Abstract
Acetonitrile is a choice of solvent for almost all chromatographic separations. In recent years, researchers around the globe have faced an acetonitrile shortage that affected routine analytical operations. Researchers have tried to counter this shortage by applying many innovative solutions, including using ultra performance liquid chromatography (UPLC) columns that are shorter and smaller in diameter than traditional high performance liquid chromatography (HPLC) columns, thus significantly decreasing the volume of eluent required. Although utilizing UPLC in place of HPLC can alleviate the solvent demand to some extent, acetonitrile is generally thought of as the solvent of choice due to its versatility. In the following communication, we describe an alternative eluent system that uses isopropanol in place of acetonitrile as an organic modifier for routine chromatographic separations. We report here the development of an isopropanol based UPLC protocol for G5 PAMAM dendrimer based conjugates that was transferred to semi-preparative applications.
The acetonitrile shortage that started in early 2008 posed enormous challenges to research laboratories both in industry and academia. This issue and the reasons associated with the shortage have been well documented and addressed by various personnel in the chromatography field.1 In a very short period of time, acetonitrile went from a few hundred dollars to a few thousand dollars per 16 litres (one case). Acetonitrile has been the choice-solvent for almost all chromatographic separations, due to its low chemical reactivity, high miscibility with water mixtures, low viscosity and low ultraviolet cut-off. Consequently, the substantial and unforeseen increase in price of acetonitrile resulted in a non-trivial financial burden on research labs across the world. Many research groups took innovative approaches to reduce their dependence on the now rationed acetonitrile, including investing in the newly developed UPLC. With a lower solvent requirement and faster analysis time the introduction of UPLC to the market could not have come at a better time.
Reverse phase HPLC is one of the major analytical techniques used to characterize, quantify and purify small molecules, proteins, oligonucleotides, macromolecules, etc. Polyamidoamine (PAMAM) dendrimers, a class of macromolecule, have found applications in various fields of drug delivery, imaging and a number of other applications.2-5 In previous efforts, reverse phase HPLC was successfully used to characterize PAMAM dendrimers and their conjugates.6 We have successfully employed the developed HPLC protocol up to a semi-preparative scale to purify functionalized forms of the dendrimer (~50 mg). As acetonitrile was used as an organic modifier in the protocol, we too faced many challenges in accomplishing our daily analytical objectives. In order to counter this challenge, we explored the use of other eluents. The choices for an organic modifier for an alternative eluent system ranged from methanol to tetrahydrofuran (THF). Due to the nature of our polymer based work, and to try and keep the analytical procedure as green as possible, we limited our choice for an organic modifier to either methanol or isopropanol. Both methanol and isopropanol are relatively similar in their physical properties as chromatographic solvents and have been used in past chromatographic eluents. Our major objective for the new elution system was to obtain separation as close to our set acetonitrile conditions that can be successfully scaled up to achieve our purification objectives. As a result, the decision to opt for isopropanol was purely based on the fact that isopropanol has a lower polarity (~50% lower dielectric constant) relative to methanol.7
UPLC, a recent advancement in the field of chromatography, can perform analysis in a matter of a few minutes as compared to the required 35 minutes to complete conventional HPLC analysis.8 Moreover, the UPLC instrument has the capability to withstand pressures up to 15 000 psi, a critical factor since isopropanol and water mixtures tend to generate high pressures. In this communication, we report that we have successfully characterized generation 5 (G5) PAMAM dendrimer and its conjugates using both isopropanol and acetonitrile-based reverse phase HPLC. We report the comparison of the separation and resolution achieved using isopropanol as an eluent relative to that achieved using acetonitrile.
Ethylene Diamine Core (EDA) G5 PAMAM dendrimer is our starting nanoparticle that serves as our drug delivery scaffold. HPLC6 and UPLC9 methods have been developed in order to characterize these nanoparticles. We have successfully transferred our optimized HPLC protocols to a UPLC set-up. In our initial experiments, G5 PAMAM dendrimer and its conjugates were characterized using our previously developed acetonitrile conditions followed by characterizing the same samples on the new eluent system with isopropanol as an organic modifier. Fig. 1 (bottom) shows the UPLC profile for G5 PAMAM dendrimer run under acetonitrile conditions whereas Fig. 1 (top) shows the UPLC profile run under isopropanol conditions. The data obtained under these two different eluent systems indicate the feasibility to use isopropanol as an organic modifier for such reverse phase UPLC analysis. The elution profile is almost identical to the one obtained with acetonitrile.
Fig. 1.
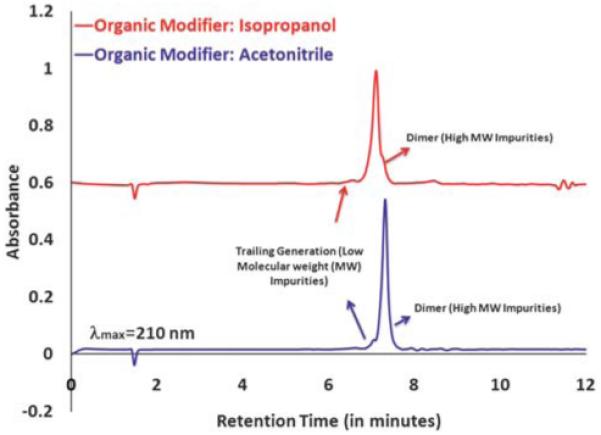
UPLC profile comparison for G5 PAMAM dendrimer ran on two different eluent system; (top) water/isopropanol/trifluoroacetic acid and (bottom) water/acetonitrile/trifluoroacetic acid.
The shoulder seen at around 7.2 minutes under isopropanol conditions, indicative of a dimer population, is not clearly seen when the same sample was run under acetonitrile conditions. This could have possibly resulted due to changes in selectivity factor resulted by different solvent strength at the respective elution times.
One of our analytical objectives is also to isolate and characterize the dendrimers with a precise number of ligands.10,11 This transfer is simple and achievable when acetonitrile is used as an eluent. To ascertain that we can replace acetonitrile with isopropanol as our choice of solvent and achieve similar resolution for such functions, we wanted to make sure that we were able to resolve such a distribution of dendrimer–ligand components in UPLC set-up. To accomplish this objective, we ran a dendrimer that was conjugated with a mean of 0.4 ligands. Dendrimer samples conjugated with 3-(4-(2-azidoethoxy)phenyl)propanoic acid (azide ligand) were run under both isopropanol and acetonitrile conditions. As seen in the UPLC profiles, shown in Fig. 2, we were able to achieve a comparable resolution under these new isopropanol conditions.
Fig. 2.
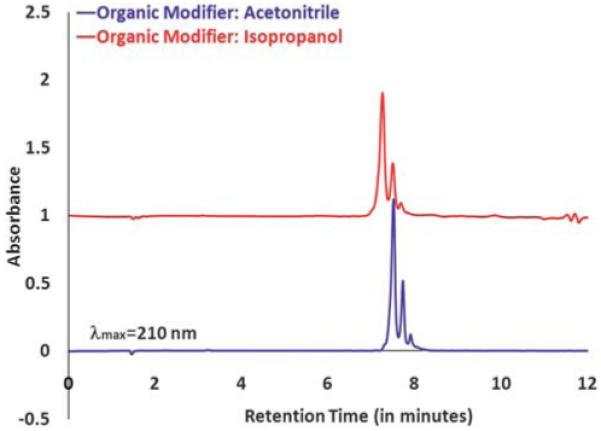
PLC profile for dendrimer samples conjugated azide ligand ran under two different eluent systems.
We also tested the ability to scale up this protocol on a semi-preparative scale. We were successful in scaling up this gradient for a 250 mm long column with a 21.2 mm internal diameter (ID) with certain modifications. Under usual conditions, with acetonitrile as an organic modifier, the flow rate is maintained at 21 ml min−1. The modifications to the protocol were done keeping the pressure limitations with the conventional HPLC systems and high pressures expected from isopropanol/water mixtures. The modified protocol included a flow rate that was adjusted to 10 ml min−1 and the usual broader gradient used for UPLC applications was replaced with a shallow gradient for semi-preparative applications. Under these operating conditions, the pressures were maintained below 3000 psi (data included in the ESI†).
We also tested the stability of the dendrimer in the isopropanol–trifluoroacetic acid eluent, the samples were made in 99/1 water/isopropanol with 0.14% trifluoroacetic acid and incubated for a period of 48 hours at room temperature. The sample was then injected twice at day 0 followed by injections at the end of 48 hours incubation. There was no significant degradation observed for the dendrimer sample over the incubation period. The precision of the developed method was measured as the repeatability on the sample ran twice within a day and running the same sample five times after 2 day incubation. The results obtained confirmed the stability of the sample in the eluent as well as helped us verify that the method is repeatable (data included in the ESI†).
Finally, we carried out characterization of our dendrimer based nanodevice that contains a targeting moiety (folic acid) and a drug (methotrexate) on a functionalized dendrimer surface. Fig. 3 shows the UPLC profile for the surface modified dendrimer conjugated with folic acid and methotrexate. The peaks at retention times 6.347 minutes and 6.719 minutes represent free folic acid and free methotrexate in the nanodevice. Calibration curves for free folic acid and methotrexate were also generated using the same protocol. This allowed us to carry out a quantitative assessment of the nanodevice to ascertain amounts of free folic acid and methotrexate within the conjugate.
Fig. 3.
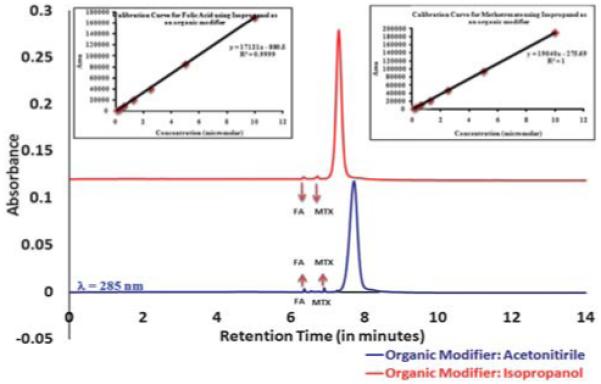
Quantitative assessment for G5 dendrimer based nanodevice. Inset images show calibration curves for free methotrexate and free folic acid using isopropanol as an organic modifier.
The calibration curves for methotrexate and folic acid are shown in the inset. Seven concentration points for the samples were analyzed, and both folic acid and methotrexate showed good linearity in the tested range. The area response obeyed the equation y = mx + C (for folic acid, y = 17121x – 880.5 with R2 = 0.999 for methotrexate y = 19040x – 275.69 with R2 = 1), where the intercept C was zero within 99.99% confidence limits and the square correlation coefficient (R2) was always close to 0.999.
Conclusions
The new eluent system incorporating isopropanol as an organic modifier gives results comparable to those obtained with acetonitrile. We have successfully employed this alternative eluent system for both quantitative assessments as well as for purification up to a semi-preparative scale (~50 mg). The developed method is repeatable and the samples made in the eluent do not degrade over the time scales employed for analysis. We understand that nothing can change the benefits that acetonitrile brings as the choice of solvent in chromatographic experiments. However, we have shown that isopropanol can be used as an alternative eluent system in lieu of acetonitrile and comparable results achieved. These data suggest that further testing of the applicability of this isopropanol eluent system for reverse phase analysis for a wide variety of samples is a worthwhile endeavor. We hope that this brief communication can bring a much needed relief to chromatographers and can open up avenues for further exploration of this eluent system.
Supplementary Material
Acknowledgements
This project has been funded in whole or in part with Federal funds from the Defense Advanced Research Projects Agency—DOD, under award W911NF-07-1-0437 and from the National Cancer Institute, National Institutes of Health, under award 1 R01 CA119409.
Footnotes
Electronic supplementary information (ESI) available: Experimental conditions and additional figures. See DOI: 10.1039/c0ay00493f
References
- 1.Majors RE. LCGC North America. 2009 June 1; [Google Scholar]
- 2.Kukowska-Latallo JF, Candido KA, Cao ZY, Nigavekar SS, Majoros IJ, Thomas TP, Balogh LP, Khan MK, Baker JR. Cancer Res. 2005;65:5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 3.Majoros IJ, Thomas TP, Mehta CB, Baker JR. J. Med. Chem. 2005;48:5892–5899. doi: 10.1021/jm0401863. [DOI] [PubMed] [Google Scholar]
- 4.Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. Eur. J. Pharm. Sci. 2009;38:185–196. doi: 10.1016/j.ejps.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Samad A, Alam MI, Saxena K. Curr. Pharm. Des. 2009;15:2958–2969. doi: 10.2174/138161209789058200. [DOI] [PubMed] [Google Scholar]
- 6.Islam MT, Majoros IJ, Baker JR. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2005;822:21–26. doi: 10.1016/j.jchromb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Li JJ, Thurbide KB. Can. J. Anal. Sci. Spectrosc. 2008;53:59–65. [Google Scholar]
- 8.Yang Y, Hodges CC. LCGC North America. 2005:31–35. [Google Scholar]
- 9.Cason CA, Oehrle SA, Fabre TA, Girten CD, Walters KA, Tomalia DA, Haik KL, Bullen HA. J. Nanomater. 2008 [Google Scholar]
- 10.Mullen DG, Desai AM, Waddell JN, Cheng XM, Kelly CV, McNerny DQ, Majoros IJ, Baker JR, Sander LM, Orr BG, Holl MMB. Bioconjugate Chem. 2008;19:1748–1752. doi: 10.1021/bc8002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullen DG, Fang M, Desai A, Baker JR, Orr BG, Holl MMB. ACS Nano. 2010;4:657–670. doi: 10.1021/nn900999c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


