully operated for a pheochromocytoma [13]. Another patient had a thoracic CT performed and a right side pulmonary tumor was identified, confirming the suspicion from CMR. No patients had a suspicion of aortic dissection from the CMR examinations.
Discussion
Twelve-lead ECG and cardiac biomarkers are basic tools in the diagnosis of acute myocardial infarction in patients with chest pain. However, patients with suspected STEMI and completely normal coronary angiography present a clinical dilemma. Further evaluation might be required to establish a diagnosis and to direct treatment strategies. CMR has become a valuable tool with the introduction of new sequences with high spatial resolution, and therefore has a potential role in the evaluation of aetiologies in patients with suspected STEMI and completely normal coronary arteries.
This study revealed an incidence of patients with suspected STEMI and normal coronary arteries of 4.3%. Previous reports have shown a prevalence of 2.6–12% [14–16]. The study may indicate that these patients often are younger men, who are less likely to have traditional risk factors for atherosclerosis, than are patients with documented coronary disease. Also, these patients may have frequent viral infections and stress symptoms (emotional and physical) prior to admission. Hypertension was documented in 25% and smoking in 53% of the patients. It is feasible that smoking may have a contributory role in the pathogenesis of this patient cohort, possibly via endothelial dysfunction and coronary artery spasm [17].
The study demonstrated that CMR, in addition to clinical findings and biomarkers, provided information in a high percentage of our patients with suspected STEMI and normal coronary arteries. The most common cause was myocarditis (29%), followed by pericarditis (27%), and takotsubo cardiomyopathy (10%). This finding is in keeping with other studies [18–20], although most other studies are retrospective. CMR was able to detect acute myocardial oedema in 40% of the patients, and the normalization of initially increased T2-ratios corresponded with a reduction of LV mass. In myocarditis the myocardial injury often proceeds from a focal to a global pattern [8], and the increase of myocardial oedema is rapid and transient [21]. This is one of the reasons why we performed a multisequential CMR approach early after admission. The early enhancement sequence (gRE), or the capillary leak phase, was not of diagnostic importance in a majority of the patients. However, serial studies demonstrate that gRE needs a longer time delay after the onset of symptoms to become positive in myocarditis patients [8], this is also shown in TTC patients [22]. Also importantly, significant infarction or fibrosis was excluded in a majority of our patients.
In the subset with myocarditis, 93% showed evidence of acute myocardial oedema as indicated by T2 imaging, and epicardial or centromyocardial LGE was seen in all of the patients. The location of the LGE allowed to distinguishing them from myocardial infarction, which is important because of different treatment strategies. Also, myocardial involvement as demonstrated by T2 imaging and LGE in addition to elevated cardiac biomarkers, allowed differentiating myocarditis from pericarditis, which is a challenge in clinical practice because they share common aetiological agents. Previous work has demonstrated that areas of LGE correlate well with biopsy findings when the site of tissue sampling is guided by the CMR findings [23]. Moreover, studies in such patients demonstrate regression of these pathological changes over time in a proportion of patients [8]. In the present study, 86% of the myocarditis patients showed complete resolution of the myocardial oedema after a period of 106 days and 36% showed persistent LGE, however, a repeat scanning after 350 days showed scarring in only one patient. These findings may underline the importance of both early and late CMR in myocarditis patients.
Comparable data on the prevalence of pericarditis is limited. The high percentage of pericarditis patients within our patient cohort might be explained by the short time delay between the onset of symptoms and CMR. Previous reports that have documented a longer time delay have shown a higher prevalence of patients with myocardial involvement [19]. The characterizing of TTC demonstrates the additional role of CMR in a disease entity that is being increasingly recognized. The therapy strategies are different from what might have been the result based on ECG and angiography alone. All patients in this group showed significant myocardial oedema as indicated by T2 imaging. The acute reduction of LVEF and moderately elevated cardiac biomarkers has been shown in several reports [22, 24]. Two patients had dilated cardiomyopathy, which may present with different ECG changes [19]. Five patients had a moderate troponin elevation as the only finding and were classified in a separate group. One patient had a tachyarrhythmia at presentation, which possibly resulted in a raised troponin. Another patient had a single elevated troponin test that was not repeated in 24 h, and a “false positive” test was considered a possibility [19].
28% of the patients had no final diagnosis at discharge or at follow-up. One possible explanation is that CMRs limited resolution might not be high enough to detect minor changes in myocardial oedema and inflammation. Microinfarctions and diffuse myocardial necrosis might be overseen for the same reason. Other explanations may require other imaging techniques, such as intravascular ultrasound. Bounhoure et al. [25] recently showed that many “normal” angiograms revealed atherosclerotic plaques, other mechanisms were coronary spasm and myocardial bridge. Among other possible causes for ST-elevation and normal coronary arteries that were not identified in this study were aortic dissection, pulmonary embolism, and syndrome X. However, it is our experience that these diagnoses have to be assessed in daily routines. We have recently shown that both aortic dissection [26] and syndrome X [27] might be represented by various ECG changes including ST-elevation.
Limitations
A limitation of the current analysis is the relatively small number of patients, although our study is large compared to previous prospective reports. The lack of endomyocardial biopsy (EMB) is a certain limitation of our study. However, the sensitivity of EMB is limited [23], and EMB may have severe complications. Also, the majority of our patients were young with an excellent prognosis, and we avoided unnecessary risks for the patients. There is a theoretical possibility that some patients suffered from undetected coronary heart disease, such as a resolved thrombus, but the lack of scarring by CMR makes an ischemic injury unlikely. Also, there is a possibility that some patients had undetected coronary spasm due to the lack of provocative tests. Ong showed that in patients with acute coronary syndromes and unobstructed coronary arteries, coronary spasm was verified by acetylcholine provocation in nearly 50% of the patients [28].
Conclusion
In conclusion, this prospective study revealed an incidence of 4.3% of patients with suspected STEMI but completely normal coronary arteries. Early CMR was valuable in the evaluation of the major diagnoses and to exclude myocardial abnormalities in patients with uncertain aetiology. Therefore, CMR is able to facilitate guidance of medical therapy in many of these patients; however, further studies are needed for the assessment of long-term outcome.
Acknowledgments
The authors are grateful for the excellent assistance of Vigdis Rosseland, Marianne Nesheim, and Grethe Hansen.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. New England Journal of Medicine. 2003;348:933–940. doi: 10.1056/NEJMra022700. [DOI] [PubMed] [Google Scholar]
- 2.Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. New England Journal of Medicine. 2003;349:2128–2135. doi: 10.1056/NEJMra022580. [DOI] [PubMed] [Google Scholar]
- 3.Fossum E, Nils-Einar K, Mangschau A (2004) Conditions associated with ST-segment elevation. N Engl J Med 350:1152–1155; author reply 1152–5 [PubMed]
- 4.Dokainish H, Pillai M, Murphy SA, DiBattiste PM, Schweiger MJ, Lotfi A, Morrow DA, Cannon CP, Braunwald E, Lakkis N. Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS-TIMI-18 substudy. Journal of the American College of Cardiology. 2005;45:19–24. doi: 10.1016/j.jacc.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, Gross M, Dietz R, Friedrich MG. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 6.Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, Noutsias M, Schultheiss HP, Kuhl U. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246:401–409. doi: 10.1148/radiol.2461062179. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. Journal of the American College of Cardiology. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802–1809. doi: 10.1161/01.cir.97.18.1802. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. Journal of the American College of Cardiology. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lange RA, Hillis LD. Clinical practice. Acute pericarditis. New England Journal of Medicine. 2004;351:2195–2202. doi: 10.1056/NEJMcp041997. [DOI] [PubMed] [Google Scholar]
- 11.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. American Heart Journal. 2008;155:408–417. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Ordovas KG, Reddy GP, Higgins CB. MRI in nonischemic acquired heart disease. Journal of Magnetic Resonance Imaging. 2008;27:1195–1213. doi: 10.1002/jmri.21172. [DOI] [PubMed] [Google Scholar]
- 13.Gronvold T, Evang JA, Stensaeth KH. A 51-year-old woman with acute chest pain. Tidsskrift for den Norske Laegeforening. 2008;128:2836–2838. [PubMed] [Google Scholar]
- 14.Kang WY, Jeong MH, Ahn YK, Kim JH, Chae SC, Kim YJ, Hur SH, Seong IW, Hong TJ, Choi DH, Cho MC, Kim CJ, Seung KB, Chung WS, Jang YS, Rha SW, Bae JH, Cho JG, Park SJ (2009) Are patients with angiographically near-normal coronary arteries who present as acute myocardial infarction actually safe? Int J Cardiol. doi: 10.1016/j.ijcard.2009.07.001 [DOI] [PubMed]
- 15.Widimsky P, Stellova B, Groch L, Aschermann M, Branny M, Zelizko M, Stasek J, Formanek P. Prevalence of normal coronary angiography in the acute phase of suspected ST-elevation myocardial infarction: experience from the PRAGUE studies. Canadian Journal of Cardiology. 2006;22:1147–1152. doi: 10.1016/s0828-282x(06)70952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen AI, Galbraith PD, Ghali WA, Norris CM, Graham MM, Knudtson ML. Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. American Journal of Cardiology. 2005;95:261–263. doi: 10.1016/j.amjcard.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Gehani AA, al-Mulla AW, Chaikhouni A, Ammar AS, Mahrous F, Tirkawi R, Ashraf A, Hajar HA. Myocardial infarction with normal coronary angiography compared with severe coronary artery disease without myocardial infarction: the crucial role of smoking. Journal of Cardiovascular Risk. 2001;8:1–8. doi: 10.1097/00043798-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Prasad SB, Richards DA, Sadick N, Ong AT, Kovoor P. Clinical and electrocardiographic correlates of normal coronary angiography in patients referred for primary percutaneous coronary intervention. American Journal of Cardiology. 2008;102:155–159. doi: 10.1016/j.amjcard.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Assomull RG, Lyne JC, Keenan N, Gulati A, Bunce NH, Davies SW, Pennell DJ, Prasad SK. The role of cardiovascular magnetic resonance in patients presenting with chest pain, raised troponin, and unobstructed coronary arteries. European Heart Journal. 2007;28:1242–1249. doi: 10.1093/eurheartj/ehm113. [DOI] [PubMed] [Google Scholar]
- 20.Larson DM, Menssen KM, Sharkey SW, Duval S, Schwartz RS, Harris J, Meland JT, Unger BT, Henry TD. “False-positive” cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA. 2007;298:2754–2760. doi: 10.1001/jama.298.23.2754. [DOI] [PubMed] [Google Scholar]
- 21.Zagrosek A, Wassmuth R, Abdel-Aty H, Rudolph A, Dietz R, Schulz-Menger J. Relation between myocardial edema and myocardial mass during the acute and convalescent phase of myocarditis—a CMR study. Journal of Cardiovascular Magnetic Resonance. 2008;10:19. doi: 10.1186/1532-429X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eitel I, Lucke C, Grothoff M, Sareban M, Schuler G, Thiele H, Gutberlet M. Inflammation in takotsubo cardiomyopathy: insights from cardiovascular magnetic resonance imaging. European Radiology. 2010;20:422–431. doi: 10.1007/s00330-009-1549-5. [DOI] [PubMed] [Google Scholar]
- 23.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 24.Koeth O, Mark B, Kilkowski A, Layer G, Cornelius B, Kouraki K, Bauer T, Zahn R, Senges J, Zeymer U. Clinical, angiographic and cardiovascular magnetic resonance findings in consecutive patients with Takotsubo cardiomyopathy. Clin Res Cardiol. 2008;97:623–627. doi: 10.1007/s00392-008-0661-x. [DOI] [PubMed] [Google Scholar]
- 25.Bounhoure JP, Ouldzen H, Carrie D, Alibelli MJ, Puel J (2007) Myocardial infarction with “angiographycally normal coronary arteries” myth or reality? Bull Acad Natl Med 191:815–824; discussion 824–5 [PubMed]
- 26.Fossum E, Ata B, Eritsland J, Klow NE, Mangschau A. Aortic dissection—a differential diagnosis in patients with chest pain and ECG changes. Tidsskrift for den Norske Laegeforening. 2003;123:2430–2432. [PubMed] [Google Scholar]
- 27.Bohmer E, Seljeflot I, Arnesen H, Hoffmann P, Abdelnoor M, Halvorsen S. The association between metabolic syndrome and infarct size in patients with acute myocardial infarction. Scandinavian Journal of Clinical and Laboratory Investigation. 2010;70:287–293. doi: 10.3109/00365513.2010.481819. [DOI] [PubMed] [Google Scholar]
- 28.Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M, Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: the CASPAR (Coronary Artery Spasm in Patients with Acute Coronary Syndrome) study. Journal of the American College of Cardiology. 2008;52:523–527. doi: 10.1016/j.jacc.2008.04.050. [DOI] [PubMed] [Google Scholar]