To the Editor
Surgical implantation of electrodes into deep brain structures for the management of conditions such as chronic pain provides an opportunity for assessment of the physiological impact of stimulation of focal brain nuclei in humans (1). Recent studies in humans have suggested that stimulation of the periventricular (PVG)/periaqueductal grey (PAG) regions can result in changes in systemic blood pressure (BP) (2, 3). In the present case, we examined the physiological impact of such stimulation in humans by obtaining continuous measures of limb blood flow, peripheral resistance, stroke volume and heart rate (HR) during deep brain stimulation (DBS) of the PVG/PAG. The patient, a 55 year old woman, was referred for management of recalcitrant chronic neuropathic, phantom limb pain (affecting both lower limbs) in 2004, subsequently leading to PVG/PAG DBS surgery in 2005 at the Radcliffe Infirmary, Oxford.
Assessments and procedures were approved by the Oxford Ethics Committee and performed in a quiet thermostatically controlled room (26°C). The patient was placed in a semi-recumbent position at which point the stimulator was turned off for an initial 20 min rest period. Following a baseline BP assessment using a Finometer PRO (Finapres Medical Systems, Netherlands), the stimulator was turned on and BP, stroke volume, total peripheral resistance and brachial artery diameter and blood flow velocity (Terason T3000, Teratech Corporation, Burlington, MA) were recorded for five minutes of this ‘on’ period. Activation of the two proximal contacts in single bipolar arrangement on the model 3389 quadripolar lead (Medtronic, MN) was set to a frequency of 40 Hz with a pulse width of 450 μs and amplitude of 3.1 V.
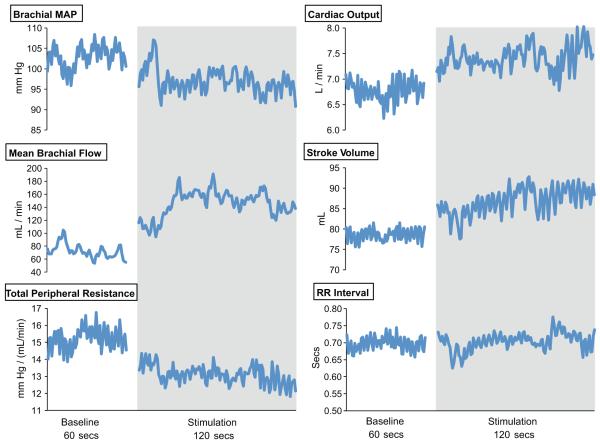
The patient’s mean brachial BP increased transiently when the stimulator was turned on, but then persistently decreased below resting values (103 ± 2 mmHg to 96 ± 2 mmHg; see Figure 1). This decrease in BP was associated with a contemporaneous increase in brachial artery blood flow (72 ± 11 to 148 ± 12 ml/min) and decrease in total peripheral resistance (15.2 ± 0.6 to 12.9 ± 0.5 mm Hg.mL−1min−1). Stroke volume increased (79 ± 2 to 89 ± 3 ml) along with cardiac output (6.7 ± 0.2 to 7.5 ± 0.3 L/min), whilst RR intervals (and HR) remained relatively stable throughout the rest and stimulation periods (0.70 ± 0.02 vs 0.71 ± 0.02 secs).
Figure 1.
Brachial blood flow, mean brachial arterial pressure, heart rate, cardiac output, stroke volume and total peripheral resistance before and during stimulation.
Although this report is consistent with previous studies describing changes in BP in awake humans during PVG/PAG stimulation (2, 3), it is the first to our knowledge to report changes in vascular tone and peripheral resistance in response to DBS in humans. We observed an initial brief spike in BP, possibly relating to transient facial pain lasting 5 secs when the stimulator was activated. Once the pain resolved, BP dropped rapidly and consistently, accompanied by a decrease in total peripheral resistance. Blood flow increased through the brachial artery whilst brachial diameter remained relatively constant, suggesting that changes in the vasomotor tone of resistance vessels downstream were responsible for the changes in flow and pressure.
We interpret the data from this subject as being consistent with a primary impact of DBS on vascular tone, as both BP and blood flow data rapidly and simultaneously changed following stimulation. Blood pressure is, of course, a highly regulated variable and any decrease would be expected to induce reflex homeostatic responses. In this context, we might have expected some compensatory increase in cardiac output and/or peripheral resistance as stimulation continued, with emphasis on the cardiac change if the vasculature is indeed primarily modulated by stimulation. The persistent impact on both total peripheral resistance and blood flow throughout stimulation, in contrast to the gradual rise in cardiac output across this period, argues for a primary role of DBS upon the vasculature. Baroreflex activation might have been expected to modify HR as well as stroke volume, however the modest drop in BP may have been insufficient to stimulate a reflex response as HR did not appreciably change during stimulation, consistent with the previous report (2).
An important limitation of case presentations lies in their limited generalisability. We cannot assume, from this report, that all patients undergoing this procedure will exhibit similar hemodynamic changes upon stimulation. It is likely, as with other physiological responses, that “responders” and “non-responders” will exist and such variability in hemodynamic responses is accentuated by small inconsistencies in electrode positioning. Indeed, the original report describing the BP effects of PAG DBS in humans observed responses in some, but not all, subjects (2).
In summary, these combined blood flow and pressure data suggest that stimulation of the PVG/PAG can impact upon vasomotor control. This finding compliments previous reports pertaining to the impact of DBS on BP and extends findings to the vasculature in humans (2, 3).
Acknowledgements
We sincerely thank the patient involved in this experiment and the nursing and surgical staff who assisted.
The study was support by the NIHR Biomedical Research Centre Award to the University of Oxford.
Prof Green’s research is supported by the Australian Research Council and National Heart Foundation.
Mr Carter was supported by a University of Western Australia Research Collaboration Award.
Dr Dawson was supported by a Liverpool John Moores University Research Development Award.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Green AL, Paterson DJ. Identification of neurocircuitry controlling cardiovascular function in humans using functional neurosurgery: implications for exercise control. Exp Physiol. 2008;93:1022–1028. doi: 10.1113/expphysiol.2007.039461. [DOI] [PubMed] [Google Scholar]
- 2.Green AL, Wang S, Owen SLF, Xie K, Liu X, Paterson DJ, Stein JF, Bain PG, Aziz TZ. Deep brain stimulation can regulate arterial blood pressure in awake humans. NeuroReport. 2005;16:1741–1745. doi: 10.1097/01.wnr.0000183904.15773.47. [DOI] [PubMed] [Google Scholar]
- 3.Green AL, Wang S, Bittar RG, Owen SLF, Paterson DJ, Stein JF, Bain PG, Shlugman D, Aziz TZ. Deep brain stimulation: A new treatment for hypertension? J Clin Neurosci. 2007;14:592–595. doi: 10.1016/j.jocn.2006.04.015. [DOI] [PubMed] [Google Scholar]