Abstract
Epigenetic inactivation of tumor suppressor genes is common in human cancer. Using a large-scale whole-genome approach in an earlier study, the authors identified epigenetically silenced genes with potential tumor suppressor function in glioblastoma (GBM). Three genes identified in this analysis—DKK1, SFRP1, and WIF1—are potent inhibitors of the Wnt signal transduction pathway. Here, the authors confirm decreased expression of these genes in GBM tumor tissue samples relative to nontumor brain tissue samples using real-time PCR. They then show that expression of all 3 genes is restored in T98 GBM cells by treatment with the histone deacetylase inhibitor Trichostatin A (TSA), but only DKK1 expression is restored by treatment with the demethylating agent 5-azacytidine. Bisulfite sequencing did not reveal significant methylation in the promoter region of DKK1, whereas histone acetylation and chromatin accessibility increased significantly for all 3 genes after TSA treatment. Ectopic expression of DKK1 significantly reduces colony formation and increases chemotherapy-induced apoptosis in T98 cells. Ectopic expression of the canonical Wnt pathway inhibitors WIF1 and SFRP1 shows a relative lack of response. Chronic Wnt3a stimulation only partially reverses growth suppression after DKK1 reexpression, whereas a specific inhibitor of the JNK pathway significantly reverses the effect of DKK1 reexpression on colony formation and apoptosis in T98 cells. These results support a potential growth-suppressive function for epigenetically silenced DKK1 in GBM and suggest that DKK1 restoration could modulate Wnt signaling through both canonical and noncanonical pathways.
Keywords: epigenetics, histone modifications, methylation, glioblastoma
Introduction
Glioblastoma multiforme (GBM), the most malignant form of brain cancer, remains incurable and rapidly fatal despite multimodality therapies, including surgery, chemotherapy, and radiation treatment. We previously described a large-scale whole-genome approach for the identification of epigenetically silenced genes with potential tumor suppressor function in GBM.1 Three genes identified through this approach—Dickkopf-1 (DKK1), secreted frizzled-related protein 1 (SFRP1), and Wnt inhibitory factor-1 (WIF1)—are potent inhibitors of the Wnt signal transduction pathway in normal development and a variety of human cancers.2,3 Microarray analysis revealed that the expression of DKK1, SFRP1, and WIF1 increased significantly in immortalized and primary GBM cell lines after treatment with the histone deacetylase (HDAC) inhibitor Trichostatin A (TSA). The epigenetic inactivation of extracellular inhibitors of the Wnt pathway, including DKK1, SFRP1, and WIF1, has been shown to correlate with progression in a number of human cancers, including colorectal, cervical, prostate, and breast cancer.4-10 Little is known about the incidence or functional consequence of epigenetically silenced WNT pathway inhibitors in GBM.
The Wnt signaling pathway plays a central role in cell fate determination and is closely linked to oncogenesis, primarily through alterations in key components that lead to the loss of β-catenin regulation.2,3 The binding of secreted Wnt glycoproteins to frizzled (Fz) transmembrane receptors activates both canonical and noncanonical Wnt pathways.11 Activation of the canonical pathway results in the stabilization and accumulation of cytoplasmic β-catenin through the inhibition of glycogen synthetase-3 (GSK-3), an important component of the activated destruction complex that normally phosphorylates β-catenin and leads to its rapid degradation. Increased levels of cytoplasmic β-catenin result in its translocation to the nucleus, where it binds to T cell factor/lymphocyte enhancer factor (TCF/LEF) transcription factors. This results in the expression of multiple Wnt target genes important for cell proliferation and differentiation, including c-myc, c-jun, and cyclin D1. Wnt signaling is inhibited by extracellular secreted antagonists that block Wnt-Fz binding by two distinct mechanisms. WIF1, the SFRPs, and Cerberus block Wnt signaling by binding directly to the Wnt glycoprotein, preventing its binding to Fz receptors. DKK1 and other members of the DKK family block Wnt signaling by sequestering low-density lipoprotein (LDL) receptor-related protein 5/6 (LRP 5/6), a coreceptor required by Fz receptors to activate the canonical pathway. DKK1 binds to the Kremen receptor, which also requires LRP 5/6 as a coreceptor. The DKK1-Kremen-LRP 5/6 complex is then actively removed from the cell membrane by endocytosis. Many proteins and genes involved in the canonical Wnt pathway have been implicated in cancer where activation of the pathway leads to inhibition of apoptosis and increased cell proliferation.3 Most studies of WNT pathway inhibitors have supported their role as potential tumor suppressor genes due to their ability to inhibit the canonical Wnt signaling pathway.12,13 The role of noncanonical Wnt pathways, which transduce Wnt signals independent of β-catenin, is less well defined in human cancer.
Several reports describe modulation of the Wnt signaling pathway through epigenetic silencing of WNT antagonists in various other human cancers.4-10,14-17 These studies have shown an association between hypermethylation of promoter-associated CpG islands and transcriptional inactivation of extracellular inhibitors of the Wnt pathway, including DKK1, SFRPs, and WIF1. The epigenetic silencing of DKK1, SFRP1, and WIF1 has not been previously described in GBM. In this report, we define the incidence and functional consequence of DNA methylation and histone modification in the promoter regions of DKK1, SFRP1, and WIF1 in relation to transcriptional repression in GBM. We show that DKK1, SFRP1, and WIF1 have decreased expression in human tumor samples relative to nontumor brain tissue samples. Although expression of all 3 genes is restored in T98 cells by treatment with the histone deacetylase inhibitor TSA, only DKK1 expression is restored by treatment with the demethylating agent 5-azacytidine. Therefore, we chose to further characterize the degree of DKK1 promoter methylation and its relation to transcriptional silencing in a panel of human tumor and nontumor normal brain samples. Our studies reveal an important role for histone modification in the epigenetic modulation of WNT antagonist expression. We then describe the significant functional consequence of DKK1 reexpression in T98 cells and compare that to the relative lack of response after reexpression of WIF1 and SFRP1. We investigate whether DKK1 reexpression results in decreased colonogenecity through inhibition of the canonical or noncanonical WNT signaling pathway. We observed that both pathways appear to be significantly modulated following DKK1 expression in T98 cells.
Results
Epigenetic Regulation of DKK1 in Human GBM: Promoter Methylation and Histone Modifications
In our earlier study, we identified 3 Wnt signaling pathway genes with increased expression in primary malignant GBM cell lines after treatment with the HDAC inhibitor TSA (Supplementary Figure S1). To investigate if these genes were also aberrantly silenced in GBM tissue samples, we first compared the expression level of each gene in a panel of histologically confirmed GBM tissue samples relative to nontumor brain tissue samples. The expression levels of 3 Wnt antagonists—DKK1, SFRP1, and WIF1—were significantly reduced in tumor tissue samples (Figure 1A). We then investigated whether transcriptional activation of these genes by TSA was a direct result of HDAC inhibition or due to a secondary effect. It is well documented that chromatin structure is altered in the neighborhood of epigenetically regulated genes and that treatment with TSA results in opening of the chromatin structure by promoting acetylation of histone H3 at lysine 9 (acetyl H3-K9), allowing the DNA sequence to become accessible to transcriptional machinery.18-20 The chromatin accessibility of the promoter region for all 3 WNT pathway inhibitors increased after TSA treatment (Figure 1B), suggesting that increased transcriptional activity is associated with opening of the chromatin structure. We next used antibodies specific for acetyl H3-K9 to monitor chromatin acetylation and found a significant increase in acetylated histone H3 binding in the promoter regions of all 3 genes following TSA treatment (Figure 1C). These data suggest that the expression of Wnt antagonists in GBM is regulated by direct epigenetic mechanisms involving histone modification.
Figure 1.
(A) Differential expression of WNT antagonists Dickkopf-1 (DKK1), secreted frizzled-related protein 1 (SFRP1), and Wnt inhibitory factor-1 (WIF1) in histologically confirmed glioblastoma (GBM) tissues relative to normal brain tissues. Total RNA was extracted from tissue samples, and relative expression of 3 WNT antagonists in GBM samples (o) with respect to expression in a nontumor brain tissues samples (●) was determined using real-time PCR (10 each, P < 0.05). The expression of housekeeping gene human glutathione synthetase (hGUS) was used as endogenous control. Y-axis units are arbitrary expression units used to calculate fold change relative to expression in 1 nontumor brain sample. (B) GBM cells display a more open chromatin structure after treatment with Trichostatin A (TSA). Nuclei isolated from TSA-treated (1 μM for 24 h) or control-treated (DMSO) T98 cells were incubated with DNase I. Real-time PCR was performed using primer pairs specific for DKK1, SFRP1, or WIF1 promoter regions to determine the number of target sequences after DNase I digestion. Relative accessibility of promoter regions in TSA-treated cells was calculated by taking the ratio of their accessibility indices relative to DMSO-treated cells. Relative chromatin accessibility significantly increased after treatment with TSA (P < 0.05). (C) TSA treatment markedly increases histone acetylation in the promoter regions of 3 Wnt antagonists. The changes in level of histone H3 lysine 9 were assessed using anti-K9 acetylated H3 antibodies. The relative changes in acetylation were calculated from the amount of histone acetylated with respect to input DNA and were found to be significantly higher (P < 0.05) in TSA-treated cells. (D) Changes in expression levels of DKK1, SFRP1, and WIF1 in T98 GBM cells following DNMT inhibition by AzaC. Total RNA was extracted after treatment with AzaC (5 μM for 72 h) or phosphate-buffered saline (PBS; n = 3, biological replicates). Expression levels of DKK1, SFRP1, and WIF1 were determined using real-time PCR, and relative expression levels in AzaC-treated cells were calculated relative to PBS-treated cells. Y-axis units are arbitrary expression units used to calculate fold change.
Of the 3 Wnt pathway inhibitors investigated in this report, only DKK1 was found to be significantly reexpressed (>2-fold) after treatment with 5-AzaC, suggesting the presence of promoter hypermethylation. We confirmed microarray data using real-time PCR (Figure 1D). To explore a potential link between promoter methylation and DKK1 expression, we used bisulfite sequencing to assess the methylation status of the CpG islands in the promoter region of DKK1 in a panel of tumor (n = 30) and nontumor (n = 19) tissue samples. Using methylation-sensitive PCR and bisulfite-treated genomic DNA, a CpG-rich region starting 1.25 kb upstream of the transcriptional start site was analyzed. DNA sequence analysis of 16 individual clones from each PCR product was performed to determine the methylation status of individual CpG sites (Supplementary Figure S2). Although CpG methylation was detected in some tumor tissue samples, there was no statistically significant difference in the methylation indices of CpG islands between tumor and nontumor tissue samples. These findings suggest that epigenetic regulation of the DKK1 gene is primarily driven by changes in histone tail modifications rather than promoter hypermethylation in GBM. In a similar fashion, DKK1 expression has recently been shown to be regulated primarily by promoter-associated histone modifications as opposed to DNA hypermethylation in lung cancer and medulloblastoma.21,22
DKK1 Inhibits GBM Cell Growth and Sensitizes Cells to Apoptosis
We next sought to characterize the functional consequences of restoring individual WNT antagonist function in T98 GBM cells. Using expression plasmids coding for DKK1, WIF1, and SFRP1, we determined the effect of their expression on the growth of T98 cells in vitro. Expression of DKK1 markedly inhibited colony formation in a colony formation assay relative to empty vector or lacZ control transfected cells. In contrast, ectopic expression of the canonical pathway inhibitors SFRP1 or WIF1 had a negligible (SFRP1) or much smaller effect (WIF1) on the colonogenicity of T98 cells (Figure 2A). We examined whether restoration of DKK1 expression could increase the sensitivity of T98 cells to apoptosis using a terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) assay. There was no increase in apoptotic cells with DKK1 reexpression alone. After treatment with a subtherapeutic dose of camptothecin and etoposide, there was a marked increase in the number of cells undergoing apoptosis in DKK1-transfected cells relative to control vector–treated cells (Figure 2B).
Figure 2.
(A) Effect of increased expression of 3 WNT antagonists on the growth of T98 glioblastoma (GBM) cells. T98 cells were transfected with plasmids coding for Dickkopf-1 (DKK1), secreted frizzled-related protein 1 (SFRP1), and Wnt inhibitory factor-1 (WIF1), or control (no insert), and G418-resistant colonies were quantified in 3 independent experiments. (B) Increased DKK1 expression sensitizes GBM tumor cells to camptothecin- and etoposide-induced apoptosis. Terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) staining of T98 cells transduced with DKK1 or control vector, with and without camptothecin/etoposide treatment. Percentages of TUNEL-positive cells are shown as a mean of 3 independent experiments.
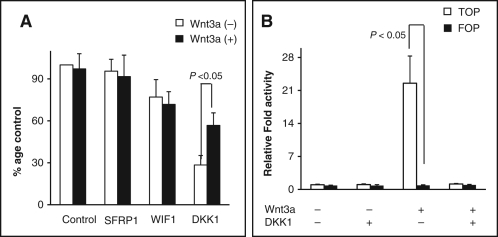
We next investigated whether DKK1 reexpression resulted in decreased colonogenecity through inhibition of the canonical WNT signaling pathway. A colony formation assay of T98 cells chronically stimulated with WNT3a did not show an increase in colonogenecity with WNT pathway activation alone. Reexpression of DKK1 in the setting of chronic WNT3a stimulation resulted in growth suppression, which was less marked than in the absence of WNT3a stimulation (Figure 3A). As shown in Figure 3B, addition of Wnt3a to T98 cells induced activation of a luciferase reporter carrying TCF binding sites (TOP), a widely used functional assay for the canonical Wnt pathway. DKK1 reexpression completely abolished Wnt3a activation of the TOP reporter. In the absence of Wnt3a, addition of DKK1 in conjunction with exogenous β-catenin partially reduces the effect of exogenous β-catenin on the TOP reporter (Supplementary Figure S3). No significant changes were observed in the activity of the control reporter with mutated TCF sites (FOP) in all experiments. Taken together, these data indicate that the canonical Wnt signaling pathway is intact and responsive to Wnt3a, DKK1, and, to a lesser extent, WIF1 in T98 cells. Given the marked amount of growth suppression seen with DKK1 reexpression in the absence of WNT3a stimulation, combined with the negligible or relatively modest suppression seen with the purely canonical pathway inhibitors SFRP1 and WIF1, we hypothesized that the marked growth inhibition observed with DKK1 reexpression could be at least partially regulated by a noncanonical WNT signaling pathway. In support of this hypothesis, we did not detect changes in cytoplasmic or nuclear β-catenin protein levels after DKK1 reexpression (Supplementary Figure S4).
Figure 3.
(A) Key components of the canonical WNT pathway exist and are responsive in T98 glioblastoma (GBM) cells. Colony formation assays were carried out as described in transfected and nontransfected T98 cells chronically stimulated with Wnt3a. Expression of Dickkopf-1 (DKK1) suppressed colony formation to a lesser extent following chronic Wnt3a stimulation (P < 0.05, analysis of variance). (B) Analysis of Wnt pathway activity in DKK1-transfected T98 cells. Luciferase reporter construct plasmids containing either wild-type TCF binding sites (TOP) or mutated TCF binding sites (FOP) were used to determine Wnt pathway activity. For stimulation of Wnt activity, transfected cells were cultured in Wnt3a-conditioned medium before determining luciferase activity. Exposure to Wnt3a increased transcriptional activity of the TOPflash reporter, which was abolished on DKK1 expression in T98 GBM cells (P < 0.05).
DKK1 Suppresses Colony Formation and Activates Apoptosis through the JNK Pathway
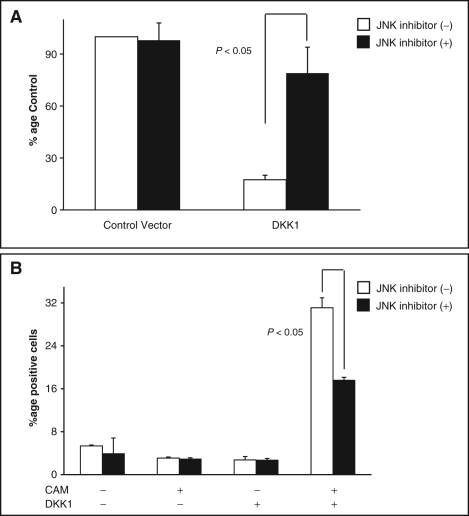
Recent studies have shown a functional correlation between DKK1 and activation of the JNK pathway in human mesothelioma and cervical carcinoma.23,24 To investigate the role of DKK1-mediated JNK signaling in the observed in vitro growth suppression, we assessed the combined effect of ectopic DKK1 expression and JNK inhibition on the rate of colony formation using the colony formation assay. Disruption of the JNK signaling pathway by the specific JNK inhibitor SP600125 nearly abolished the growth-suppressive effect previously seen upon restoration of DKK1 expression. DKK1-transfected cells treated with SP600125 demonstrated a marked 4.5-fold increase in colony formation compared to control-treated DKK1 transfected cells (Figure 4A), a rate of colony formation similar to T98 cells transfected with control vector alone. We hypothesized that the growth-inhibiting effects of DKK1 reexpression might be associated with increased sensitivity to apoptotic stimuli. We next evaluated the effect of JNK signaling pathway inhibition on the increased sensitivity to apoptosis seen with ectopic DKK1 expression. T98 cells transfected with DKK1 were treated with a subtherapeutic dose of camptothecin and etoposide in the presence of SP600125 or vehicle control. The increased rate of apoptosis seen with DKK1 expression was diminished significantly by JNK pathway inhibition (Figure 4B). Taken together, these data suggest that DKK1-mediated growth suppression and increased sensitivity to chemotherapy-induced apoptosis are modulated by activation of the JNK signaling pathway in T98 GBM cells.
Figure 4.
(A) Inhibition of the JNK pathway restores the colony formation rate of T98 cells following Dickkopf-1 (DKK1) expression. Colony formation assays were performed on T98 cells transfected with expression plasmids coding for DKK1 or empty vector control in the presence and absence of the JNK inhibitor SP600125 (Sigma, St. Louis, MO). Quantification of the number of G418 selected colonies in 3 independent experiments was determined relative to empty vector control. Treatment with SP600125 (5 μM) significantly blocked the suppression of colony formation seen after DKK1 expression (P < 0.05). (B) The observed increased rate of apoptosis following DKK1 expression decreased significantly (P < 0.05) following JNK pathway inhibition by SP600125.
Discussion
The regulation of cell growth and survival can be subverted by a variety of genetic or epigenetic alterations that modulate transcriptional programs normally responsible for controlling cell number. Several reports have shown that dysregulation of the Wnt pathway is associated with progression in malignant tumors.25,26 In GBM, modulation of Wnt signaling has been shown to affect growth and motility.27-33 Of note, mutations in Wnt antagonists have not been detected in GBM,34 suggesting that other mechanisms might play a role in their decreased expression. We recently identified a large cohort of epigenetically silenced genes in GBM,1 including several genes known to be antagonists of the Wnt network. This suggested that perturbation of the Wnt network due to epigenetic silencing of key Wnt antagonists might contribute to malignant behavior in GBM.
Our data suggest that expression of the extracellular Wnt inhibitors DKK1, SFRP1, and WIF1 is epigenetically regulated by chromatin modifications in GBM. We found that HDAC inhibition of GBM cells results in an increase in acetylation of histone H3 lysine and opens chromatin at the promoter region of DKK1. This results in a greater accessibility of the promoter region to the various elements of transcriptional machinery, leading to increased expression levels. Abundant evidence currently supports a synergistic link between promoter hypermethylation and histone deacetylation in the active suppression of gene transcription.35-39 In cancer, promoter hypermethylation has been associated with reduced expression of genes with potential tumor suppressor function involving apoptotic and/or proliferative pathways.1,40,41 Genes silenced in association with promoter hypermethylation can be reactivated by inhibition of DNA methyl transferase (DNMT) activity using the specific DNMT inhibitor 5-Aza-cytidine (AzaC).42-44 Because DKK1 expression increased on treating GBM cells with AzaC, we investigated if methylation of promoter-associated CpG islands played a role in regulating DKK1 expression. Using methylation-sensitive PCR and bisulfite-treated genomic DNA, we analyzed a CpG-rich region containing 57 CpG sites extending 1.24 kb upstream of the transcriptional start site. All cytosines at non-CpG sites were converted to uracils by bisulfite treatment, and those present at CpG sites either remained as cytosines or were converted to uracil. The Wilcoxon one-way analysis of variance (ANOVA) test demonstrated that there were no statistically significant differences in the methylation indices of the CpG islands in the DKK1 promoter region. These findings support our earlier observations that chromatin modifications play a dominant role in transcriptional regulation of epigenetically silenced genes in GBM.1,45
Of the 3 epigenetically silenced WNT antagonists, only restoration of DKK1 expression resulted in growth suppression, indicating a possible tumor suppressor function. After observing the relative lack of growth suppression with the direct WNT inhibitors SFRP1 and WIF1, we focused our functional studies on DKK1. Several reports also suggest a potential tumor suppressor function for DKK1 in a variety of other human cancers, including breast, cervical carcinoma, melanoma, mesothelioma, and choriocarcinoma.23,24,46-48 Most studies of DKK1 have supported its role as a potential tumor suppressor gene due to its ability to inhibit the canonical Wnt signaling pathway.47,49 We found that DKK1 chemosensitizes GBM cells to apoptotic stimuli and exerts its growth-suppressive effects at least partially through a noncanonical WNT pathway. The role of noncanonical Wnt pathways has not been described previously in GBM. DKK1 has been shown to modulate a noncanonical Wnt pathway in human mesothelioma and cervical carcinoma, possibly mediated by JNK pathway activation.23,24 Based on our data, it will be interesting to determine whether pharmacologic or antibody-based therapies targeting the Wnt pathway will affect tumor growth. Success will require further assessment of the molecular properties of the DKK1, their specific activities in target cells, and thorough understanding of the regulatory loops between signaling pathways and cell populations affected by Wnt signaling in additional tumor samples, glioma cell lines, and in vivo mouse models.
Materials and Methods
Tissue Samples and Cell Lines
Tumor and nontumor brain specimens were obtained from the Central Nervous System Tissue Bank at the University of Iowa Hospitals and Clinics. All patients gave informed consent prior to collection of specimens according to institutional guidelines. Each tumor specimen was histologically verified by a board-certified neuropathologist and archived for further DNA, RNA, and protein studies. The immortalized T98 cell line was obtained from American Type Culture Collection (Manassas, VA) and was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum as described previously.1 Cell lines were treated with 1 μmol/L TSA (Sigma, St. Louis, MO) or a control volume of DMSO for 24 h. For AzaC, cells lines were treated with 5 μmol/L AzaC (Sigma) dissolved in phosphate-buffered saline (PBS) containing 1 μmol/L acetic acid or PBS for 72 h as described earlier.1
Real-Time PCR Analysis
Total RNA was extracted from T98 cell lines or tissue samples using Trizol (Invitrogen, Carlsbad, CA). After purification with RNeasy (Qiagen, Valencia, CA), its quality was assessed with the Agilent Bioanalyzer (Palo Alto, CA). Purified RNA (1 μg) was reverse transcribed using random primers per the manufacturer's protocol (High Capacity cDNA Archive Kit, Applied Biosystems, Foster City, CA). The resulting cDNA was diluted 20-fold and used as template. Assay-on-Demand gene expression reagents (Applied Biosystems) were used to determine expression levels of DKK1, SFRP1, or WIF1. Real-time PCR was performed on the ABI PRISM 7900 HT Sequence Detection System under the following default conditions: 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The expression of human glutathione synthetase (hGUS) was used as the endogenous control as constant expression level of hGUS was observed in tumor and nontumor tissue samples. Comparative cycle threshold (Ct) method was used for quantification of the transcripts per the manufacturer's protocol. Measurement of ΔCt was performed in triplicate.
Chromatin Immunoprecipitation Assays
Formaldehyde cross-linking and ChIP assays in T98 cells treated with DMSO or 1 μM TSA were performed as described earlier.1 The antibodies used in the ChIP assays included anti-K9 acetylated H3 (Upstate Biotechnology, Lake Placid, NY) or normal rabbit IgG (Santa Cruz Biotechnology, Lake Placid, NY). The cross-links were reversed by heating the samples at 65°C for 4 h with NaCl. The samples were then treated with proteinase K overnight, and DNA was extracted by the phenol chloroform method, ethanol precipitated, and resuspended in 50 μL of water. The PCR primers were designed to yield 250-bp products. Their sequences can be found in Supplementary Table S1. To ensure that PCR amplification was in linear range, each reaction was set up at different dilutions of DNA for varying amplification cycle numbers, and final PCR conditions were selected accordingly. The PCR mixture contained 20 pM of each primer, 1 μL of extracted DNA, 0.5 units of Taq DNA Polymerase (Eppendorf, Pittsburg, PA), 0.2 mM dNTPs (each), and 2 mM MgSO4 in a final volume of 50 μL. The PCR was performed with the following cycling parameters: an activation step of 94°C for 3 min followed by 30 cycles of 94°C for 2 min, 50°C for 2 min, and 68°C for 3 min, with a final extension step of 68°C for 10 min. The PCR products were visualized by 1.8% agarose gel electrophoresis and quantitated by densitometry. Each assay was done in triplicate. Statistical analysis was performed using the paired t test.
Chromatin Accessibility Assay
Chromatin accessibility assays were performed as described earlier.50 Nuclei isolated from TSA-treated cells (1 μM for 24 h) or control-treated (DMSO) T98 cells were suspended in 100 μL of digestion buffer (50 mM Tris [pH 8.0], 100 mM NaCl, 3 mM MgCl2, 1 mM CaCl2, 0.15 mM Spermine, and 0.5 mM spermidine) and 20 μg of RNAse I (Qiagen). Nuclease digestion was carried out by adding 20 U of RQ1 DNase (Promega, Madison, WI) and incubating for 2 min at 37°C. DNA was purified with phenol/chloroform extraction and ethanol precipitated. Real-time PCR was performed using a primer pair specific for DKK1, SFRP1, or WIF1 promoter regions to determine the number of target sequences after DNase I digestion. The primer sequences along with their positions relative to transcriptional start sites are provided in Supplementary Table S1. Reactions contained 5 pmol of each primer, 20 ng of DNA, and 2× SYBR Green PCR Master Mix (Applied Biosystems). The PCR conditions used were as follows: 10-min denaturation at 95°C, followed by 40 cycles of 94°C for 30 sec and 60°C for 30 sec. Amplification of the target amplicon was monitored as a function of increased SYBR green fluorescence. An analysis threshold was set, and Ct was computed for each sample. The accessibility index was determined by the following formula: AI = 2[(Ct Dnase treated) − (Ct Untreated)]. Relative accessibility of DKK1 promoter in TSA-treated cells was calculated relative to DMSO-treated cells by taking the ratio of their accessibility indices. Statistical analysis was performed using the paired t test.
CpG-Island Bisulfite Analysis
Bisulfite sequencing was used to determine the methylation status of CpG islands in the promoter regions of DKK1 in 30 tumor and 19 nontumor samples as described earlier.1 Briefly, genomic DNA isolated from GBM tissue, normal brain tissue samples, or T98 cell lines were bisulfite treated. Using methylation-sensitive PCR, promoter-associated CpG island was amplified using primers described in Supplementary Table S2. The PCR mixture contained 20 pM of each primer, 40 ng of bisulfite-treated DNA (or 2 μL of first-round product), 1.25 units of Taq DNA Polymerase (Eppendorf, Pittsburg, PA), 0.2 mM dNTPs (each), and 2 mM MgSO4 in a final volume of 50 μL. The PCR was performed with the following cycling parameters: an activation step of 94°C for 3 min followed by 50 cycles of 94°C for 2 min, 50°C for 2 min, and 68°C for 3 min, with a final extension step of 68°C for 10 min. Methylated PCR products were cloned into TOPO TA (Invitrogen). Plasmid DNA from 16 independent clones was prepared and sequenced from both ends with BIG Dye terminators v3.1.
Transfection and Colony Formation Assays
Full-length open reading frames for DKK1, WIF1, and SFRP1 were PCR amplified from MGC clones, cloned into the pcDNA3.1D/V5-His-TOPO vector (Invitrogen), and sequence verified. Colony formation assays were performed in monolayer culture. Cells were plated at 1.5 × 105 per well using 6-well plates and transfected with pcDNA3.1D/V5-His-TOPO/DKK1, pcDNA3.1D/V5-His-TOPO/WIF1, pcDNA3.1D/V5-His-TOPO/SFRP1, pcDNA3.1D/V5-His-TOPO/lacZ, or pcDNA3.1D/V5-His-TOPO with no insert (mock control) using Trans It-Neural transfection reagents (Mirus, Madison, WI). The cells were selected in G418 (1 mg/mL) supplemented media at 24 h posttransfection. Some cells were simultaneously harvested to confirm increased expression of the transfected gene by real-time PCR. G418-resistant cells were maintained for 2 weeks in culture. Cells were detached, resuspended in media containing 0.3% agarose, and overlaid on 0.6% agarose. Then, 0.5 mL media was added to the plates every 4 days, and colony formation was quantitated after fixation and staining with methylene blue after 3 weeks. To investigate the role of canonical Wnt pathway in GBM, we carried out colony formation assays in transfected and untransfected T98 cells chronically stimulated with Wnt3a. Wnt-3a- and L-conditioned media (CM) were prepared as previously reported51 with recombinant human Wnt3a (100 ng/mL, R&D Systems, Minneapolis, MN) freshly added to the culture media every 3 days. The role of noncanonical pathways was studied by performing colony formation assays on T98 cells transfected with expression plasmids coding for DKK1 or empty vector control in the presence and absence of the JNK inhibitor 5 μM SP600125 (Sigma). Quantification of the number of G418-selected colonies in 3 independent experiments was determined. Statistical analysis was performed using the paired t test.
Terminal Deoxynucleotidyl Transferase–Mediated dUTP Nick End Labeling
PCR-amplified full-length open reading frames for DKK1 were cloned into pacAD5CMVIRESeGFPpA and sequence verified to generate adenoviral vectors. These clones were recombined in HEK293 cells with pacAD5 9.2-100 to produce recombinant adenovirus particles as described earlier.52 T98 cells transduced with adenovirus-expressing DKK1 or control virus were treated with either camptothecin (1 μM) and etoposide (3 μM) or DMSO control for 40 h in the presence or absence of SP600125 (5 μM). TUNEL reaction was carried out with the APO-BrdU TUNEL assay kit (Molecular Probes, Raleigh, NC) using Alexa fluor 647 conjugated monoclonal Ab and Hoechst 33342 for staining DNA. Cells were recorded with LSRII (Becton Dickinson, Franklin Lakes, NJ). Statistical analysis was performed using the paired t test.
Luciferase Reporter Assays
Luciferase reporter construct plasmids containing either wild-type TCF binding sites (TOP) or mutated TCF binding sites (FOP) were used for transfection, and T98 cells (1 × 106) were plated into each well of a 48-well dish (Corning, Corning, NY). After 24 h, cells were transfected simultaneously with 0.1 μg of DKK1 and 0.1 μg of pTOPflash or pFOPflash expression vectors/well. Nontransfected T98 cells and cells transfected with only TOP or FOP luciferase reporter constructs were used as controls. Luciferase activity was determined in transfected cells relative to controls using the Dual-Luciferase Reporter Assay system (Promega) in triplicate. For stimulation of Wnt activity, transfected cells were cultured in Wnt3-conditioned medium for 4 h, after which luciferase activity was determined. All assays were performed in triplicate unless stated otherwise. Statistical analysis was performed using the paired t test.
Supplementary Material
Acknowledgments
We thank the Swedish Medical Foundation for providing financial assistance for these studies and Prof. Randy Moon, University of Washington, for providing pTOPflash and pFOPflash plasmids. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors received no financial support for the research and/or authorship of this article.
Supplementary material for this article is available on the Genes & Cancer Web site at http://ganc.sagepub.com/supplemental.
References
- 1. Foltz G, Ryu GY, Yoon JG, Nelson T, Fahey J, Frakes A, et al. Genome-wide analysis of epigenetic silencing identifies BEX1 and BEX2 as candidate tumor suppressor genes in malignant glioma. Cancer Res 2006;66:6665-74 [DOI] [PubMed] [Google Scholar]
- 2. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell 2006;127:469-80 [DOI] [PubMed] [Google Scholar]
- 3. Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer 2008;8:387-98 [DOI] [PubMed] [Google Scholar]
- 4. Ai L, Tao Q, Zhong S, Fields CR, Kim WJ, Lee MW, et al. Inactivation of Wnt inhibitory factor-1 (WIF1) expression by epigenetic silencing is a common event in breast cancer. Carcinogenesis 2006;27:1341-8 [DOI] [PubMed] [Google Scholar]
- 5. Lee J, Yoon YS, Chung JH. Epigenetic silencing of the WNT antagonist DICKKOPF-1 in cervical cancer cell lines. Gynecol Oncol 2008;109:270-4 [DOI] [PubMed] [Google Scholar]
- 6. Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res 2005;65:4218-27 [DOI] [PubMed] [Google Scholar]
- 7. Suzuki H, Toyota M, Carraway H, Gabrielson E, Ohmura T, Fujikane T, et al. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer 2008;98:1147-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene 2006;25:4116-21 [DOI] [PubMed] [Google Scholar]
- 9. He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, et al. Blockade of Wnt-1 signaling induces apoptosis in human colorectal cancer cells containing downstream mutations. Oncogene 2005;24:3054-8 [DOI] [PubMed] [Google Scholar]
- 10. Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 2004;36:417-22 [DOI] [PubMed] [Google Scholar]
- 11. Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors 2005;23:111-6 [DOI] [PubMed] [Google Scholar]
- 12. Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, et al. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. Oncogene 2009;28:2569-80 [DOI] [PubMed] [Google Scholar]
- 13. Chen G, Jingbo A, Wang M, Farley S, Lee LY, Lee LC, et al. Menin promotes the Wnt signaling pathway in pancreatic endocrine cells. Mol Cancer Res 2008;6:1894-907 [DOI] [PubMed] [Google Scholar]
- 14. Chung MT, Sytwu HK, Yan MD, Shih YL, Chang CC, Yu MH, et al. Promoter methylation of SFRPs gene family in cervical cancer. Gynecol Oncol 2009;112:301-6 [DOI] [PubMed] [Google Scholar]
- 15. Maehata T, Taniguchi H, Yamamoto H, Nosho K, Adachi Y, Miyamoto N, et al. Transcriptional silencing of Dickkopf gene family by CpG island hypermethylation in human gastrointestinal cancer. World J Gastroenterol 2008;14:2702-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S, et al. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis 2007;28:2459-66 [DOI] [PubMed] [Google Scholar]
- 17. Uhm KO, Lee ES, Lee YM, Kim HS, Park YN, Park SH. Aberrant promoter CpG islands methylation of tumor suppressor genes in cholangiocarcinoma. Oncol Res 2008;17:151-7 [DOI] [PubMed] [Google Scholar]
- 18. Lafon-Hughes L, Di Tomaso MV, Mendez-Acuna L, Martinez-Lopez W. Chromatin-remodelling mechanisms in cancer. Mutat Res 2008;658:191-214 [DOI] [PubMed] [Google Scholar]
- 19. Shukla V, Vaissiere T, Herceg Z. Histone acetylation and chromatin signature in stem cell identity and cancer. Mutat Res 2008;637:1-15 [DOI] [PubMed] [Google Scholar]
- 20. Verdone L, Agricola E, Caserta M, Di Mauro E. Histone acetylation in gene regulation. Brief Funct Genomic Proteomic 2006;5:209-21 [DOI] [PubMed] [Google Scholar]
- 21. Hussain M, Rao M, Humphries AE, Hong JA, Liu F, Yang M, et al. Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res 2009;69:3570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vibhakar R, Foltz G, Yoon JG, Field L, Lee H, Ryu GY, et al. Dickkopf-1 is an epigenetically silenced candidate tumor suppressor gene in medulloblastoma. Neuro Oncol 2007;9:135-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee AY, He B, You L, Xu Z, Mazieres J, Reguart N, et al. Dickkopf-1 antagonizes Wnt signaling independent of beta-catenin in human mesothelioma. Biochem Biophys Res Commun 2004;323:1246-50 [DOI] [PubMed] [Google Scholar]
- 24. Peng S, Miao C, Li J, Fan X, Cao Y, Duan E. Dickkopf-1 induced apoptosis in human placental choriocarcinoma is independent of canonical Wnt signaling. Biochem Biophys Res Commun 2006;350:641-7 [DOI] [PubMed] [Google Scholar]
- 25. Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003;1653:1-24 [DOI] [PubMed] [Google Scholar]
- 26. Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol 2003;129:199-221 [DOI] [PubMed] [Google Scholar]
- 27. Lau YK, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q. Merlin is a potent inhibitor of glioma growth. Cancer Res 2008;68:5733-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu J, Zhang F, Zhao D, Hong L, Min J, Zhang L, et al. ATRA-inhibited proliferation in glioma cells is associated with subcellular redistribution of beta-catenin via up-regulation of Axin. J Neurooncol 2008;87:271-7 [DOI] [PubMed] [Google Scholar]
- 29. Palos TP, Zheng S, Howard BD. Wnt signaling induces GLT-1 expression in rat C6 glioma cells. J Neurochem 1999;73:1012-23 [DOI] [PubMed] [Google Scholar]
- 30. Pu P, Zhang Z, Kang C, Jiang R, Jia Z, Wang G, et al. Downregulation of Wnt2 and beta-catenin by siRNA suppresses malignant glioma cell growth. Cancer Gene Ther 2009;16:351-61 [DOI] [PubMed] [Google Scholar]
- 31. Roth W, Wild-Bode C, Platten M, Grimmel C, Melkonyan HS, Dichgans J, et al. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene 2000;19:4210-20 [DOI] [PubMed] [Google Scholar]
- 32. Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene 2002;21:878-89 [DOI] [PubMed] [Google Scholar]
- 33. Wang J, Shou J, Chen X. Dickkopf-1, an inhibitor of the Wnt signaling pathway, is induced by p53. Oncogene 2000;19:1843-8 [DOI] [PubMed] [Google Scholar]
- 34. Mueller W, Lass U, Wellmann S, Kunitz F, von Deimling A. Mutation analysis of DKK1 and in vivo evidence of predominant p53-independent DKK1 function in gliomas. Acta Neuropathol 2005;109:314-20 [DOI] [PubMed] [Google Scholar]
- 35. Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet 1999;21:103-7 [DOI] [PubMed] [Google Scholar]
- 36. Ghoshal K, Datta J, Majumder S, Bai S, Dong X, Parthun M, et al. Inhibitors of histone deacetylase and DNA methyltransferase synergistically activate the methylated metallothionein I promoter by activating the transcription factor MTF-1 and forming an open chromatin structure. Mol Cell Biol 2002;22:8302-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wischnewski F, Pantel K, Schwarzenbach H. Promoter demethylation and histone acetylation mediate gene expression of MAGE-A1, -A2, -A3, and -A12 in human cancer cells. Mol Cancer Res 2006;4:339-49 [DOI] [PubMed] [Google Scholar]
- 38. Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res 2001;61:7025-9 [PubMed] [Google Scholar]
- 39. Zhang Y, Fatima N, Dufau ML. Coordinated changes in DNA methylation and histone modifications regulate silencing/derepression of luteinizing hormone receptor gene transcription. Mol Cell Biol 2005;25:7929-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agirre X, Vilas-Zornoza A, Jimenez-Velasco A, Martin-Subero JI, Cordeu L, Garate L, et al. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res 2009;69:4443-53 [DOI] [PubMed] [Google Scholar]
- 41. Veeriah S, Brennan C, Meng S, Singh B, Fagin JA, Solit DB, et al. The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc Natl Acad Sci USA 2009;106:9435-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bonazzi VF, Irwin D, Hayward NK. Identification of candidate tumor suppressor genes inactivated by promoter methylation in melanoma. Genes Chromosomes Cancer 2009;48:10-21 [DOI] [PubMed] [Google Scholar]
- 43. Ostrow KL, Park HL, Hoque MO, Kim MS, Liu J, Argani P, et al. Pharmacologic unmasking of epigenetically silenced genes in breast cancer. Clin Cancer Res 2009;15:1184-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shen WJ, Dai DQ, Teng Y, Liu HB. Regulation of demethylation and re-expression of RASSF1A gene in gastric cancer cell lines by combined treatment of 5-Aza-CdR and NaB. World J Gastroenterol 2008;14:595-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Foltz G, Yoon JG, Lee H, Ryken TC, Sibenaller Z, Ehrich M, et al. DNA methyltransferase-mediated transcriptional silencing in malignant glioma: a combined whole-genome microarray and promoter array analysis. Oncogene 2009;28:2667-77 [DOI] [PubMed] [Google Scholar]
- 46. Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang BH, Bosserhoff AK. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene 2006;25:5027-36 [DOI] [PubMed] [Google Scholar]
- 47. Mikheev AM, Mikheeva SA, Maxwell JP, Rivo JV, Rostomily R, Swisshelm K, et al. Dickkopf-1 mediated tumor suppression in human breast carcinoma cells. Breast Cancer Res Treat 2008;112:263-73 [DOI] [PubMed] [Google Scholar]
- 48. Mikheev AM, Mikheeva SA, Rostomily R, Zarbl H. Dickkopf-1 activates cell death in MDA-MB435 melanoma cells. Biochem Biophys Res Commun 2007;352:675-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, et al. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene 2005;24:1098-103 [DOI] [PubMed] [Google Scholar]
- 50. Rao S, Procko E, Shannon MF. Chromatin remodeling, measured by a novel real-time polymerase chain reaction assay, across the proximal promoter region of the IL-2 gene. J Immunol 2001;167:4494-503 [DOI] [PubMed] [Google Scholar]
- 51. Endo Y, Wolf V, Muraiso K, Kamijo K, Soon L, Uren A, et al. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J Biol Chem 2005;280:777-86 [DOI] [PubMed] [Google Scholar]
- 52. Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther 2000;7:1034-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.