Abstract
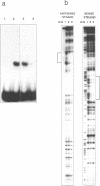
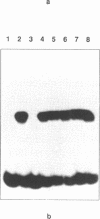
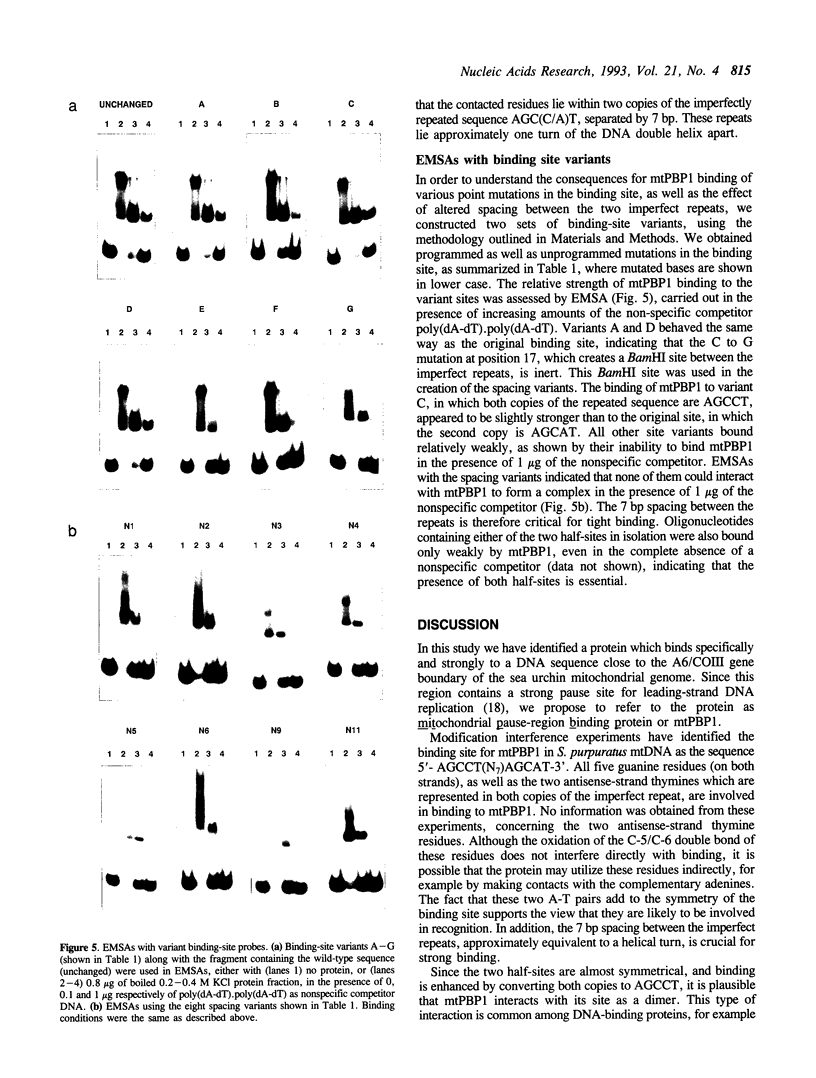
Based on electrophoretic mobility shift assays, DNase I footprinting and modification interference analyses we have identified a sequence-specific DNA-binding protein in blastula stage mitochondria of the sea urchin Strongylocentrotus purpuratus, which interacts with a binding site around the major pause site for DNA replication. This region straddles the boundary of the genes for ATP synthase subunit 6 and cytochrome c oxidase subunit III, and contains also a prominent origin of lagging-strand synthesis. The protein is thermostable, and its natural high-affinity binding site comprises the sequence 5'-AGCCT(N7)AGCAT-3'. Binding studies have demonstrated that two copies of the imperfect repeat, as well as the 7 bp spacing between them, are essential for tight binding. Based on the location of its binding site, we tentatively designate the protein mitochondrial pause-region binding protein (mtPBP) 1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calzone F. J., Hög C., Teplow D. B., Cutting A. E., Zeller R. W., Britten R. J., Davidson E. H. Gene regulatory factors of the sea urchin embryo. I. Purification by affinity chromatography and cloning of P3A2, a novel DNA-binding protein. Development. 1991 May;112(1):335–350. doi: 10.1242/dev.112.1.335. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Roberti M., Rainaldi G., Gadaleta M. N., Saccone C. The complete nucleotide sequence, gene organization, and genetic code of the mitochondrial genome of Paracentrotus lividus. J Biol Chem. 1989 Jul 5;264(19):10965–10975. [PubMed] [Google Scholar]
- Chang D. D., Fisher R. P., Clayton D. A. Roles for a promoter and RNA processing in the synthesis of mitochondrial displacement-loop strands. Biochim Biophys Acta. 1987 Jul 14;909(2):85–91. doi: 10.1016/0167-4781(87)90029-7. [DOI] [PubMed] [Google Scholar]
- Christianson T. W., Clayton D. A. A tridecamer DNA sequence supports human mitochondrial RNA 3'-end formation in vitro. Mol Cell Biol. 1988 Oct;8(10):4502–4509. doi: 10.1128/mcb.8.10.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7864–7868. doi: 10.1073/pnas.88.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. P., Clayton D. A. Purification and characterization of human mitochondrial transcription factor 1. Mol Cell Biol. 1988 Aug;8(8):3496–3509. doi: 10.1128/mcb.8.8.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. P., Lisowsky T., Breen G. A., Clayton D. A. A rapid, efficient method for purifying DNA-binding proteins. Denaturation-renaturation chromatography of human and yeast mitochondrial extracts. J Biol Chem. 1991 May 15;266(14):9153–9160. [PubMed] [Google Scholar]
- Fisher R. P., Topper J. N., Clayton D. A. Promoter selection in human mitochondria involves binding of a transcription factor to orientation-independent upstream regulatory elements. Cell. 1987 Jul 17;50(2):247–258. doi: 10.1016/0092-8674(87)90220-0. [DOI] [PubMed] [Google Scholar]
- Gartenberg M. R., Crothers D. M. DNA sequence determinants of CAP-induced bending and protein binding affinity. Nature. 1988 Jun 30;333(6176):824–829. doi: 10.1038/333824a0. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Elliott D. J., Math V. B., Farquharson A. Nucleotide sequence and gene organization of sea urchin mitochondrial DNA. J Mol Biol. 1988 Jul 20;202(2):185–217. doi: 10.1016/0022-2836(88)90452-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H. T., Herbert E. R., Rankine J. Sea urchin egg mitochondrial DNA contains a short displacement loop (D-loop) in the replication origin region. Nucleic Acids Res. 1989 Nov 25;17(22):8949–8965. doi: 10.1093/nar/17.22.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Heyman R. A., Mangelsdorf D. J., Dyck J. A., Evans R. M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. H., Kornberg A., Hidaka M., Kobayashi T., Horiuchi T. Escherichia coli replication termination protein impedes the action of helicases. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9104–9108. doi: 10.1073/pnas.86.23.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowsky T., Michaelis G. A nuclear gene essential for mitochondrial replication suppresses a defect of mitochondrial transcription in Saccharomyces cerevisiae. Mol Gen Genet. 1988 Oct;214(2):218–223. doi: 10.1007/BF00337714. [DOI] [PubMed] [Google Scholar]
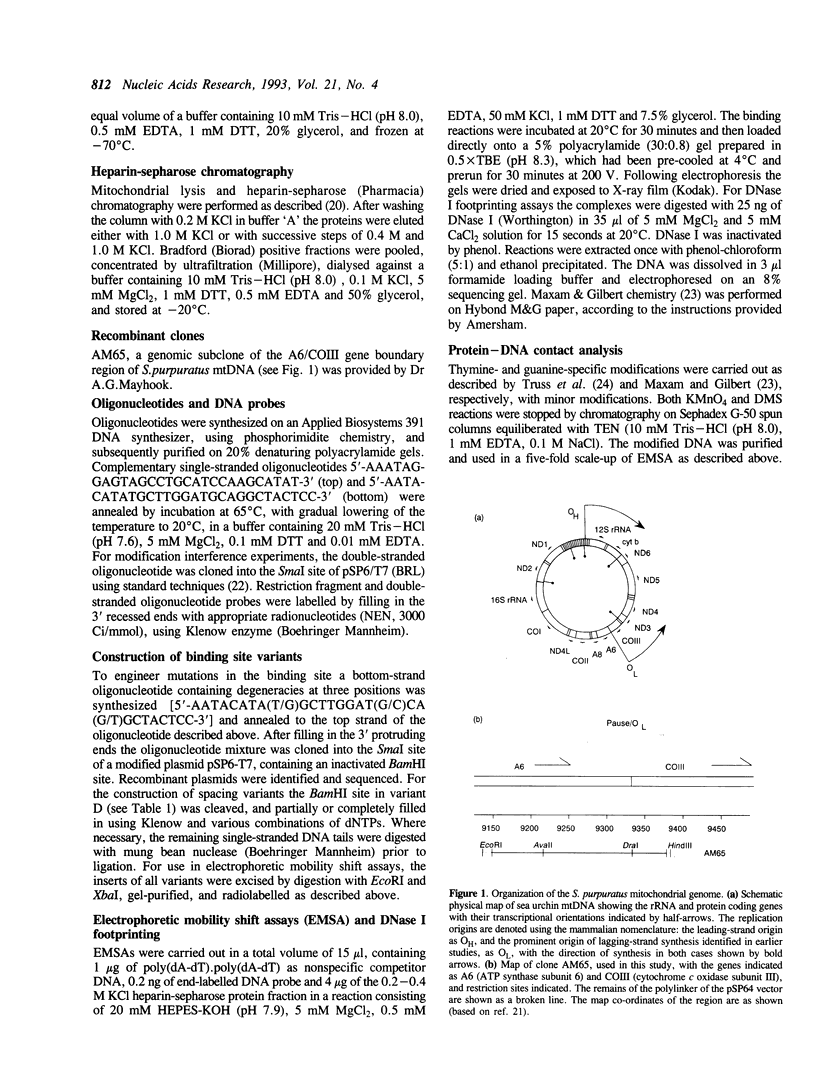
- Mayhook A. G., Rinaldi A. M., Jacobs H. T. Replication origins and pause sites in sea urchin mitochondrial DNA. Proc Biol Sci. 1992 Apr 22;248(1321):85–94. doi: 10.1098/rspb.1992.0046. [DOI] [PubMed] [Google Scholar]
- Rinaldi A. M., Carra E., Salcher-Cillari I., Oliva A. O. The nucleus negatively controls the synthesis of mitochondrial proteins in the sea urchin egg. Cell Biol Int Rep. 1983 Mar;7(3):211–218. doi: 10.1016/0309-1651(83)90228-x. [DOI] [PubMed] [Google Scholar]
- Roberti M., Mustich A., Gadaleta M. N., Cantatore P. Identification of two homologous mitochondrial DNA sequences, which bind strongly and specifically to a mitochondrial protein of Paracentrotus lividus. Nucleic Acids Res. 1991 Nov 25;19(22):6249–6254. doi: 10.1093/nar/19.22.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel A. H., Groot Koerkamp M. J., Tabak H. F. Mitochondrial RNA polymerase of Saccharomyces cerevisiae: composition and mechanism of promoter recognition. EMBO J. 1988 Oct;7(10):3255–3262. doi: 10.1002/j.1460-2075.1988.tb03192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truss M., Chalepakis G., Beato M. Contacts between steroid hormone receptors and thymines in DNA: an interference method. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7180–7184. doi: 10.1073/pnas.87.18.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]