Abstract
Background
Older adults have low rates of physical activity participation but respond positively to telephone-mediated support programs. Programs are often limited by reliance on professional staff. This study tested telephone-based physical activity advice delivered by professional staff versus trained volunteer peer mentors.
Design
A 12-month, randomized, controlled clinical trial was executed from 2003–2008. Setting/participants: Twelve volunteer peer mentors and 181 initially inactive adults ages 50 years and older were recruited from the San Francisco Bay Area.
Intervention
Participants were randomized to: (1) telephone-based physical activity advice delivered by professional staff, (2) telephone-based physical activity advice delivered by trained volunteer peers, or (3) an attention-control arm of staff-delivered telephone support for nutrition. Main Outcome Measures: Moderate-intensity or more vigorous physical activity (MVPA) was assessed at baseline, 6, and 12 months with the CHAMPS Questionnaire, with accelerometry validation (Actigraph) in a randomly selected subsample. Treatment fidelity was examined through analysis of quantity and quality of intervention delivery.
Results
At 6 and 12 months, both physical activity arms significantly increased MVPA relative to the control arm. Both physical activity arms were comparable in quantity of intervention delivery, but peers demonstrated more versatility and comprehensiveness in quality of intervention content.
Conclusions
This study demonstrates that trained peer volunteers can effectively promote physical activity increases through telephone-based advice. The results support a program delivery model with good dissemination potential for a variety of community settings.
Keywords: physical activity, telephone, peer mentors, volunteers
Introduction
Given the persistent low rates of physical activity among American midlife and older adults (National Center for Health Statistics, 2009), many efforts have been made to develop programs to increase regular physical activity. Efficacious, empirically-based programs that are grounded in behavior modification and self-management principles and that can be individually tailored are strongly recommended for implementation at the community level (Guide to Community Preventive Services, 2009). Programs aimed specifically at midlife and older adults that rely on the telephone as the main delivery vehicle have been successfully validated in rigorous research and disseminated in diverse community settings (King et al., 2007; Wilcox et al., 2008). Such telephone-based programs provide the individual tailoring and support that are critical to long-term behavior change, while allowing for program delivery that is typically more flexible and convenient than face-to-face or group-based formats (Castro & King, 2002).
While telephone-based programs have many advantages, widespread dissemination has been limited by reliance on professional staff to implement the program; this can increase costs and reduce delivery capacity (Sevick et al., 2007). The use of volunteers as peer mentors is a popular, economical method to provide education and support to a variety of populations (Chambre, 1993). According to socio-cultural and communication theories, people are more receptive to assistance when it is delivered by someone perceived as similar to oneself (e.g., of comparable age, background, and life experience; Bonk, 1998, Corrigan, Dell, Lewis & Schmidt, 1980). Peer-delivered telephone programs designed specifically for older adults have resulted in increased pneumococcal and influenza vaccinations (Krieger, Castorina, Walls, Weaver & Ciske, 2000), and mammography and colorectal cancer screening (Derose, Fox, Reigadas & Hawes-Dawson, 2000, Weinrich, Wienrich, Stromborg, Boyd & Weiss, 1993).
A small but growing body of research has begun to examine peer mentors to promote physical activity. One 14-week experimental study tested the effects of a group-based physical fitness training program led by either trained older peer mentors or un-paid, undergraduate kinesiology students (Dorgo, King & Brickey, 2009). Both classes resulted in pretest to post-test fitness improvements. Although participants in the student-led classes had better participation, participants in the peer-led classes had greater physical, mental, and social functioning (Dorgo, Robinson & Bader, 2009). In a 4-month quasi-experimental study, the validated Strong for Life home-based strength training program was implemented by volunteers through the Robert Wood Johnson Foundation Faith in Action initiative (Etkin, Prohaska, Harris, Latham & Jette, 2006). The program successfully trained a large number of volunteers in many communities, and a substantial percentage of participants engaged in at least 2 exercise sessions per week. However, the study was limited by the lack of a comparison group and high mentor and participant attrition (> 35%). Finally, a quasi-experimental study evaluated peer telephone support for older adults with diabetes delivered through a program partnership between a neighborhood senior center, a social services provider, two community clinics, and a university research center (Batik, Phelan, Walwick, Wang & LoGerfo, 2008). Peer mentors were recruited through the senior center, conducted the work at the center, and were trained by a study consultant and a program coordinator employed by the social services provider. Over 6 months, the percentage of physically active participants increased from 21 to 43%, but the study were limited by a small sample, difficulty enrolling patients, and lack of a comparison condition. Other quasi-experimental studies support the use of trained peer volunteers in delivering telephone-mediated physical activity advice for midlife and older adults (Hooker et al. 2005; Wilcox et al., 2008) but similarly suffer from a lack of comparison conditions and other methodological constraints.
While these studies provide encouraging results related to the efficacy of peer models, no experimental trial has directly compared the effects of a physical activity program delivered by trained peer mentors versus trained, paid professionals. The purpose of this study was to test these two delivery models using a rigorous research design and an extended time frame relative to the current evidence base. Given the previous literature on the impact of peer mentors, it was hypothesized that the trained mentors would be as effective as professional staff in increasing rates of physical activity among participants relative to an attention-control condition.
Methods
Procedures
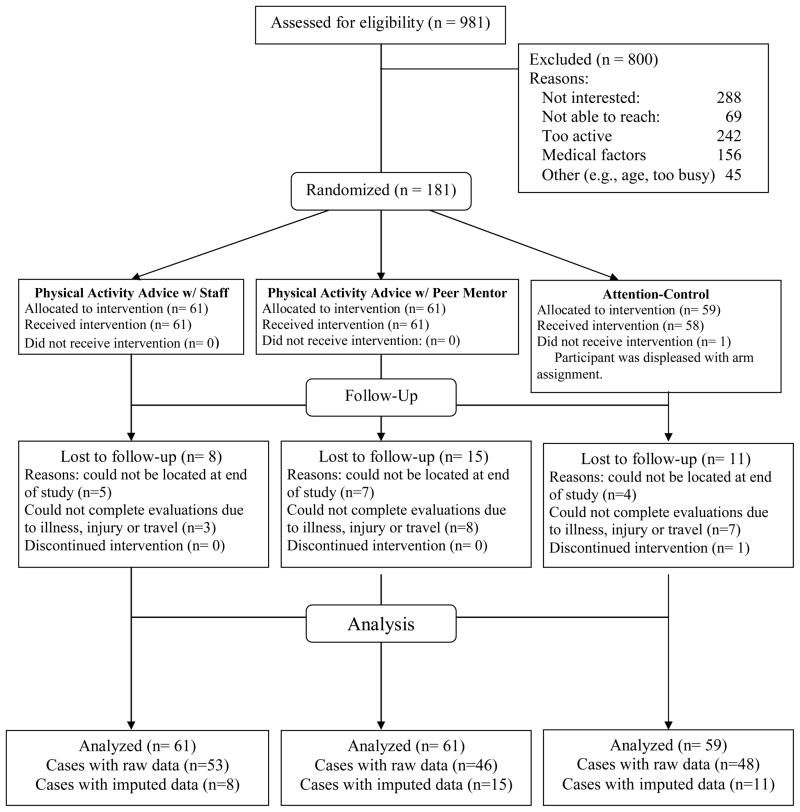
The TEAM (Telephone Advice and Mentoring) study was a 12-month randomized controlled trial. See Figure 1 for a summary of the flow of trial activities. Data were collected between 2004 and 2007 and analyzed in 2008 to 2009. Participants were recruited from mass mailings and advertisements in the San Francisco Bay Area. People were invited to call the study office to undergo telephone screening. Participants had to meet the following eligibility criteria: (1) age of 50 years or older; (2) under-active (defined as less than 60 minutes of physical activity per week); (3) stable on any medications for at least 3 months; (4) free of any condition that would prohibit physical activity without direct supervision, and (5) willing to be randomly assigned to study arm. If eligible based on the telephone screen, individuals were invited to an information session, and then underwent written informed consent procedures and baseline measurements during which study eligibility was confirmed. A computerized Efron procedure (Efron, 1971) with gender stratification was used to randomly allocate participants to one of the three study arms. Intervention staff ran the randomization program and tracked assignment to study arms. Measurement staff members were blinded to study arm assignment.
Figure 1.
Flow of TEAM study activities
Intervention
There were three intervention arms in the study: (1) telephone-based physical activity advice delivered by a trained professional staff member, (2) identical advice delivered by a trained volunteer peer mentor, or (3) an attention-control arm of telephone-based heart-healthy nutrition advice delivered by a trained professional staff member that was identical to the other arms in intervention format, staff time, and attention. All arms were 12 months long from randomization to post-test. All participants received an initial face-to-face session during which they were introduced to their study arm, met their assigned staff person or peer mentor, discussed relevant health history and motivation for changing their behavior, set initial goals, and established a regular telephone appointment time. Telephone calls were delivered twice per month for the first 2 months and then monthly for the remainder of the year for a total of 14 telephone contacts in 12 months (Castro & King, 2002).
The physical activity intervention was the well-validated Stanford Active Choices Program (Stanford Health Promotion Resource Center, 2005), a guided self-management program delivered via one face-to-face session followed by scheduled telephone contacts. The emphasis of Active Choices is to increase leisure-time physical activity of moderate or more vigorous aerobic intensity (MVPA) to achieve current recommendation of at least 150 minutes per week, spread across most days of the week (Nelson et al., 2007, Physical Activity Guidelines Advisory Committee, 2008). The program is grounded in social cognitive theory (Bandura, 1998) and facilitates behavior change by helping participants learn, use, and reinforce self-management skills to adopt and maintain physical activity (e.g., self-efficacy enhancing skills including realistic goal-setting, problem solving related to physical activity-relevant barriers, vicarious learning, self-monitoring via simple calendars, behavioral feedback via pedometers, and social support enhancement). The Transtheoretical Model (Prochaska & Marcus, 1994) is employed at each contact to identify the participant’s current stage of motivational readiness; this enables the interventionist to tailor the discussion to the cognitive or behavioral processes that are most relevant at that point in time (Marcus, Rossi, Selby, Niaura & Abrams, 1992). The regular telephone contacts are supplemented as needed by mailings of monthly newsletters and tip sheets with more in-depth, topic-specific advice (e.g., staying active in inclement weather, on vacation, etc.).
Professional Staff
Three research assistants were hired and trained to deliver the physical activity and attention-control interventions. These staff members had bachelor’s degrees in science-related fields, and a minimum of 2 years of experience working with adults in health research settings. One staff member was fluent in Spanish. Each staff person maintained an average caseload of 20–30 participants. Two had experience delivering Active Choices in previous trials. Regardless of their experience, all staff underwent a standard full-day training in the implementation of Active Choices with the Intervention Director (Dr. Castro). In training, they reviewed the basic roles and responsibilities of being a facilitator, learned the fundamentals of social cognitive theory and motivational readiness, practiced essential counseling skills (e.g., active listening, creative problem-solving, asking open-ended questions), and actively rehearsed and practiced counseling sessions. Throughout the trial, all staff routinely audio taped all sessions (with participant consent), and participated in weekly case conference supervision to review audiotapes, discuss participant challenges or successes, and support each other.
Peer Mentors
Peer mentors were recruited from mailings to previous research participants and announcements to local active aging community groups. Mentors were eligible if they were: physically active (at least 150 minutes of MVPA per week), willing to participate in all training and supervision, and willing to volunteer 4–6 hours per week for a minimum of one year. Participants completed a written application and interviewed with the volunteer coordinator (Ms. French). If selected, they were invited to an orientation session and asked to commit to training. They underwent 8 hours of Active Choices training identical to that conducted with the professional staff. Peer mentors were assigned post-training practice sessions identical to the professional staff, including assignments to rehearse the advice and counseling components and practice completing forms to document the content and delivery of the interventions. Practice sessions were either observed in person by the volunteer coordinator or audio taped to give constructive feedback on skills and execution of the program protocol. Over 6 months, 12 peer mentors were recruited from approximately 30 applicants. Following training and post-training practice, mentors were assigned 3–10 participants depending upon their time availability. They were matched to participants based on participants’ preferred language (2 mentors were bilingual in Spanish), and schedule convenience. Peer mentors shared one desk in the research office for use in making phone contacts but were also provided pre-paid telephone charge cards if they wished to make their calls from home. All private information was kept in the research offices, but mentors were allowed to keep minimal notes to make their contacts at home. Identical to the professional staff, peer mentors completed information sheets to document every contact with participants, and audio taped their sessions with participant consent. Biweekly required supervision meetings were held to retrain on behavioral principles and review case notes and audiotapes throughout the study. The volunteer coordinator also maintained weekly email and telephone contact with mentors.
Attention-Control Arm
Participants in this arm were given support to adopt a heart-healthy diet focused on reducing saturated fat and increasing fruits and vegetables, based on an evidence-supported telephone nutrition advice program evaluated in a previous randomized trial (King, Baumann, O’Sullivan, Wilcox & Castro, 2002). Nutrition content was updated and finalized by a registered dietician using a variety of educational materials from the American Heart Association, American Cancer Society, and American Dietetic Association. Similar to the introductory participant session conducted in the other two arms, trained staff conducted a one-on-one session with participants to establish nutrition goals, discuss relevant health background, and schedule telephone contacts. Participants were asked to track their food intake and behavior change efforts (e.g., replace butter with olive oil, etc.). Participants were asked to maintain their usual patterns of physical activity and not discuss physical activity with their interventionist during the study so as to remain focused on heart-healty nutritional changes and not contaminate the delivery of the assigned interventions. After post-test data collection, they were offered one physical activity counseling session, a pedometer, tracking calendar, and all written information sheets from the PA arms.
Measurements
Measurements were collected at baseline prior to randomization, and at 6 and 12 months. Staff members conducting assessments were blinded to assigned study arm.
Physical Activity
The main outcome was leisure-time moderate-intensity or more vigorous physical activity (MVPA) measured via the CHAMPS questionnaire (Stewart et al., 2001). This self-report measure is extensively validated for use in middle- and older-aged adults, correlates with other standardized measures in the field (e.g., the Stanford Physical Activity Recall), has been validated in both English and Spanish, and has shown sensitivity to change in a wide range of intervention trials (Hooker et al., 2005, King et al. 2007, Wilcox et al, 2008). Respondents indicate both the quantity (frequency per week and minutes per day) and type of activity. Activities are assigned metabolic equivalents (METS) according to standard classifications adjusted slightly in some cases for older populations (Stewart et al., 2001). Because the CHAMPS questionnaire involves self-reporting of ‘usual activity levels’ over the previous 4-week period and has participants choose categories reflecting varying ranges of minutes/week engaged in each activity (as opposed to specifying specific minutes/week for each activity item), it is most appropriately used to reflect relative differences in physical activity across individuals and/or across time as opposed to absolute physical activity levels. (King et al, 2007). The main outcome calculated from the CHAMPS was minutes per week of moderate or greater intensity leisure-time physical activity (MVPA). Household and occupational activities were excluded because few individuals in this age group engaged in occupational physical activities and the intervention focused specifically on increasing leisure time activity. Study participants completed the CHAMPS at baseline, 6, and 12 months. For purposes of evaluating how serving as a peer mentor for others could impact the mentor’s own physical activity levels, peer mentors completed the CHAMPS after their initial program-training period (prior to working with participants) and at the completion of work with participants.
Objective Physical Activity validation
At baseline, a symptom-limited graded treadmill test was used to establish the moderate-intensity heart rate range for the physical activity interventions. Participants walked on the treadmill at an initial speed of 1.5 mph (0% incline) with speed gradually increased by 0.2–0.5 mph increments. As the treadmill speed increased, heart rate was continually monitored until 65–70% of age-predicted maximal heart rate (MHR) was attained and/or the participant reported a rating of perceived exertion (RPE) of 15–17 on a scale of 6 to 20 (6 = no exertion at all, 20 = maximal exertion). This treadmill speed (i.e., the individualized moderate-intensity walking pace) was then used as part of the treadmill testing protocol. The testing protocol began with the participant walking at the predetermined pace with 0% incline. The incline was increased by 2% every 2 minutes with no change in treadmill speed. Heart rate, blood pressure, and RPE were recorded during the last minute of each 2-minute stage until the participant reached 75% - 85% of age-predicted MHR and/or an RPE of 15–17. The MTI Actigraph accelerometer was worn during treadmill testing. Accelerometry activity counts per minute from the last minute of the first treadmill stage (0% incline) was used to establish an individualized moderate-intensity activity count threshold when the accelerometer was later worn by participants in the field (Pruitt et al., 2008).
A random sample of 30 participants (10 from each arm) completed accelerometer monitoring at the 6-month interim time point to verify their self-reported physical activity. The monitor collects continuous data via a battery-operated microprocessor that senses motion with a piezoelectric linear accelerometer. Participants wore the extensively validated MTI Actigraph (MTI Actigraph, Fort Walton Beach, FL) for a minimum of 8 hours a day for at least 5 days in a 7-day period. To detect activity of at least moderate intensity, participants wore the Actigraph while undergoing the baseline treadmill testing as described above. The individualized moderate-intensity activity count threshold determined during treadmill testing was used to detect and characterize activity of at least moderate intensity performed during daily routines (Pruitt, et al., 2008). Given the physical activity goals described earlier, only activity that registered at or above the moderate-intensity threshold that was sustained for at least 10 minutes was counted (Physical Activity Guidelines Advisory Committee, 2008).
Intervention Fidelity
All staff and peer mentors completed structured contact sheets to quantify the intervention content delivered in each session. Intervention content included both quantity (length of session) and quality (self-management concepts discussed, tip sheets sent to participant; goals set) of the intervention delivered. Ten content areas were identified as core self-management and social-cognitive counseling topics for use in the intervention: (1) perceived benefits of physical activity, (2) problem-solving barriers to physical activity; (3) discussion of lessons learned from previous physical activity experiences; (4) the individualized decisional balance between the “pros” versus “cons” of being active; (5) injury prevention; (6) enhancing enjoyment of physical activity; (7) creating self-rewards for being active; (8) enhancing self-efficacy through overcoming obstacles and building success; (9) eliciting social support; and (10) implementing relapse prevention. The Intervention Director (Dr. Castro) verified the intervention fidelity data through weekly review of audio taped sessions and contact sheets.
Participant Perceptions of Advisor
Participants rated the perceived quality and competence of their respective telephone advisors across the two physical activity intervention arms using a 39-item scale that was adapted from previous studies (King, Haskell, Taylor, Kraemer & DeBusk, 1991). The scale was comprised of items that assessed participants’ perceived trust, competence, communication, empowerment, and connection to their advisor. Cronbach’s coefficient alpha at baseline was .98, indicating high internal consistency of items.
Sample Size and Statistical Analysis
Data analysis focused on testing differences among the three arms across the 12-month intervention period. The primary variable was CHAMPS average weekly minutes of leisure-time moderate-intensity or more vigorous physical activity (MVPA). Based on previous trials (King et al., 2007; Wilcox et al., 2008), in order to detect between-group differences as small as 30 minutes with a standard deviation of 50 minutes, the effect size (standardized mean difference) was calculated to be 0.60. The sample size needed to detect an effect size of 0.60 or greater with alpha at .05 and power of .80 was 50 subjects per arm. To protect against a drop-out rate across the 12-month period of as much as 20%, 60 subjects per arm needed to be randomized. Thus, the total sample size required was 180. Demographics (age, education, primary language) were evaluated at baseline using correlation analysis and t-tests for descriptive purposes. Given the typical lack of equivalency concerning regression lines, a repeated measures, between-groups ANCOVA (General Linear Models Procedure, SAS 9.2, SAS Institute, Inc., Cary, NC) was used to test the primary hypothesis that both intervention groups would significantly increase PA relative to the comparison arm, but there would be no between-group differences between the Peer Mentor and Professional Staff arms. Analyses were conducted using both raw data with case-wise deletion of missing values, and intent-to-treat analyses. For participants missing 6-month data only (n = 7) and both 6-month and 12-month data (n = 15), the baseline value was carried forward. For participants missing 12-month data only (n = 19), secondary data from telephone advisor intervention logs from months 7–12 of the intervention were inspected. If these data indicated the participant was at least as active as the 6-month data collection point, then the 6-month values were carried forward. Of the 19 participants missing 12-month data, 13 had evidence from telephone advisor logs to indicate physical activity levels at or greater than that reported at their 6-month CHAMPS. For those participants, the 6-month data value was carried forward. For the 6 without corroborating data, their baseline values were carried forward.
Equivalency testing was performed to establish if changes observed in the Peer Mentor arm were equivalent to changes observed in the Professional Staff arm, as opposed to simply “not statistically different” (Rogers, Howard & Vessey, 1993). In equivalency testing, an a priori criterion is set to determine the clinically meaningful difference needed to conclude non-equivalence. One-sided inference tests (90% Confidence Interval) are then computed around the observed differences between the two groups. If the a priori criterion falls within the 90% Confidence Intervals, the groups are considered essentially equivalent (Rogers et al., 1993). The equivalency criterion was set at ± 20% of the average increase in CHAMPS minutes of MVPA per week for the participants in the Professional Staff arm, calculated for baseline to 6 months and baseline to 12 months. This criterion was chosen because it has been used in similar studies of physical activity equivalency (Steele, Mummery & Dwyer, 2009) and approximated 30 minutes of additional physical activity. This has clinical meaning as it represents one more day per week towards reaching current recommended guidelines (Nelson, et al., 2007). Any change in physical activity for the Peer Mentor arm that fell outside of the ± 20% change range for the Professional Staff arm would be considered substantively different.
Intervention delivery information was evaluated to examine if the quantity and quality of the interventions varied between the physical activity peer mentors and professional staff across the 12-month period. T-tests examined between-group differences in quantity of intervention delivered (total calls and average length of calls), and topics discussed during the calls. A between-groups, repeated measures ANOVA was used to test changes over time between the two physical activity groups in their ratings of their advisors’ skills and competence.
Results
A total of 981 were approached for eligibility screening. Of the 800 who were excluded at the screening stage, 36% (n=288) expressed disinterest in study participation and declined to undergo in the eligibility interview. People who completed the interview and were determined to be ineligible did not differ from randomized participants by gender or number or type of excluding health conditions, but they were older (61.5 vs. 59.1years, t[530] = 3.32, p =.001), and were more likely to be an ethnic or racial minority (57.2% vs. 32.6%, X2 [2, 620] = 32.4, p=.001). Descriptive information of the randomized sample is reported in Table 1. The sample was ethnically representative of the age group in this geographic region, with approximately a third of participants representing the prevalent Latino and Asian minority groups in the Bay Area (U.S. Census Bureau, 2008). The majority (65.8%) were women, employed outside the home, and well educated. There were no statistically significant differences between study arms in age, education level, gender distribution, race/ethnicity, marital status, or employment status (p values > .05). The peer mentors were demographically similar to the participants, with the exception being that most were retired, thus providing the flexibility and time to volunteer (See Table 1).
Table 1.
Demographic Characteristics.
| Variable | Total Participant Sample (N=181) | Peer Mentors (n=9) |
|---|---|---|
| Mean Age in Years (SD) | 59.1 (6.1) | 64.4 (5.8) |
| Mean Years of Education (SD) | 15.6 (2.6) | 17.0 (1.0) |
| % Women | 65.8% | 88.9% |
| Marital Status | ||
| Married/Cohabitating | 59.8% | 55.6% |
| Divorced/Separated | 26.8% | 22.2% |
| Never Married | 8.9% | 11.1% |
| Widowed | 4.5% | 11.1% |
| Race/Ethnicity | ||
| Non-Hispanic White/Caucasian | 67.4% | 88.9% |
| Latino/Hispanic | 15.2% | 11.1% |
| Asian/Pacific Islander | 13.5% | ------ |
| Employment Status | ||
| Employed outside the home | 70.8% | 11.1% |
| Retired | 16.0% | 77.8% |
| Unemployed, seeking work | 8.6% | 0% |
| Homemaker, student, volunteer | 4.6% | 11.1% |
One participant dropped out immediately after baseline due to dissatisfaction with assignment to the comparison arm. Twelve percent (n = 22) of subjects were lost to follow up at 6 months; 19% (n = 34) were lost at 12 months. There were no differences in arm assignment between participants who provided data and those who were lost to follow-up at 12 months (X2 [2,181] = 2.63, p =.27). Those lost to follow up were generally younger than those who provided 12-month data, t (181) = 2.2, p = .03, but otherwise there were no differences based on follow-up status in gender, employment status, race/ethnicity, educational status, or baseline physical activity. No serious adverse events occurred during the trial.
Of the 12 mentors that were recruited, one discontinued immediately after training due to discomfort with the level of responsibility. Another discontinued before being assigned a caseload due to loss of interest. One peer mentor resigned shortly after being assigned a caseload due to a serious health condition; participants assigned to this mentor were reassigned to others. Late in the first year of the intervention, another peer mentor was diagnosed with terminal cancer; she maintained her caseload for as long as she was able, and eventually transferred her cases to other mentors. The remaining 8 mentors stayed committed for the entire 3-year study period, and completed all of their assigned caseloads.
Leisure Time Physical Activity Outcomes
Results of physical activity outcomes were the same using either the raw data or intent-to-treat data. Intent-to-treat values are reported. Age, education, employment status, ethnicity, and language preference were not significantly correlated with baseline CHAMPS physical activity (r values > .10). The sample was underactive at baseline, averaging 59.9 MVPA minutes per week (SD = 112.1). From baseline to 6 months and baseline to 12 months, participants receiving physical activity advice from either peer mentors or professional staff significantly increased MVPA relative to the control arm (study arm × time interaction effect F (4,354) = 3.79, p = .005). See Figure 1 for an illustration of the increase in MVPA over time. Participants in the peer mentor arm reported an average increase of MVPA of 216.2 minutes (SD = 355.7) per week from baseline to 12 months, compared to a 178.0 (SD = 234.9) average minutes per week increase among participants in the professional staff arm. The comparison arm increased an average of 71.5 minutes per week (SD = 179.1) from baseline to 12 months. These baseline-to-12-month differences translate to a .51 effect size (Cohen’s d) between the Peer Mentor arm and the Control arm, a .50 d effect size between the Professional Staff arm and the Control arm, and a .13 d effect size between the Peer Mentor and Professional Staff arms. There were no between-group differences in MVPA between the peer professional staff arms at either 6 months (p = .4) or 12 months (p = .5).
In tests of statistical equivalency, the Professional Staff and Peer Mentor arms were equivalent in their average increases in MVPA from baseline to 6 months, but were not equivalent from baseline to 12 months (see Table 2). From baseline to 6 months, the mean increase in MVPA in the Peer Mentor arm was within 20% of the mean increase in the Professional Staff arm (i.e., the 90% Confidence Interval was contained within the equivalency interval). However, the baseline to 12-month mean increase in MVPA in the Peer Mentor arm was greater than 20% of the mean increase in the Professional arm (i.e., the 90% Confidence Interval was not contained within the equivalency interval).
Table 2.
Equivalency Test Results for Differences between Peer Mentor versus Professional Staff arms in Physical Activity Increases.
| Peer Mentor (n = 61) | Professional Staff (n=61) | Difference | Equivalency Interval* | 90% CI Lower | 90% CI Upper | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SE | ||||
| Baseline to 6 months | 192.8 | 269.6 | 165.5 | 229.7 | −27.3 | 2.9 | ± 33.1 | −32.0 | −22.6 |
| Baseline to 12 months | 216.2 | 355.7 | 178.0 | 234.9 | −38.2 | 3.1 | ± 35.6 | −43.3 | −33.1 |
±20% of Mean of Professional Staff arm; CI = Confidence Interval
The peer mentors remained regularly physically active throughout the study. Results of a paired comparison t-test showed no significant changes between baseline and post-test CHAMPS physical activity, t(9) = 1.75, p = .12. Peers reported an average of 489.4 minutes per week of MVPA at baseline (SD = 238.6), and 354.4 minutes per week of MVPA at the end of their study participation (SD = 236.1). Mentors were active at least an hour every day (with some being active up to 90 minutes per day), and most reported walking as their primary activity.
Actigraphy Validation
At the 6-month interim time point, 17% of participants were randomly selected across all study arms (n = 30, approximately 10 in each arm) to wear the MTI Actigraph. Participants wore it for an average of 7 days (range = 5–7) and for an average of 13 hours, 34 minutes per day (SD = 2 hours, 1 minute). There were no significant differences across arms in numbers of days and hours worn per day (p > .52). Minutes of actigraphy-measured MVPA were significantly correlated with the CHAMPS MVPA minutes per week (r = .44, p = .01). Given the reduced sample size for the accelerometer sub-study (n = 30), and the fact that the two physical activity arms did not differ in the Actigraph-derived measures, (t (18) = −1.89, p = .07), the two physical activity arms were combined and compared to the Control arm. Physical Activity Intervention arms had significantly greater mean minutes of physical activity per hour at or above their moderate-intensity threshold compared to the Control arm. The physical activity arms averaged 22.4 (SD = 19.3) minutes per hour of MVPA, compared to 5.7 minutes per hour (SD = 3.9) in the Control arm, t (30) = 2.94, p = .007. These data provide an objective indicator that, on the days the accelerometer was worn, Physical Activity Intervention participants engaged in more physical activity in their targeted intensity range relative to Controls.
Evaluation of Intervention Delivery and Fidelity
Table 3 illustrates the quantity of intervention delivered between the peer mentors and professional staff. Peer mentors and staff delivered equal amounts of the intervention, completing an average of 11 of 14 planned telephone calls across the year, t (122) = 0.79, p = 0.4. The average length of the telephone calls was equivalent between arms, averaging approximately 15–16 minutes, t (122) = 1.07, p = 0.3. Relative to professional staff, peer mentors more frequently discussed by phone the balance of pros and cons of physical activity t (122) = 2.8, p = .008; the perceived benefits of PA, t (122) = 4.0, p = .001, physical activity history t (122) = 3.7, p = .0005, and self-rewards with their participants t (122) = 2.0, p = .05. Professional staff more frequently discussed self-efficacy in the phone contacts relative to the peer mentors, t(122) = −6.3, p = .0001. In rating their perceptions of their advisor’s skill and competency, there were no significant between-group differences or changes over time across the two arms, F (2, 122) = 3.10, p = .06.
Table 3.
Intervention Delivery Activities for Peer Mentor and Professional Staff Intervention Arms
| Variable | Peer Mentor Arm (N=61) | Professional Staff Arm (n=61) | Mean Difference | 95% Confidence Interval |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Total Telephone Calls Completed | 11.4 (2.6) | 11.0 (3.3) | 0.4 | −0.6 – 1.5 |
| Average Minutes per Call | 16.0 (5.5) | 15.0 (4.7) | 1.0 | −.8–2.8 |
| Percent of calls with specific content discussed: | ||||
| Decisional Balance | 7.4 (19.5) | 0.5 (2.3) | 6.9 | 1.9–11.8* |
| Perceived Benefits | 46.7 (24.0) | 30.4 (16.9) | 16.2 | 8.1–24.4* |
| Perceived Barriers | 43.7 (26.0) | 51.0 (18.9) | −7.3 | −16.5–1.9 |
| PA History | 23.2 (13.2) | 12.3 (7.8) | 10.9 | 5.0−16.8* |
| Self-Efficacy | 26.9 (13.5) | 51.5 (22.4) | −24.6 | −32.3 – -16.8* |
| Injury Prevention | 30.5 (19.7) | 38.9 (28.8) | −8.3 | −18.9–2.27 |
| Enjoyment | 31.0 (22.7) | 23.3 (15.2) | 7.7 | −0.2–15.6 |
| Social Support | 32.1 (23.8) | 24.7 (12.6) | 7.4 | −2.1–17.0 |
| Self-Rewards | 33.0 (19.9) | 10.3 (4.7) | 22.6 | 15.7–30.0* |
| Relapse Prevention | 20.4 (14.5) | 23.1 (16.0) | −2.7 | −10.2–4.9 |
p < .05
Conclusions
These results demonstrate that peer mentors can successfully promote the initial adoption and 12-month maintenance of leisure-time moderate to more vigorous intensity physical activity among underactive midlife and older adults. Compared to trained professional staff, the volunteer mentors were at least as (if not more) effective in encouraging meaningful increases in physical activity among their assigned participants over one year. The peer mentors were also successful in maintaining comparable program delivery fidelity relative to the paid program staff. This result is important when considering translation and dissemination of evidence-based research. The fact that program fidelity was not compromised when non-professionals delivered the program is an encouraging sign that research-based interventions can be delivered through alternate, potentially lower-cost sources while maintaining program quality and effectiveness.
We attribute the success of the peer mentor arm to at least two things: (1) the reasonably adaptable nature of the Active Choices program; and (2) the strong pool of peer mentors utilized in this study. While experience with health counseling or facilitation are assets in using the Active Choices program, it is a program that lends itself to a variety of community settings because it does not demand high levels of specialized education or experience (Griffin et al, 2010; Hooker et al., 2005; Wilcox et al., 2008). Good “people skills” such as listening and communication may be the most important attributes of the telephone advisor that facilitate physical activity changes (Castro & King, 2002). The mentors involved in this project were also given the greatest potential for success through careful selection, training, and support throughout the trial. The peer mentors involved in the TEAM study were already reasonably physically active and committed to the activities required by the study. They were chosen for these reasons to ensure the trial was staffed with high quality mentors, but the authors recognize that this type of volunteer pool may not be easily available in some community settings. Good mentors may be harder to locate and require additional investment to train and support, but as this study found, this investment of resources can reap great rewards in terms of volunteer retention, program fidelity, and ultimately, program efficacy. Of relevance to this issue, dissemination studies in this area that have used community volunteers and professional staff to deliver Active Choices programs have reported increases in physical activity levels of similar magnitude to those reported in this investigation (Hooker et al., 2005; Wilcox et al., 2008).
While the outcomes of this trial were positive, some limitations to the research should be considered. The self-report CHAMPS outcome measure, while reliable and validated, carries the risk of reporting errors that may result in inflated estimates of physical activity (Hekler, et al., 2009). However, in this trial we were able to verify the self-report outcome with objective accelerometry data and found the measures to be well correlated at levels comparable to other self-report measures and objective actigraphy (Pruitt et al, 2009). This type of verification against objective measures of activity combined with targeting a specified portion of the physical activity intensity range increase our confidence that the reported values from the CHAMPS are good relative indicators of leisure-time physical activity.
The success of the physical activity interventions may have also been enhanced by the message to attention-control participants to maintain their usual pattern of physical activity. A comparable statement was made to the physical activity conditions (i.e., maintain your usual nutrition patterns). While this one-time statement may have influenced the natural patterns of behavior change that may have occurred, it was important to discourage participants from seeking help from their advisor for other behaviors to prevent contamination of their assigned intervention. Despite the message, the attention-control condition made modest increases in PA, which suggest that the “maintain your usual habits” message did not restrict participant’s attempts at behavior changes on their own.
As mentioned previously, the trial also benefited from the dedicated involvement of a select group of peer mentors. While our peer mentor sample was demographically representative of the geographic area where the study was conducted, they were generally highly educated and experienced with physical activity. However, other volunteer models, such as the Experience Corps model (Martinez, et al., 2006), show that older adults from a variety of backgrounds and settings possess life experiences that can be harnessed and applied successfully to new roles, including volunteer roles (Martinez, et al., 2006). Many of the TEAM peer mentors reported that they were drawn to volunteer for the project because it was aligned with their personal values of healthy and active living, and it provided an opportunity to learn new skills and share experiences. Approaching potential volunteers with this type of message may carry substantial weight for attracting new volunteers for community based health promotion efforts.
Based on the experience of TEAM and current evidence supporting similar telephone-based physical activity interventions (Eakin, Lawler, Vandelanotte & Owen, 2007), further translation and dissemination efforts for this type of program are indicated. Cost analysis of peer vs. professional delivery programs is a fruitful next target for investigation. While both delivery mechanisms can produce similar outcomes, data on the potential resource investments and savings to be gained (both in terms of financial and health-related benefits) would be valuable to help guide organizations and program planners in the decision-making about program feasibility and capacity. As this program expands in dissemination, the flexible approach also creates opportunities to adapt it to other important physical activity goals. More recent data underscores the importance of cardiorespiratory fitness, not just physical activity per se (Lee, et al., 2010) in the reduction of all-cause mortality. Thus, the advice and support of an Active Choices advisor could target more vigorous intensity activity to specifically improve cardiovascular fitness. Likewise, emerging research has shown the increased risks of sedentary activity, particularly time spent sitting (Patel, et al., 2010). Reductions in sitting and sedentary time are other feasible and worthwhile outcomes that could be tested with the Active Choices intervention.
The data from the TEAM trial should encourage those already using telephone-based programs to consider involving volunteers in program delivery, as long as they are carefully selected and consistently trained, supported, and supervised. Given that a manualized program is available for Active Choices, along with a supplementary training guide for working with Peer Mentors, a “train-the-trainer” model could be implemented to facilitate such dissemination efforts, as was done in the Robert Wood Johnson Foundation Active for Life Initiative (Wilcox et al., 2008). With continued refinement, these programs can provide an accessible dissemination strategy for more segments of the inactive population, and provide midlife and older adults with an avenue to do community volunteer service to enrich their own lives and those whom they mentor.
Figure 2. Between-group differences in CHAMPS MVPA† at baseline, 6, and 12 months.
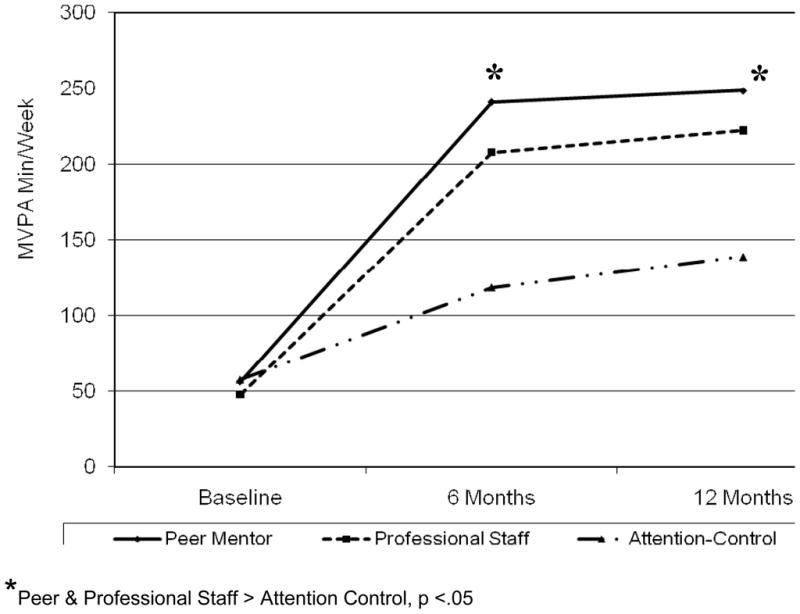
† Based on intent-to-treat data; MVPA = Leisure-time moderate or more vigorous intensity physical activity
Acknowledgments
The research was supported by Public Health Service Grant HL072489 from the National Heart Lung and Blood Institute.
The authors thank Sarah French for coordinating the peer mentor volunteers, Catherine Cassayre, Julia Wu, Arturo Fernandez, and Suzanne Plank for implementing the staff intervention, Ms. Carolyn Prosak for her assistance with developing the nutrition comparison arm, and Dr. David Ahn for his assistance with statistical analysis. We are grateful to Louise Doying, Iby Heller, Sylvia Hughes, Ruthanne Lowe, Esther Ludena, Clare Smith, Gayle Snyder, Garry Thomas and the late Susan Musick for their tireless volunteer work as peer mentors.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/HEA
Contributor Information
Cynthia M. Castro, Stanford Prevention Research Center, Stanford University School of Medicine
Leslie A. Pruitt, Stanford Prevention Research Center, Stanford University School of Medicine
Matthew P. Buman, Stanford Prevention Research Center, Stanford University School of Medicine
Abby C. King, Departments of Health Research & Policy and Medicine, Stanford University School of Medicine
References
- Bandura A. Health promotion from the perspective of social cognitive theory. Psychology and Health. 1998;13(4):623–649. [Google Scholar]
- Batik O, Phelan EA, Walwick JA, Wang G, LoGerfo JP. Translating a community-based, motivational support program to increase physical activity among older adults with diabetes at community clinics: a pilot study of Physical Activity for a Lifetime of Success (PALS) Preventing Chronic Disease. 2008;5(1) Retrieved from http://www.cdc.gov/pcd/issues/2008/jan/07_0142.htm. [PMC free article] [PubMed]
- Bonk CJ. Extending sociocultural theory to adult learning. In: Smith MC, Pourchot T, editors. Adult learning and development: Perspectives from Educational Psychology. Mahwah, NJ: Lawrence Erlbaum and Associates; 1998. [Google Scholar]
- Castro CM, King AC. Telephone-assisted counseling for physical activity. Exercise and Sport Sciences Reviews. 2002;30(2):64–68. doi: 10.1097/00003677-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Chambre SM. Volunteerism by elders: past trends and future prospects. The Gerontologist. 1993;33(2):221–228. doi: 10.1093/geront/33.2.221. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Dell DM, Lewis KN, Schmidt LD. Counseling as a social influence process: a review. Journal of Counseling Psychology Monograph. 1980;27:395–441. [Google Scholar]
- Derose KP, Fox SA, Reigadas E, Hawes-Dawson J. Church-based telephone mammography counseling with peer counselors. Journal of Health Communication. 2000;5:175–88. doi: 10.1080/108107300406884. [DOI] [PubMed] [Google Scholar]
- Dorgo S, King GA, Brickey GD. The application of peer mentoring to improve fitness in older adults. Journal of Aging and Physical Activity. 2009;17:344–361. doi: 10.1123/japa.17.3.344. [DOI] [PubMed] [Google Scholar]
- Dorgo S, Robinson KM, Bader J. The effectiveness of a peer-mentored older adult fitness program on perceived physical, mental, and social function. Journal of the American Academy of Nurse Practitioners. 2009;21(2):116–122. doi: 10.1111/j.1745-7599.2008.00393.x. [DOI] [PubMed] [Google Scholar]
- Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. American Journal of Preventive Medicine. 2007;32(5):419–434. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- Etkin CD, Prohaska TR, Harris BA, Latham N, Jette A. Feasibility of implementing the Strong for Life program in community settings. The Gerontologist. 2006;46(2):284–292. doi: 10.1093/geront/46.2.284. [DOI] [PubMed] [Google Scholar]
- Griffin SF, Wilcox S, Ory MG, Lattimore D, Leviton L, Castro C, Carpenter RA, Rheaume C. Results from the Active for Life Process Evaluation: Program Delivery Fidelity and Adaptations. Health Education Research. 2010;25(2):325–342. doi: 10.1093/her/cyp017. [DOI] [PubMed] [Google Scholar]
- Guide to Community Preventive Services. Behavioral and social approaches to increase physical activity: individually-adapted health behavior change programs. Retrieved from http://www.thecommunityguide.org/pa/behavioral-social/individuallyadapted.html.
- Hekler EB, Buman M, Haskell W, Sallis JF, Frank L, Saelens BE, King AC. Descriptive Analysis of the CHAMPS physical activity questionnaire within a large sample of older adults. Poster session presented at the annual meeting of the Society of Behavioral Medicine; Montreal, Quebec, Canada. 2009. Apr, [Google Scholar]
- Hooker SP, Seavey W, Weidmer CE, Harvey DJ, Stewart AL, Gillis DE, Nicholl KL, King AC. The California active aging community grant program: translating science into practice to promote physical activity in older adults. Annals of Behavioral Medicine. 2005;29(3):155–65. doi: 10.1207/s15324796abm2903_1. [DOI] [PubMed] [Google Scholar]
- King AC, Friedman RH, Marcus B, Castro C, Napolitano M, Ahn D, Baker L. Ongoing physical activity advice by humans versus computers: The Community Health Advice by Telephone (CHAT) Trial. Health Psychology. 2007;26(6):718–727. doi: 10.1037/0278-6133.26.6.718. [DOI] [PubMed] [Google Scholar]
- King AC, Baumann K, O'Sullivan P, Wilcox S, Castro C. Effects of moderate-intensity exercise on physiological, behavioral, and emotional responses to family caregiving: A randomized controlled trial. Journal of Gerontology: Biological Sciences & Medical Sciences. 2002;57:M26–36. doi: 10.1093/gerona/57.1.M26. [DOI] [PubMed] [Google Scholar]
- King AC, Haskell WL, Taylor CB, Kraemer HC, DeBusk RF. Group- vs. home-based exercise training in healthy older men and women: A community-based clinical trial. JAMA. 1991;266(11):1535–1542. [PubMed] [Google Scholar]
- Krieger JW, Castorina JS, Walls ML, Weaver MR, Ciske S. Increasing influenza and pneumococcal immunization rates: A randomized controlled study of a senior center-based intervention. American Journal of Preventive Medicine. 2000;18(2):123–31. doi: 10.1016/s0749-3797(99)00134-8. [DOI] [PubMed] [Google Scholar]
- Lee DC, Sui X, Ortega FB, Kim YS, Church TS, Winett RA, Ekelund U, Katzmarzyk PT, Blair SN. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. British Journal of Sports Medicine. 2010 doi: 10.1136/bjsm.2009.066209. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Rossi JS, Selby VC, Niaura RS, Abrams DB. The stages and processes of exercise adoption and maintenance in a worksite sample. Health Psychology. 1992;11(6):386–395. doi: 10.1037/0278-6133.11.6.386. [DOI] [PubMed] [Google Scholar]
- Martinez IL, Frick K, Glass TA, Carlson M, Tanner E, Ricks M, Fried LP. ngaging Older Adults in High Impact Volunteering that Enhances Health: Recruitment and Retention in the Experience Corps\Baltimore. Journal of Urban Health. 2006;83(5):941–953. doi: 10.1007/s11524-006-9058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. DHHS Publication No 2009–1232. Hyattsville, MD: U.S. Department of Health and Human Services; 2009. Health, United States, 2008 with Chartbook. [Google Scholar]
- Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Medicine & Science in Sports & Exercise. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- Patel AV, Bernstein L, Deka A, Feigelson HS, Campbell PT, Gapstur SM, Colditz GA, Thun MJ. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. American Journal of Epidemiology. 2010;172(4):419–429. doi: 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Report of the Physical Activity Guidelines Advisory Committee. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- Prochaska JO, Marcus BH. The transtheoretical model: Applications to exercise. In: Dishman RK, editor. Advances in Exercise Adherence. Champaign, IL: Human Kinetics; 1994. [Google Scholar]
- Pruitt LA, Glynn NW, King AC, Guralnik JM, Aiken EK, Miller G, Haskell WL. Use of accelerometry to measure physical activity in older adults at risk for mobility disability. Journal of Aging and Physical Activity. 2008;16(4):416–434. doi: 10.1123/japa.16.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, Howard KI, Vessey JT. Using significance tests to evaluate equivalence between two experimental groups. Psychological Bulletin. 1993;113(3):553–565. doi: 10.1037/0033–2909.113.3.553. [DOI] [PubMed] [Google Scholar]
- Sevick MA, Napolitano MA, Papandonatos GD, Gordon AJ, Reiser LM, Marcus BH. Cost-effectiveness of alternative approaches for motivating activity in sedentary adults: Results of Project STRIDE. Preventive Medicine. 2007;45(1):54–61. doi: 10.1016/j.ypmed.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford Health Promotion Resource Center. The Stanford Active Choices Program: Telephone-Assisted Counseling for Physical Activity. Stanford, CA: Stanford Health Promotion Resource Center; 2002. [Google Scholar]
- Steele RM, Mummery WK, Dwyer T. A comparison of face-to-face or internet-delivered physical activity intervention on targeted determinants. Health Education & Behavior. 2009;36:1051–1064. doi: 10.1177/1090198109335802. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine & Science in Sports & Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Weinrich SP, Weinrich MC, Stromborg MF, Boyd M, Weiss HL. Using elderly educators to increase colorectal cancer screening. The Gerontologist. 1993;33(4):491–496. doi: 10.1093/geront/33.4.491. [DOI] [PubMed] [Google Scholar]
- Wilcox S, Dowda M, Leviton LC, Bartlett-Prescott J, Bazzarre T, Campbell-Voytal K, Carpenter RC, Castro CM, Dowdy D, Dunn AL, Griffin SF, Guerra M, King AC, Ory MG, Rheaume C, Tobnick J, Wegley S. Active for Life: Final Results from the Translation of Two Physical Activity Programs. American Journal of Preventive Medicine. 2008;35(4):340–351. doi: 10.1016/j.amepre.2008.07.001. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2006–2008 American Community Survey, Santa Clara County and San Mateo County Custom Table. 2009 Retrieved from http://factfinder.census.gov.