Abstract
Approximately 15% of patients with Sturge-Weber syndrome have bilateral intracranial involvement and the prognosis of these patients is believed to be particularly unfavorable. We reviewed the clinical and neuroimaging features of patients with Sturge-Weber syndrome and bilateral intracranial involvement. Seizure variables, presence of hemiparesis and degree of developmental impairment at last follow-up were compared to imaging abnormalities. Out of 110 Sturge-Weber syndrome patients studied, 14 had bilateral brain involvement, which always showed an asymmetric pattern on glucose metabolism positron emission tomography. Although most patients had frequent seizures initially, associated with frontal hypometabolism on positron emission tomography, six patients (43%) had achieved good seizure-control during follow-up. Bilateral frontal hypometabolism was associated with severe developmental impairment; two children with bitemporal hypometabolism showed autistic features. Hemiparesis was associated with superior frontal (motor cortex) hypometabolism. Three patients had resective surgery, resulting in improved seizure control and/or developmental outcome. The severity of neurological complications and clinical course depend highly on the extent of cortical dysfunction in bilateral Sturge-Weber syndrome; bilateral frontal and temporal hypometabolism is associated with poor developmental outcome. Nevertheless, good seizure control and only mild/moderate developmental impairment can be achieved in about half of bilateral Sturge-Weber syndrome patients, with or without resective surgery.
Keywords: Sturge-Weber syndrome, positron emission tomography, glucose metabolism, bilateral, neurological complications
INTRODUCTION
Sturge-Weber syndrome is a rare congenital neurocutaneous disorder with an estimated incidence of 1 per 50,000 live births [1]. Sturge-Weber syndrome is characterized by facial port wine stains, glaucoma and intracranial leptomeningeal angiomatosis [2]. The intracranial venous abnormality is thought to be the result of a failure of the primitive vascular plexus at the cephalic end of the neural tube to regress properly during the first trimester in utero [3]. Intracranial angiomatosis is unilateral in the majority of cases, mostly posterior, and does not correlate with the unilateral or bilateral appearance of the facial birthmark [4]. The extent of the angioma as well as the underlying cortical involvement is highly variable in Sturge-Weber syndrome. Similar to the variability in extent of angiomatous involvement, the clinical presentation and disease course in Sturge-Weber syndrome both show a wide range with respect to the presence, onset and intractability of epilepsy, motor dysfunction and neuro-cognitive impairment [5–7]. Importantly, early onset of catastrophic seizures is a bad prognostic factor for cognitive and motor deficit [8]. Previous studies focusing on cases with unilateral brain involvement attempted to find simple imaging correlates and predictors for different neurological outcomes. For instance, a semi-quantitative measure of cortical volume asymmetry correlated well with the overall clinical status [9]. Cognitive function (IQ) was strongly associated with white matter volume loss ipsilateral to the angioma, and also with cortical as well as thalamic glucose hypometabolism on 2-deoxy-2[18F]fluoro-D-glucose positron emission tomography (glucose PET) [10, 11].
Bilateral intracranial involvement, reported in about 15 % of cases, is associated with earlier onset of seizures and worse cognitive development compared to unilateral cases [2, 5]. These patients are generally not considered to be surgical candidates, although epilepsy surgery (focal resection or hemispherectomy) was reported to be beneficial both from seizure and developmental points of view in a few cases [12, 13]. Due to the rarity of Sturge-Weber syndrome with bilateral intracranial involvement, the imaging and clinical characteristics of this subset of patients have not been well characterized. Therefore, the aim of the present study was to examine the relationship between neuroimaging findings (with particular emphasis on GLUCOSE PET, which was available in all patients) and clinical characteristics (seizure severity, motor deficit, developmental outcome) in a relatively large series of patients with bilateral Sturge-Weber syndrome, collected over more than two decades. The relationship between these clinical features and cortical patterns of glucose metabolism was specifically investigated.
MATERIALS AND METHODS
Patients were selected from a PET database which included patients with the clinical diagnosis of Sturge-Weber syndrome. PET studies were performed at one of two institutions: the Division of Nuclear Medicine and Biophysics, University of California at Los Angeles (UCLA) (between 1986 and 1993) or at the PET Center, Children’s Hospital of Michigan (CHM) in Detroit (between 1994 and 2010). There were a total of 110 patients with Sturge-Weber syndrome in the database. Patients with bilateral brain involvement (n=14) were selected based on CT and/or MRI as well as GLUCOSE PET findings. Nine patients had MRI exams; patients evaluated in the late 80s and early 90s did not have MRI. Also, all selected patients had unilateral or bilateral facial port wine stain.
PET scans were performed using either the NeuroECAT positron tomograph (CTI, Knoxville, Tenn.) at UCLA, or the CTI/Siemens EXACT/HR positron tomograph at CHM. Details of the acquisition parameters have been described previously [14, 15]. All patients underwent full neurological evaluation as part of their medical care. The available medical records of the 14 selected patients were reviewed. For each patient, clinical details including age at onset of first seizure(s), type of seizures, effectiveness of seizure control, presence of motor deficit on neurological examination and degree of developmental impairment at the last contact with the patient were noted.
Control of seizures in the past and (if changed) at the time of last follow-up was categorized into 3 groups. Seizure control was considered good if the patient was seizure-free or had clinical seizures less than once per month on average. Moderate seizure control was assigned if the patient had monthly seizures, but not more than 1 seizure/week. If weekly or more frequent seizures occurred, the epilepsy was considered poorly controlled.
The degree of developmental impairment was assessed retrospectively by a neuropsychologist (M.B.) based on available interview data including the parent’s report of the child’s achievement of major developmental milestones, functional/adaptive behavior status in the various domains (communication, daily living, social, and motor skills) and school classification/placement at the last follow-up. Based on the above data, patients were categorized into 3 groups: 1) development and adaptive behavior within normal limits; studying in regular school; 2) mild-moderate developmental delay/impairment; some modifications to academic program, and/or mildly impaired adaptive behavior functioning in at least one domain; 3) severely/profoundly impaired; significantly delayed milestones across domains, classification/placement in special education setting, severely impaired adaptive behavior across domains. Age at onset of first seizures was compared between patients with severe and mild-moderate developmental impairment using independent t-test in PASW Statistics 18 software (SPSS Inc., Chicago, IL). Initial GLUCOSE PET studies were reviewed in each case and the extent and location of glucose metabolic abnormalities were qualitatively correlated with the neurological complications. Relationship between intractable seizures and developmental impairment was also investigated qualitatively.
RESULTS
During the studied period there were a total of 14 Sturge-Weber syndrome patients (12.7 % of the whole series; 9 male; average age at PET scan: 9 years) with bilateral intracranial involvement who underwent GLUCOSE PET scanning at least once. The clinical and imaging findings of these patients are provided in Table 1 and Table 2, respectively. Based on the available MRI reports and images (n=9), signs of intracranial involvement (e.g.: atrophy, vascular abnormalities) were more pronounced in one side of the brain (see Table 2). Concordantly, hemispheric involvement, as assessed by glucose PET, appeared to be asymmetric in all cases, with a predominant (more extensive) abnormality in one of the hemispheres. One entire hemisphere was hypometabolic in three patients. Patient #6 underwent right hemispherectomy at a different institution prior to PET scanning due to intractable seizures; in this case, only the contralateral hemisphere was evaluated. Eight of the 14 patients had long-term follow-up data available after the PET scan, with a follow-up period of 1–16 years (median: 9 years, Table 1).
Table 1.
Clinical data of the patients
| No/Sex | Age at PET scan | Age at last follow-up | Age at onset of first seizures | Type of seizures | Seizure control | Hemiparesis | Developmental impairment |
|---|---|---|---|---|---|---|---|
| 1/M* | 8 m | 1.7 y | 5.5 m | CPS/Gen | Poor | Left | Severe delay |
| 2/M | 1.0 y | 16 y | 6 m | CPS/Gen | Moderate | Left | Severe delay |
| 3/F | 1.7 y | 2.8 y | 3 m | CPS | Poor-Good (VNS) | Right | Severe delay |
| 4/M | 1.8 y | 1.8 y | 1.5 m | SPS, CPS | Poor (VNS) | Left | Severe delay |
| 5/M | 5.4 y | 9.1 y | 7.1 y | CPS | Moderate | None | Mild/moderate |
| 6/F | 6.4 y | 6.5y | n.a. | CPS/Gen | Poor-Good | Left | Mild/moderate delay |
| 7/M | 7.5 y | 7.5 y | 6 m | CPS/SPS | Poor | Right | Mild/moderate delay |
| 8/F | 8.3 y | 8.3 y | 5 m | CPS/SPS/Gen | Poor-Good | None | Severe delay |
| 9/M | 9.8 y | 9.8 y | 2 w | CPS | Poor-Good | Left | Mild/moderate delay |
| 10/M | 10 y | 22 y | 22 m | CPS | Good | None | Mild/moderate delay |
| 11/F* | 11 y | 27 y | 4 y | Drop/CPS/GTCS | Poor-moderate | Right | Severe – Mild/moderate delay |
| 12/F | 15 y | 15 y | n.a. | Drop, P | Poor | Right | Severe delay |
| 13/M | 26 y | 32 y | 18 m | CPS | Moderate | None | Mild/moderate delay |
| 14/M | 21 y | 35 y | 2.5 m | SPS | Good | None | Mild/moderate |
Note: Clinical data including seizure control, presence of hemiparesis and cognitive developmental delay were obtained at last follow-up. Changes (if present) during the disease course are also indicated as initial status given first followed by follow-up status. Patients indicated with asterisk underwent epilepsy surgery following their PET scans. PET: positron emission tomography; M: male; F: female; y: year; m: month; w: week; CPS: complex partial seizures; SPS: simplex partial seizures; Drop: drop attacks; Gen: secondary generalized seizures; GTCS: generalized tonic-clonic seizures; VNS: vagal nerve stimulator; n.a.: not available.
Table 2.
PET (hypometabolism, unless otherwise indicated) and MRI abnormalities of the patients
| No/Sex | Age at PET scan | Place and year of PET scan | Time difference between PET and MRI | Brain glucose PET scan findings | Brain MRI findings | ||
|---|---|---|---|---|---|---|---|
| Right | Left | Atrophy | Angioma | ||||
| 1/M | 8 m | CHM/1997 | 1 y | supF, P, O, supT | infF, mid+infT | Bilateral, generalized | Bilateral, most prominent in both P (right>left), left F, left supT, left supO, both calcarine region (right>left) |
| 2/M | 1.0 y | CHM/19953 | n.a. | P, O, supF, supT | F, infT | n.a. | n.a. |
| 3/F | 1.7 y | CHM/20103 | 1 y | F incr., (P) | T, P, O, (F) | Left T, P, O and right F | Left T, P, O and right F |
| 4/M | 1.8 y | CHM/20093 | 1 y | hemisphere | F, O | Bilateral (right>left) | Bilateral, more extensive on the right |
| 5/M | 5.4 y | CHM/20023 | 1 m | T, P, O | T, P | Bilateral with enlarged ventricles | Bilateral |
| 6/F | 6.4 y | CHM/19943 | n.a. | Hemisphere surgically removed | P, postT, O | n.a. | n.a. |
| 7/M | 7.5 y | CHM/20013 | 2 d | P (SM cortex) | Hemisphere | Left hemisphere > right | No leptomeningeal enhancement Left choroid plexus enlarged (Left P, O, F angioma at 1 y of age) |
| 8/F | 8.3 y | CHM/20103 | 1 d | F, (P), with SM preserved | Hemisphere, with SM preserved | Left hemisphere and right F | Entire left hemisphere and right F |
| 9/M | 9.8 y | CHM/20103 | 1 d | T, P, O, (supF) | T | Right hemisphere (post. quadrant predominance), left T pole | Right post. quadrant, left T |
| 10/M | 10 y | CHM/19953 | 5 y | P, latO | P, O, supT | Moderate in right, moderate-severe in left hemisphere, bilateral ventricular enlargement | Right F, P, O |
| 11/F | 11 y | UCLA/1989 | n.a. | P, O | F, T, O | n.a. | n.a. |
| 12/F | 15 y | UCLA/1989 | n.a. | P, latO | Hemisphere | n.a. | n.a. |
| 13/M | 26 y | UCLA/1991 | 4 y | P, O, infT | P,O, infF | No significant atrophy | Right F, T; smaller ones on left+right |
| 14/M | 21 y | UCLA/1992 | n.a. | T, P, O | (P) | n.a. | n.a. |
Note: PET: positron emission tomography; CHM: Children’s Hospital of Michigan; UCLA: University of California, Los Angeles M: male; F: female; y: year; m: month; d: days; F: frontal; T: temporal; P: parietal; O: occipital; SM: sensorimotor; sup: superior; inf: inferior; mid: middle; lat: lateral; post.: posterior; incr.: increased glucose metabolism; Regions in parentheses indicate mild involvement. n.a.: not available/not applicable.
Seizure characteristics
All patients experienced seizures during the course of their disease, with an onset before the PET scanning in all cases. The age at onset of first seizures was available in 12 patients and ranged from 2 weeks to 7.1 years (median: 6 months). Eight of the 12 patients had their seizure onset within the first 6 months of life. The primary seizure type was partial (mostly complex partial), with occasional secondary generalization. Interestingly, altogether 6 patients (43%) had eventually good seizure control, including four who had frequent seizures initially. None of these patients had total hemispheric hypometabolism on PET (one of these patients underwent hemispherectomy before PET). In addition, patients with poor seizure control (with or without subsequent improvement) always had frontal glucose metabolic abnormality (mostly hypometabolism) on PET.
Hemiparesis and developmental impairment: relationship to frontal hypometabolism
Nine patients (64%) had mild to severe hemiparesis. The GLUCOSE PET scans of all these 9 patients showed hemispheric or extensive multilobar hypometabolism, including superior frontal areas contralateral to the paretic extremities. In contrast, patients with no overt hemiparesis never had total hemispheric hypometabolism, and superior as well as middle frontal regions showed no hypometabolism in these patients. In one patient with bilateral frontal hypometabolism and no hemiparesis (patient #8), hypometabolism included premotor areas but bilateral sensorimotor cortex was well preserved.
All patients showed at least some degree of developmental impairment, which was mild-moderate in 7 patients (50%) initially. PET scans showed total hemispheric functional abnormality in only one of the patients with mild/moderate impairment; the bulk of the frontal lobes were relatively spared on both sides. In contrast, bilateral frontal lobe hypometabolism was always coupled with severe developmental impairment (Figure 1). Patients with severe delay (N=7) showed either unilateral total hemispheric (including frontal lobe) or unilateral/bilateral frontal metabolic abnormality (mostly hypometabolism) in addition to multilobar involvement. None of the patients had severe developmental impairment if good seizure control was maintained during the entire course of their disease. In addition, age at first seizure onset did not differ significantly between patients with severe and mild-moderate developmental impairment (p=0.49).
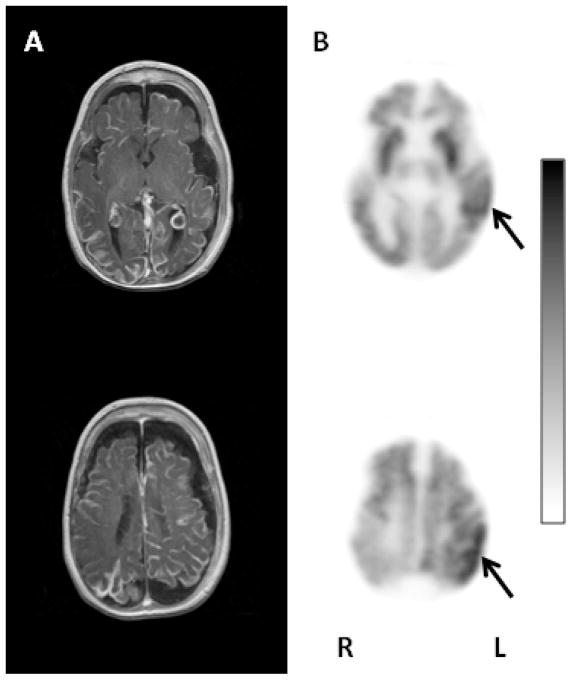
Figure 1.
Representative axial images of the T1 weighted postgadolinium MRI (A) and glucose PET images (B) of patient # 4. This child had poor seizure control, severe developmental impairment and left hemiparesis at the time of the PET scanning. The MRI showed severe bilateral brain atrophy with extensive bihemispheric leptomeningeal angiomatosis and bilateral enlargement of the choroid plexus. The glucose metabolism of the entire right hemisphere was decreased. Additionally, the left frontal and occipital cortex was also hypometabolic, and only the temporal and parietal cortex showed preserved glucose metabolism on the left side (solid arrows). L: left; R: right.
Interestingly, two patients (patient # 2 and #5) developed an autistic behavioral phenotype during the follow-up period. Both patients showed prominent bitemporal (although asymmetric) hypometabolism on PET (Figure 2), in addition to hypometabolism in some other regions (Table 1).
Figure 2.
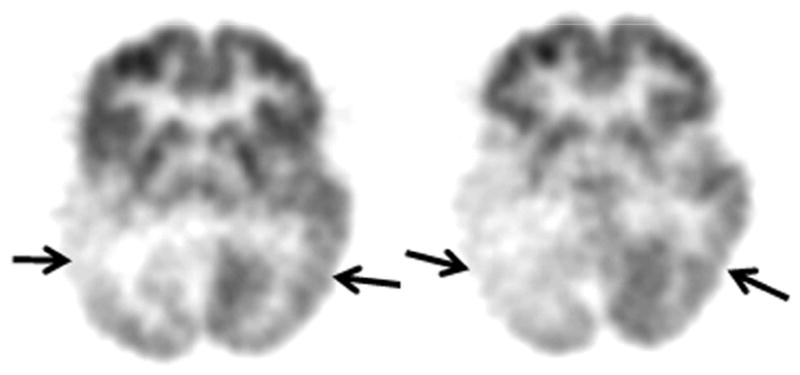
Axial image planes of the glucose PET scan of patient # 5, whose developmental impairment was mild/moderate. However, he developed autistic behavioral features. His PET scan demonstrated bilateral (right more severe than left) temporal hypometabolism (solid arrows) as well as bilateral parietal and right occipital hypometabolism.
Outcome of epilepsy surgery
Three of the 14 patients in this series underwent epilepsy surgery because of refractory seizures. Two of the patients (patients # 1 and #11) had their surgery following GLUCOSE PET scanning. Long term follow up was available in patient # 11 who showed marked improvement in seizure control as well as significant developmental improvement after left hemispherectomy. Although only 5 months follow-up was available in patient # 1, his life threatening seizures ceased after the surgery and he showed some developmental improvement (although he remained severely impaired). One of the patients (patient # 6) underwent right hemispherectomy before PET scanning. Although the surgery resulted in the cessation of her seizures, she remained moderately impaired. Post-surgical PET showed extensive hypometabolism involving portions of the non-resected left occipital, parietal and temporal lobes.
DISCUSSION
This is the first study focusing on neuroimaging correlates of neuro-developmental outcome in a series of patients with bilateral Sturge-Weber syndrome. The findings demonstrate that, although the general clinical outcome is often poor in these patients, there is a significant portion (~50%) of this group who can achieve relatively good seizure control with or without surgery, develop good gross motor function, and, most importantly, escape severe developmental impairment during the course of the disease. Our findings suggest that this relatively good outcome in these patients is likely due to the common asymmetric lobar functional involvement, allowing at least one side of the brain to support neuro-cognitive functions. In contrast, bilateral frontal lobe involvement appears to be a critical imaging marker of both developmental and motor outcome, while bitemporal hypometabolism may be a risk for autistic features. Therefore, functional neuroimaging with PET can be particularly important to provide prognostic information in children with bilateral Sturge-Weber syndrome. In addition, as respective epilepsy surgery can be considered in some cases with bilateral Sturge-Weber syndrome (and has been successfully performed in 3 of 14 patients in our cohort), GLUCOSE PET can also evaluate the functional integrity of the non-resected hemisphere before surgery.
Long-term clinical prognosis varies considerably among patients with Sturge-Weber syndrome [7, 17], and it is challenging even in unilateral Sturge-Weber syndrome to predict long-term outcome during the early course of the disease. Previous studies have, however, shed some light on potential clinical or imaging markers of unfavorable outcome. Early onset of seizures and intractable epilepsy have been shown to be associated with developmental deterioration and the need for special education, rendering epilepsy a crucial factor in developmental impairment [17]. With respect to brain damage, assessed by neuroimaging, degree of cortical atrophy appeared to be strongly associated with the overall clinical severity [9]. In addition, simple clinical markers, such as hemiparesis, may differentiate between patients with and without impaired adaptive functioning, a good indicator of everyday functioning [6]. Notably, most neuroimaging and neuro-psychological studies have focused on the majority of Sturge-Weber syndrome patients who have unilateral intracranial damage.
No studies to date have focused exclusively on the imaging and clinical characteristics of bilateral Sturge-Weber syndrome. However, some clinical and prognostic differences between patients with bilateral and unilateral brain involvement have been outlined. It has been demonstrated that bilateral hemispheric involvement renders patients more susceptible to seizures, and seizure onset may occur earlier in patients with Sturge-Weber syndrome and bilateral leptomeningeal angiomatosis than in those with unilateral lesions [5]. Indeed, lack of epilepsy is very rare within this subset of Sturge-Weber syndrome patients; concordantly, all patients in our series had a history of seizures. The rare absence of seizures in bilateral Sturge-Weber syndrome (and also in cases with unilateral involvement) is likely associated with good intellectual functioning [5]. In contrast, the developmental status and educability of the majority of patients with bilateral cerebral involvement and concomitant seizures varies considerably, but is almost invariably impaired. Our data are in accord with this concept; however, we could not find an obvious relationship between early onset seizures and more severe developmental impairment. Whether this relationship truly exists in bilateral Sturge-Weber syndrome could probably be answered by investigating even larger series in a longitudinal setting (which would be difficult to obtain unless a broad, multi-center study is performed). Nevertheless, in a large study of patients with Sturge-Weber syndrome (including uni- and bilateral cases) mental retardation and the requirement of special education showed a decreasing tendency with increasing age at seizure onset [18]. In addition, earlier PET studies suggested that chronic seizure activity plays a role in progression of cortical metabolic abnormalities in children with epilepsy (related or not related to Sturge-Weber syndrome) [19, 20].
Previous studies indicated that more extensive brain damage is associated with more severe neuro-cognitive deficit in patients with Sturge-Weber syndrome. The extent of cortical damage, thalamic hypometabolism, as well as white matter loss ipsilateral to the angioma were strong predictors of impaired cognitive function in unilateral Sturge-Weber syndrome [10, 11, 15]. In addition, our recent results of an objective voxel-wise analysis identified ipsilateral prefrontal white matter areas with anisotropy values correlating with full-scale IQ of patients with unilateral angiomatosis [21]. Importantly, severe early damage of one hemisphere may facilitate functional reorganization to the intact contralateral hemisphere [15]. In case of bilateral intracranial involvement this process is most likely significantly hindered, which explains the worse overall clinical picture in these patients. Yet, most patients with one frontal lobe showing good metabolic activity can avoid severe developmental impairment, suggesting that at least partial reorganization of critical cognitive functions can take place in these children. Another interesting finding underlining the deleterious effect of bilateral homotopic functional damage of the brain in Sturge-Weber syndrome is that two patients with bitemporal involvement developed autism. Concordantly, previous studies of childhood autism demonstrated bilateral dysfunction of temporal regions involved in language and social perception [22]. In addition, functional impairment of medial as well as lateral temporal regions, along with other brain regions, has been implicated in a subgroup of children with facial port-wine stain and autism, but without intracranial angioma [23].
Our finding that cortical hypometabolism involves superior frontal areas or even the whole hemisphere in patient with some degree of hemiparesis is concordant with a recent study, showing more diffuse cortical injury, extending to frontal areas, in hemiparetic children, regardless of the uni- or bilateral localization of the angioma [6]. That study suggested that hemiparesis may be a simple marker of adaptive functioning while indicating frontal lobe involvement. Based on our data, however, it is important to note that preserved function of the sensorimotor cortex (even in the presence of bi-frontal damage) could result in normal gross motor functions. Nevertheless, more anterior frontal injury may account for fine motor dysfunction [21].
Although our limited MRI data (in some instances reports from many years apart) preclude any definitive statement about clinical correlates of MRI abnormalities, it is clear that MRI scans including post-contrast sequences are capable of identifying bilateral structural brain damage in Sturge-Weber syndrome. As shown in Table 2, MRI-depicted abnormalities (volume loss and/or angioma), similar to PET abnormalities, were almost invariably more severe in one of the hemispheres. Since many of the available MRI scans were performed far in time from the PET scans, exact concordance or mismatch between these modalities need to be addressed in future studies using co-registration. Nevertheless, an earlier study showed that areas of glucose hypometabolism extend beyond CT-depicted abnormalities in patients with Sturge-Weber syndrome [14]. In the current series, we had three patients with MRI and PET performed within days (patients #7–9). In all 3 cases, PET provided additional information to MRI: in patient #7, no leptomeningeal enhancement was detected, despite bilateral atrophy; PET showed very focal hypometabolism on the right side indicating functional integrity of much of that hemisphere. In patient #8, PET demonstrated preservation of the sensory-motor cortex, while in patient #9, temporal lobe hypometabolism extended beyond temporal pole atrophy seen on MRI.
As opposed to the traditional concept that a bihemispheric disease precludes epilepsy surgery in Sturge-Weber syndrome, successful hemispherectomy or focal resection with good long-term seizure control and improved development have been reported in a few cases [12, 13]. Our experience, although limited, also supports the importance of considering epilepsy surgery even in bilateral Sturge-Weber syndrome cases with intractable seizures. As data support that persistence of devastating seizures contributes to neuro-cognitive decline [24, 25], early removal of the epileptic focus may be beneficial with respect to seizure control as well as neuro-cognitive development. Since brain involvement is typically asymmetric in these patients, GLUCOSE PET may play a critical role to evaluate the functional integrity of the unresected hemisphere.
Some limitations of the present study have to be noted. Firstly, the distinction between unilateral and bilateral intracranial involvement is often not unambiguous. Advanced neuroimaging techniques may depict brain abnormalities missed by other modalities [26, 27]. Importantly, we included only those patients in this study whose bilateral brain damage was confirmed by PET and another imaging method (CT and/or MRI). Since cases with Sturge-Weber syndrome and bilateral intracranial involvement are very rare, a reasonable number of patients could be collected only over a long period (24 years). Thus, it was not possible to obtain more formal and uniform quantitative neuro-psychological data in all cases, and we were only able to qualitatively correlate cortical glucose metabolic abnormalities with rather gross categories of developmental impairment as well as seizure control. Importantly, the frequency, type and severity of seizures as well as occurrence of stroke-like episodes (which were not systematically analyzed in this study) may also contribute to the variable outcome of patients with bilateral Sturge-Weber syndrome. In addition, the broad age range of the studied patients and the lack of long-term outcome data in some cases were further limitations in our study.
Acknowledgments
This study was supported partially by a grant from the National Institutes of Health (R01 NS041922 to C.J.). We also thank the Sturge-Weber Foundation for referring patients to us. We are grateful to the families and children who participated in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas-Sohl KA, Vaslow DF, Maria BL. Sturge-Weber syndrome: a review. Pediatr Neurol. 2004;30:303–10. doi: 10.1016/j.pediatrneurol.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Bodensteiner JB, Roach ES. Overview of Sturge-Weber syndrome. In: Roach E, Bodensteiner J, editors. Sturge-Weber syndrome. 2. The Sturge-Weber Foundation; Mt. Freedom, NJ: 2010. pp. 19–32. [Google Scholar]
- 3.Comi AM. Pathophysiology of Sturge-Weber syndrome. J Child Neurol. 2003;18:509–16. doi: 10.1177/08830738030180080701. [DOI] [PubMed] [Google Scholar]
- 4.Pascual-Castroviejo I, Diaz-Gonzalez C, Garcia-Melian RM, Gonzalez-Casado I, Munoz-Hiraldo E. Sturge-Weber syndrome: study of 40 patients. Pediatr Neurol. 1993;9:283–8. doi: 10.1016/0887-8994(93)90064-j. [DOI] [PubMed] [Google Scholar]
- 5.Bebin EM, Gomez MR. Prognosis in Sturge-Weber disease: comparison of unihemispheric and bihemispheric involvement. J Child Neurol. 1988;3:181–4. doi: 10.1177/088307388800300306. [DOI] [PubMed] [Google Scholar]
- 6.Reesman J, Gray R, Suskauer SJ, Ferenc LM, Kossoff EH, Lin DD, Turin E, Comi AM, Brice PJ, Zabel TA. Hemiparesis is a clinical correlate of general adaptive dysfunction in children and adolescents with Sturge-Weber syndrome. J Child Neurol. 2009;24:701–8. doi: 10.1177/0883073808329529. [DOI] [PubMed] [Google Scholar]
- 7.Zabel TA, Reesman J, Wodka EL, Gray R, Suskauer SJ, Turin E, Ferenc LM, Lin DD, Kossoff EH, Comi AM. Neuropsychological features and risk factors in children with Sturge-Weber syndrome: four case reports. Clin Neuropsychol. 2010;24:841–59. doi: 10.1080/13854046.2010.485133. [DOI] [PubMed] [Google Scholar]
- 8.Maton B, Krsek P, Jayakar P, Resnick T, Koehn M, Morrison G, Ragheb J, Castellano-Sanchez A, Duchowny M. Medically intractable epilepsy in Sturge-Weber syndrome is associated with cortical malformation: implications for surgical therapy. Epilepsia. 2010;51:257–67. doi: 10.1111/j.1528-1167.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 9.Kelley TM, Hatfield LA, Lin DD, Comi AM. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. J Child Neurol. 2005;20:867–70. doi: 10.1177/08830738050200110201. [DOI] [PubMed] [Google Scholar]
- 10.Juhasz C, Lai C, Behen ME, Muzik O, Helder EJ, Chugani DC, Chugani HT. White matter volume as a major predictor of cognitive function in Sturge-Weber syndrome. Arch Neurol. 2007;64:1169–74. doi: 10.1001/archneur.64.8.1169. [DOI] [PubMed] [Google Scholar]
- 11.Alkonyi B, Chugani HT, Behen M, Halverson S, Helder E, Makki MI, Juhasz C. The role of the thalamus in neuro-cognitive dysfunction in early unilateral hemispheric injury: A multimodality imaging study of children with Sturge-Weber syndrome. Eur J Paediatr Neurol. 2010;14:425–33. doi: 10.1016/j.ejpn.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bye AM, Matheson JM, Mackenzie RA. Epilepsy surgery in Sturge-Weber syndrome. Aust Paediatr J. 1989;25:103–5. doi: 10.1111/j.1440-1754.1989.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 13.Tuxhorn IE, Pannek HW. Epilepsy surgery in bilateral Sturge-Weber syndrome. Pediatr Neurol. 2002;26:394–7. doi: 10.1016/s0887-8994(01)00414-3. [DOI] [PubMed] [Google Scholar]
- 14.Chugani HT, Mazziotta JC, Phelps ME. Sturge-Weber syndrome: a study of cerebral glucose utilization with positron emission tomography. J Pediatr. 1989;114:244–53. doi: 10.1016/s0022-3476(89)80790-5. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, Asano E, Muzik O, Chugani DC, Juhasz C, Pfund Z, Philip S, Behen M, Chugani HT. Sturge-Weber syndrome: correlation between clinical course and FDG PET findings. Neurology. 2001;57:189–95. doi: 10.1212/wnl.57.2.189. [DOI] [PubMed] [Google Scholar]
- 16.Sparrow SS, Cicchetti DV, Balla DA. Vineland-II Vineland Adaptive Behavior Scales. 2. AGS Publishing; Circle Pines, MN: 2005. [Google Scholar]
- 17.Sujansky E, Conradi S. Outcome of Sturge-Weber syndrome in 52 adults. Am J Med Genet. 1995;57:35–45. doi: 10.1002/ajmg.1320570110. [DOI] [PubMed] [Google Scholar]
- 18.Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49–58. doi: 10.1177/088307389501000113. [DOI] [PubMed] [Google Scholar]
- 19.Benedek K, Juhasz C, Chugani DC, Muzik O, Chugani HT. Longitudinal changes in cortical glucose hypometabolism in children with intractable epilepsy. J Child Neurol. 2006;21:26–31. doi: 10.1177/08830738060210011101. [DOI] [PubMed] [Google Scholar]
- 20.Juhasz C, Batista CE, Chugani DC, Muzik O, Chugani HT. Evolution of cortical metabolic abnormalities and their clinical correlates in Sturge-Weber syndrome. Eur J Paediatr Neurol. 2007;11:277–84. doi: 10.1016/j.ejpn.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkonyi B, Govindan RM, Chugani HT, Behen ME, Jeong J. Focal white matter abnormalities related to neurocognitive dysfunction: An objective diffusion tensor imaging study of children with Sturge-Weber syndrome. Pediatr Res. 2010;69:74–9. doi: 10.1203/PDR.0b013e3181fcb285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boddaert N, Zilbovicius M. Functional neuroimaging and childhood autism. Pediatr Radiol. 2002;32:1–7. doi: 10.1007/s00247-001-0570-x. [DOI] [PubMed] [Google Scholar]
- 23.Chugani HT, Juhasz C, Behen ME, Ondersma R, Muzik O. Autism with facial port-wine stain: a new syndrome? Pediatr Neurol. 2007;37:192–9. doi: 10.1016/j.pediatrneurol.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PJ, Duncan JS. Cognitive decline in severe intractable epilepsy. Epilepsia. 2005;46:1780–7. doi: 10.1111/j.1528-1167.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 25.Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. 2010;14:146–9. doi: 10.1016/j.ejpn.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Yu Y, Juhasz C, Kou Z, Xuan Y, Latif Z, Kudo K, Chugani HT, Haacke EM. MR susceptibility weighted imaging (SWI) complements conventional contrast enhanced T1 weighted MRI in characterizing brain abnormalities of Sturge-Weber Syndrome. J Magn Reson Imaging. 2008;28:300–7. doi: 10.1002/jmri.21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juhasz C, Chugani HT. An almost missed leptomeningeal angioma in Sturge-Weber syndrome. Neurology. 2007;68:243. doi: 10.1212/01.wnl.0000242581.43024.0a. [DOI] [PubMed] [Google Scholar]