Abstract
The Pdcd4 (programmed cell death gene 4) gene has been implicated as a novel tumor suppressor gene in the development of several types of human cancer. The Pdcd4 protein is believed to act as a translation suppressor of mRNAs containing structured 5′ UTRs. Pdcd4 contains 2 copies of so-called MA3 domains that mediate tight interactions with the translation initiation factor eIF4A, resulting in the inhibition of the eIF4A helicase activity. The N-terminal part of Pdcd4, which has been less well characterized, binds RNA in vitro, but as yet, it has not been clear whether RNA binding by Pdcd4 plays a role in vivo. Here, the authors have identified 2 highly conserved clusters of basic amino acid residues that are essential for the RNA binding activity of Pdcd4. They also show that a substantial fraction of Pdcd4 is present, together with small ribosomal subunits, in translation preinitiation complexes. Using mutants that disrupt RNA binding or the Pdcd4-eIF4A interaction, they demonstrate that the ribosomal association of Pdcd4 is dependent on its RNA binding activity as well as on its ability to interact with eIF4A. Their work provides the first direct evidence for an essential role of the Pdcd4 RNA binding activity in vivo and suggests that RNA binding is required for recruiting Pdcd4 to the translation machinery.
Keywords: Pdcd4, RNA binding, eIF4A, ribosome
Introduction
The tumor suppressor gene Pdcd4 (programmed cell death 4) was initially identified in a search of genes whose expression increases during apoptosis.1 Subsequent work showed that Pdcd4 is a novel tumor suppressor gene. Pdcd4 has been demonstrated to suppress cell transformation in an in vitro mouse keratinocyte model of tumor promotion2 and tumor formation in an in vivo mouse model of skin carcinogenesis.3 Decreased expression of Pdcd4 has been strongly implicated in the development and progression of a variety of human tumors, including lung, colon, liver, and breast cancer.4-8 Downregulation of Pdcd4 expression in tumor cells has been linked to the expression of oncogenic micro-RNA miR-21, which targets the 3′ untranslated region of Pdcd4 mRNA.9-11 On the protein level, Pdcd4 expression is regulated by S6K-mediated phosphorylation, which triggers the ubiquitination of Pdcd4 via the E3 ubiquitin ligase complex SCF(betaTRCP) and its subsequent degradation.12,13 Downregulation of Pdcd4 expression affects cells in two major ways, both of which are presumed to contribute to tumor development: a number of studies have shown that decreased Pdcd4 expression increases the mobility and invasiveness of tumor cells.8,11,14,15 In addition, decreased Pdcd4 expression has been shown to deregulate the cellular DNA damage response.16,17
Pdcd4 encodes a highly conserved, predominantly nuclear protein that is able to shuttle between nucleus and cytoplasm18 and whose subcellular localization is controlled by protein kinase Akt-mediated phosphorylation.19 So far, two main activities have been attributed to Pdcd4. Several studies have shown that Pdcd4 affects the transcription of specific genes by modulating the activities of certain transcription factors, such as c-Jun,20,21 Sp1,15 and p53.16 An example is the upregulation of the p21(Waf1/Cip1) gene after Pdcd4 knockdown, which was found to be due to abrogation of Pdcd4-dependent inhibitory effects on the p300/CBP-dependent acetylation of p53.16 In addition, Pdcd4 is thought to act as a translation suppressor of specific mRNAs. Pdcd4 has been shown to interact with the eukaryotic translation initiation factor eIF4A, a member of the DEAD-box protein family that functions as an adenosine triphosphate (ATP)–dependent RNA helicase and catalyzes the unwinding of mRNA secondary structures at the 5′ untranslated regions (5′-UTRs).22,23 Binding of Pdcd4 to eIF4A is mediated by 2 so-called MA-3 domains that occupy the central and C-terminal part of the protein and whose structure and complex formation with eIF4A have been analyzed in detail by crystallography and nuclear magnetic resonance (NMR).24-28 It was found that binding to Pdcd4 inhibits the helicase activity of eIF4A,22,23 which is required to unwind stable secondary structures in the 5′-UTRs of certain mRNAs during initiation of translation.29 It was therefore assumed that Pdcd4 acts as a suppressor of cap-dependent translation of mRNAs with structured 5′-UTRs. This assumption was supported by studies assessing the effects of Pdcd4 on the translation of artificial RNAs containing 5′ hairpin structures,22,23 but up to now, no natural translational target mRNAs for Pdcd4 have been unequivocally identified.
We have previously shown that the N-terminal domain of Pdcd4 binds RNA in vitro,18 but as yet, there is no direct evidence that RNA binding by Pdcd4 plays a role in vivo. We have now analyzed the RNA binding domain in more detail. We have identified 2 conserved clusters of basic amino acid residues as playing essential roles in Pdcd4 RNA binding. Our results show that a substantial fraction of Pdcd4 is associated, together with the small ribosomal subunit, with translation preinitiation complexes. Interestingly, the ribosomal association of Pdcd4 is dependent on its RNA binding activity. Our work, therefore, provides the first direct evidence for an essential role of the Pdcd4 RNA binding activity in vivo and suggests that RNA binding is required for recruiting Pdcd4 to the translational machinery.
Results and Discussion
Mapping of amino acid residues involved in RNA binding by Pdcd4
We have previously shown that the N-terminal part of Pdcd4 is able to bind to RNA in vitro.18 This suggested that this part of the protein might function as an RNA binding domain. To further explore the function of the N-terminal domain of Pdcd4 and to be able to address the role of RNA binding in vivo, we set out to map the amino acid residues that are crucial for the RNA binding activity. As a first step, we expressed different parts of Pdcd4 as glutathione-S-transferase (GST) fusion proteins and analyzed them by an RNA binding assay. As illustrated in Figure 1, the purified proteins were run on a sodium dodecyl sulfate (SDS)–polyacrylamide gel, blotted onto nitrocellulose, and incubated with radiolabeled RNA. Consistent with our previous work, full-length Pdcd4 but not an N-terminally truncated form of Pdcd4 lacking the first 150 amino acids was able to bind RNA. Analysis of the RNA binding activity of subregions of the N-terminal domain revealed that 2 regions located between amino acids 50 to 100 and 100 to 159 showed RNA binding activity, whereas a protein corresponding to amino acids 1 to 50 of Pdcd4 did not bind RNA. We therefore concluded that 2 regions within the N-terminal domain of Pdcd4 are involved in RNA binding.
Figure 1.
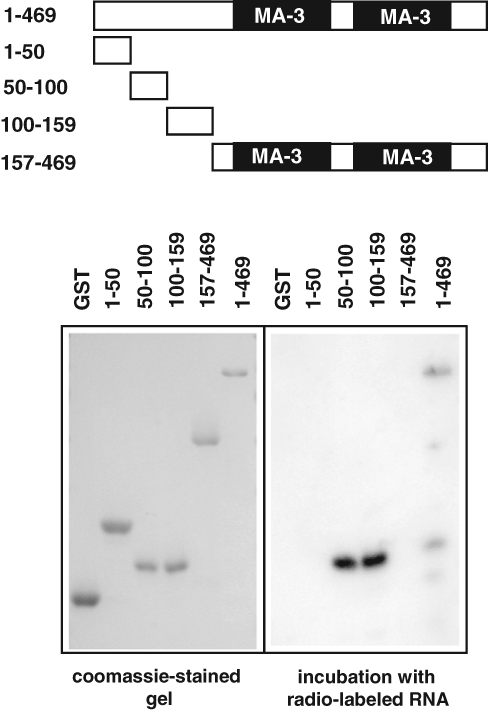
RNA binding by Pdcd4 is mediated by 2 subregions within the N-terminal domain of Pdcd4. Glutathione-S-transferase (GST) fusion proteins containing full-length Pdcd4 or different parts of Pdcd4 were displayed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto nitrocellulose, and subjected to the RNA binding assay as described in Materials and Methods. The right panel shows an autoradiogram of the blot after incubation with in vitro synthesized, radiolabeled RNA. The left panel shows a Coomassie brilliant blue–stained gel containing the same amounts of the different GST-proteins. The GST fusion proteins are illustrated schematically at the top (numbers indicate Pdcd4 amino acids that are fused to GST in each construct).
Inspection of the amino acid sequence of Pdcd4 revealed 2 clusters of arginine and lysine residues centered around amino acids 63 and 105 (Fig. 2). We refer to these clusters as RBM1 and RBM2, respectively. Both regions show significant amino acid sequence conservation between vertebrate Pdcd4 proteins as well as between Pdcd4 homologs of very divergent species such as Suberites domuncula (a sponge), Drosophila melanogaster, Xenopus laevis, Danio rerio, and the mouse and humans (Fig. 2A). In the case of RBM2, this conservation is especially obvious and extends C-terminally beyond the cluster of basic residues. We reasoned that these clusters, because of their overall positive charge, might be involved in binding RNA. To address this possibility, we mutated several of the basic residues in both clusters to alanine. We then expressed these Pdcd4 mutants as GST fusion proteins and subjected them to the in vitro RNA binding assay. Figure 2B shows that the RNA binding activity of the proteins mutated in RBM1 or RBM2 or in both clusters (RBM1+2) was virtually undetectable, confirming that both clusters of basic amino acid residues are crucial for RNA binding by full-length Pdcd4.
Figure 2.

Identification of 2 conserved amino acid motifs involved in RNA binding by Pdcd4. (A) Schematic structure of Pdcd4 and the location of 2 clusters of basic amino acids (referred to as RBM1 and RBM2). The amino acid sequences of RBM1 and RBM2 in Pdcd4 proteins from the sponge Suberites domuncula (S.d.), Drosophila melanogaster (D.m.), Xenopus laevis (X.l.), Danio rerio (D.r.), Mus musculus (M.m.), and Homo sapiens (H.s.) are shown below. Amino acids identical to human Pdcd4 are marked by black boxes. The asterisks mark the positions of arginine and lysine residues in human Pdcd4. (B) Fusion proteins of glutathione-S-transferase (GST) and full-length Pdcd4 carrying the indicated mutations in RBM1 (mutRBM1), RBM2 (mutRBM2), or both motifs (RBM1+2) were analyzed for RNA binding activity as described in Figure 1. The upper panel shows Coomassie brilliant blue staining of the fusion proteins to confirm equal loading of the proteins. The lower panel shows an autoradiogram of a blot of the same proteins subjected to the RNA binding assay. In both panels, the arrows mark the GST-Pdcd4 fusion proteins. (C) GST-Pdcd4-wt (top) or GST-Pdcd4-mutRBM 1+2 (bottom) proteins were analyzed by chromatography on poly(U)-agarose. Bound proteins were eluted from the column with binding buffer containing increasing amounts of NaCl. Samples of input, flow-through, 2 successive washes, and salt-eluate fractions were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with Pdcd4 antiserum. Equal aliquots were loaded for input, flow-through, and wash fractions, whereas aliquots of the salt-eluate fractions were 5 times larger.
As an alternative RNA binding assay that avoids the denaturation and renaturation of the protein, we subjected GST-Pdcd4-wt and GST-Pdcd4-mutRBM1+2 proteins purified in soluble form to chromatography on poly(U)-agarose. We have demonstrated before that the endogenous Pdcd4 protein binds to poly(U)-agarose and is eluted in buffers of increasing ionic strength.18 Figure 2C shows that bacterially expressed wild-type Pdcd4 was able to bind to poly(U)-agarose efficiently, whereas the mutant Pdcd4 protein failed to bind. Most of the wild-type Pdcd4 was eluted in the presence of 200 to 300 mM NaCl, suggesting that it binds to RNA under physiological salt conditions.
RNA-binding activity of Pdcd4 is required for recruiting Pdcd4 to ribosomal complexes
Next, we were interested to know whether the disruption of the in vitro RNA binding activity affects the behavior of Pdcd4 in vivo. A previous report has suggested that Pdcd4 cosediments with ribosomes and/or polysomes in sucrose density gradients30; we therefore set out to compare the ribosomal association of wild-type and mutant Pdcd4 proteins. As a first step, we examined the ribosomal association of endogenous Pdcd4. We prepared cytoplasmic extracts from HeLa cells and analyzed them by sucrose density gradient centrifugation. Figure 3A illustrates the distribution of Pdcd4 and of the 18S and 28S ribosomal RNAs throughout the gradient fractions. A significant amount of Pdcd4 appeared in fractions that contained small ribosomal subunits and monomeric ribosomes with the highest amount of Pdcd4 sedimenting slightly faster than small ribosomal subunits and slower than complete ribosomes. These fractions also contained eIF4A. This suggested that Pdcd4 together with the small 40S ribosomal subunit is part of translation preinitiation complexes and is consistent with the role of Pdcd4 as an eIF4A interacting protein. There was no Pdcd4 in fractions containing polysomes. A second peak of Pdcd4 was detected at the top of the gradient, presumably representing soluble cytoplasmic protein. Similar sedimentation patterns of Pdcd4 were observed when cytoplasmic extracts from other cell lines were analyzed (Fig. 3B and data not shown). To substantiate that Pdcd4 is associated with ribosomes, we treated cytoplasmic extract from HeLa cells with EDTA, which is known to dissociate ribosomes into small and large subunits and to disrupt preinitiation complexes. Under these conditions, all of the Pdcd4 appeared at the top of the gradient (Fig. 3C). Similarly, treatment of cell extract with RNase abolished the peak of Pdcd4 cosedimenting with ribosomal complexes (Fig. 3D). To further corroborate the notion that Pdcd4 is associated with ribosomal complexes, we prepared cytoplasmic extracts from HeLa cells and immunoprecipitated them with Pdcd4-specific antiserum or with preimmune serum. We then analyzed the immunoprecipitates by agarose gel electrophoresis to visualize ribosomal RNAs. We confirmed that the Pdcd4-specific antiserum was able to immunoprecipitate Pdcd4 (see Supplementary Fig. S1). As shown in Figure 3E, 18S and 28S ribosomal RNAs were present in the specific but not in the control immunoprecipitate. The ethidium bromide stainable material was RNA (and not DNA), as demonstrated by its sensitivity toward RNase treatment. Note that the relative amounts of 18S and 28S RNAs were changed in the immunoprecipitate versus the input samples, consistent with the preferential association of Pdcd4 with small ribosomal subunits. Taken together, the data illustrated in Figure 3 demonstrate that Pdcd4 is associated with complexes containing small ribosomal subunits.
Figure 3.

Ribosomal association of Pdcd4. (A) Cytoplasmic extracts isolated from HeLa cells were analyzed by sucrose density gradient centrifugation. Individual fractions of the gradient were then analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting using antibodies against Pdcd4 (top) and eIF4A (middle) or by agarose gel electrophoresis and staining with ethidium bromide to detect 18S and 28S ribosomal RNAs (bottom). Fractions containing predominantly small ribosomal subunits, complete ribosomes, and polysomes are marked. (B) Cytoplasmic extracts from HEK293 cells were analyzed as described in panel A. (C) HeLa cells were analyzed as described in panel A except that EDTA was used to disrupt ribosomal complexes. (D) HeLa cells were analyzed as described in panel A except that the cell extract was treated with RNAse before centrifugation. (E) Cytoplasmic extracts of HeLa cells, pretreated for 30 min with or without cycloheximide, were immunoprecipitated with Pdcd4-specific antiserum or with preimmune serum. Coprecipitated 18S and 28S ribosomal RNAs were then visualized by agarose gel electrophoresis and staining with ethidium bromide. Aliquots of the samples were treated with DNase or RNase before gel electrophoresis. The bottom panel shows aliquots of the cytoplasmic extracts before immunoprecipitation.
Having established the ribosomal association of wild-type Pdcd4, we next examined the ability of the mutant proteins to associate with ribosomes. We expressed mutant Pdcd4 proteins deficient in RNA binding in HeLa cells and analyzed cytoplasmic extracts by sucrose density gradient centrifugation. In these experiments, coexpressed hemagglutinin (HA) tagged wild-type Pdcd4 served as an internal control for the mutant proteins, all of which were Flag tagged. Figure 4 shows that deletion of the entire N-terminal domain completely abrogated the ribosomal association of Pdcd4, consistent with the notion that RNA binding plays an important role in the binding of Pdcd4 to ribosomal subunits. Mutation of RBM1 only slightly diminished the ribosomal association of the protein, whereas mutation of RBM2 had a much more significant effect on the interaction of Pdcd4 with ribosomes. When RBM1 and RBM2 were mutated simultaneously, the protein was unable to associate with ribosomes.
Figure 4.

Ribosomal association of mutant Pdcd4 proteins. HeLa cells were cotransfected with expression vectors for HA-tagged wild-type Pdcd4 and Flag-tagged mutant Pdcd4 lacking the N-terminal domain (Pdcd4-ΔRBD), carrying mutations in RBM1 (Pdcd4-mutRBM1), RBM2 (Pdcd4-mutRBM2), or both RBM motifs (Pdcd4-mutRBM1+2) or carrying the D418A substitution (Pdcd4-D418A). Cytoplasmic extracts were prepared 20 h after transfection and analyzed by sucrose density gradient sedimentation as described in Figure 3. In each panel, the distribution of Flag-tagged mutant and HA-tagged wild-type proteins is shown at the top and the middle, respectively. The distribution of the ribosomal RNAs is shown at the bottom.
Ribosomal association of Pdcd4 also requires its interaction with eIF4A
Because the interaction of Pdcd4 and eIF4A has been strongly implicated in the translation suppressor activity of Pdcd4,22 we were interested to know whether the ribosomal association of Pdcd4 was also dependent on the Pdcd4-eIF4A interaction. To address this issue, we made use of the D418A mutant of Pdcd4, which has a strongly reduced affinity for eIF4A.23 We transfected HeLa cells with an expression vector for the D418A mutant, followed by sedimentation analysis of cytoplasmic extracts. As illustrated in Figure 4, the mutant protein no longer cosedimented ahead of the small ribosomal subunit but was distributed in the upper part of the gradient. We therefore concluded that the interaction with eIF4A is necessary for the stable ribosomal association of Pdcd4.
Implications for the cytoplasmic function of Pdcd4
Our work leads to several interesting conclusions regarding the role of RNA binding for the cytoplasmic function of Pdcd4. First, our work confirms that Pdcd4 binds RNA via its N-terminal domain and identifies 2 clusters of positively charged amino acids, RBM1 and RBM2, as being essential for the RNA binding activity of Pdcd4. Both clusters are conserved between Pdcd4 homologs from extremely divergent species such as sponges, insects, and vertebrates. This strongly suggests that the ability to bind RNA is an evolutionary conserved feature of Pdcd4. Interestingly, the region located immediately C-terminally to RBM2 is also very highly conserved, suggesting that it plays an important role, either in RNA binding or in another interaction of Pdcd4.
Second, our work demonstrates that a significant fraction of the cytoplasmic Pdcd4 is associated with ribosomal complexes. It has previously been claimed that Pdcd4 cosediments with the polysome fraction in a sucrose density gradient,30 but our data now clearly show that Pdcd4 is stably associated with a complex sedimenting slightly faster than small ribosomal (40S) subunits and slower than complete (80S) ribosomes. Because of this sedimentation pattern, it appears likely that Pdcd4 is primarily associated with the 48S translation preinitiation complex. This is consistent with the fact that Pdcd4 interacts with eIF4A (which is part of this complex) and is further supported by the observation that the D418A mutant of Pdcd4 disrupts the stable association of Pdcd4 with the ribosomal complex. Asp 418 of Pdcd4 is involved in making direct contacts with eIF4A, and its replacement by alanine has been shown to substantially weaken the Pdcd4-eIF4A interaction.23-25 Our data, therefore, indicate that the ribosomal complex with which Pdcd4 interacts contains eIF4A and, furthermore, that this interaction is necessary for the stable association of Pdcd4 with this complex. Taken together, our data demonstrate that Pdcd4 interacts via eIF4A with the translation preinitiation complex.
Third, our results demonstrate that mutations of the RNA binding motifs affect the ribosomal association of Pdcd4 and thus provide the first evidence that RNA binding by Pdcd4 plays a crucial role in vivo. Mutation of RBM1 had only a minor effect in vivo, whereas replacement of only 2 arginine residues within RBM2 had a significantly stronger effect on the ribosomal association of Pdcd4. The combination of both RBM mutants eventually led to complete disruption of the ribosomal association of Pdcd4. Although both single RBM mutants had virtually completely lost RNA binding activity in vitro, they showed residual association with ribosomes. Presumably, the ability of both mutant proteins to still bind to eIF4A and to RNA with the intact RBM explains why they are still associated with the ribosomal complexes, at least to some extent. In conclusion, therefore, our data demonstrate that the association of Pdcd4 with translation preinitiation complexes is mediated by protein-protein interactions between Pdcd4 and eIF4A as well as protein-RNA interactions between Pdcd4 and, possibly, the mRNAs present in these complexes. Whether the Pdcd4-RNA interaction is specific for certain mRNAs and whether RNA binding mediates the recruitment of Pdcd4 to preinitiation complexes containing specific mRNAs are very interesting questions that can be addressed once specific target mRNAs for Pdcd4 have been identified.
Materials and Methods
Eukaryotic expression vectors
A human Pdcd4 expression vector was generated by amplifying the coding region of human Pdcd4 from total cDNA of T47D breast cancer cells using 5′-CTGGATCCGCCACCATGGATGTAGAAAATGAGCAG-3′ and 5′-GACTCGAGTTTAGTAGCTCTCTGGTTTA-3′ as forward and reverse primers and inserting the PCR product between the BamHI and XhoI sites of pCDNA4. A C-terminally HA-tagged expression vector for human Pdcd4 was generated by a subsequent PCR using the reverse primer 5′-GACTCGAGTTAGGCGTAGTCGGG CACGTCGTAGGGGTAGTAGCTCTCTGGTTTA-3′ and cloning the PCR product between the BamHI and XhoI sites of pCDNA4. An N-terminally Flag-tagged version was constructed by replacing pCDNA4 polylinker sequences between the KpnI and BamHI sites immediately upstream of the Pdcd4 coding region by the sequence GGTACCGAATTCGCCACCATGGATTACAA GGACGACGATGACAAGGGATCC. pCDNA4-Flag-hPdcd4- D418A is a point-mutated derivative in which Asp 418 was changed to Ala using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and appropriate primers. In plasmid pCDNA4-Flag-hPdcd4-RBD-Mut1, the human Pdcd4 coding sequence was changed from AAGGCAAAAAGGCGACTAAGGAAAAACTCA to AAGGCAGCGGCCGCACTAGCGGCAAACTCA, resulting in mutation of the RBM1 from KAKRRLRKN to KAAAALAAN. In plasmid pCDNA4-Flag-hPdcd4-RBD-Mut2, the human Pdcd4 coding sequence was changed from GCTGGATAGGCGATCCAGAT to GCTGGATGCGGCAT CCAGAT, resulting in mutation of the RBM2 from LDR RSRSG to LDAASRSG. Plasmid pCDNA4-Flag-hPdcd4-RBD-DM contains a combination of both mutations. pCD NA4-hPdcd4-ΔRBD encodes an internally deleted human Pdcd4 protein lacking amino acids 16 to 150. All mutants were verified by sequencing.
HeLa cells were transfected with the desired expression vectors using the calcium-phosphate coprecipitation method, as described before.31 Cells were harvested 16 to 20 h after transfection.
Bacterial expression vectors
Expression vectors coding for different GST-Pdcd4 fusion proteins were generated by cloning the full-length or different parts of the mouse Pdcd4 coding region between the BamHI and NotI sites of the expression vector pGex-6P-2. The respective parts of the coding region were amplified by PCR using appropriately designed primers, and the final constructs were verified by sequencing. Point mutants of the RNA binding motifs RBM1, RBM2, or both were generated as fusion proteins of GST and full-length mouse Pdcd4. In the case of RBM1, the amino acid sequence was changed from KAKRRLRKN to KAAAALAAN; in the case of RBM2, the sequence was changed from LDRRSRSG to LDAASRSG. GST fusion proteins were expressed and purified as described.32 To detect GST-Pdcd4 by Western blotting, a Pdcd4 antiserum reacting with the C-terminus of human Pdcd4 (Acris, Herford, Germany) was used.
RNA-binding assays
RNA binding assays of GST-Pdcd4 fusion proteins were performed as described before.18 GST fusion proteins were fractionated by SDS-PAGE and blotted onto nitrocellulose. Radiolabeled RNA was synthesized in vitro using T7 RNA polymerase, [32P]-UTP, and the control template (pGEM Express) provided in the Riboprobe System T7 kit (Promega, Madison, WI), as described previously.18 Blots were incubated first with 6 M urea, 0.2% NP40 for 10 min; washed 4 times 15 min each with RNA binding buffer (10 mM Tris-HCl, pH 7.5; 5 mM KCl; 2 mM MgCl2) lacking RNA; and then incubated for 15 min in RNA binding buffer containing radiolabeled RNA. Subsequently, blots were washed 3 times with binding buffer lacking RNA (10 min each wash) and analyzed by autoradiography. All incubations were performed at room temperature. For chromatography on poly(U)-agarose (all steps at 4°C), GST-Pdcd4 fusion proteins were purified by binding to glutathione-sepharose and eluting them overnight with 15 mM glutathione in 10 mM Tris-HCl, pH 7.5. Eluted proteins were then bound to poly(U)-agarose (Sigma, P8563) equilibrated in RNA binding buffer. Proteins not bound (flow-through), eluted with 2 successive washes with binding buffer (wash fractions 1 and 2), and eluted with binding buffer supplemented with increasing concentrations of NaCl were analyzed by SDS-PAGE and Western blotting.
Sucrose density gradient centrifugation of cytoplasmic extracts
Cells were treated with 50 µg/mL cycloheximide for 30 min before harvesting. Cells were then washed with ice-cold phosphate-buffered saline (containing 50 µg/mL cycloheximide) and lysed in hypotonic buffer (10 mM HEPES, pH 7.5; 5 mM KCl; 2 mM MgCl2; 1 mM dithiothreitol [DTT]; 0.5% NP40; 1 mM phenylmethanesulfonyl fluoride [PMSF]; supplemented with 40 U/mL RNaseOUT [Invitrogen, Carlsbad, CA] and a protease inhibitor cocktail containing 5 ng/mL pepstatin A, 1 ng/mL leupeptin hemisulfate, and 5 ng/mL aprotinin) for 20 min on ice. The lysed cells were pelleted at 14,000 rpm for 15 min at 4°C. The supernatant was collected as cytoplasmic fraction and layered on top of a 10% to 50% sucrose density gradient made in hypotonic buffer. Gradients were centrifuged in a SW41 rotor at 36,000 rpm for 2 h at 4°C. The gradients were fractionated, and aliquots of the fractions were analyzed by 10% SDS-PAGE and Western blotting to determine the distribution of Pdcd4 and eIF4A or treated with Proteinase K and 1% SDS and analyzed by agarose gel electrophoresis to determine the distribution of ribosomal RNAs. Rabbit anti-Pdcd4 serum was described before16; anti-eIF4A, HA, and Flag antibodies were from Abcam (Cambridge, UK), Hiss Diagnostics (Freiburg, Germany), and Sigma (Munich, Germany), respectively. For EDTA treatment of ribosomal complexes, 25 mM EDTA (final concentration) was added to the cytoplasmic extracts. In this case, the sucrose gradient was made in hypotonic buffer lacking MgCl2 and supplemented with 25 mM EDTA. For RNase treatment of ribosomal complexes, the extract was digested with 100 µg/mL RNaseA for 1 h at 37°C.
Immunoprecipitation
Cells were pretreated with or without cycloheximide, and cytoplasmic extracts were prepared as described above. Aliquots of the extracts were immunoprecipitated with rabbit anti-Pdcd4 serum16 or with preimmune serum for 3 h on ice. Immunoprecipitates were collected on protein A sepharose, washed with hypotonic buffer, and finally eluted from the sepharose with 1% SDS. Samples of the immunoprecipitates or the input material were treated with Proteinase K before they were analyzed by agarose gel electrophoresis followed by ethidium bromide staining. Samples were optionally treated with RNase-free DNase or with RNase before gel electrophoresis.
Supplementary Material
Acknowledgments
The authors thank M. Plischka and N. Bitomsky for constructing the Pdcd4-D418A mutant and Heike Most for performing some of the RNA binding assays.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
This work was supported by grants from the Deutsche Krebshilfe and the Wilhelm-Sander-Stiftung. LW was supported by a fellowship from the Graduate School of Chemistry (GSC-MS) at the University of Münster.
Supplementary material for this article is available on the Genes & Cancer Web site at http://ganc.sagepub.com/supplemental.
References
- 1. Shibahara K, Asano M, Ishida Y, Aoki T, Koike T, Honjo T. Isolation of a novel mouse gene MA-3 that is induced upon programmed cell death. Gene 1995;166:297-301 [DOI] [PubMed] [Google Scholar]
- 2. Cmarik JL, Min H, Hegamyer G, Zhan S, Kulesz-Martin M, Yoshinaga H, et al. Differentially expressed protein Pdcd4 inhibits tumor promoter-induced neoplastic transformation. Proc Natl Acad Sci USA 1999;96:14037-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jansen AP, Camalier CE, Colburn NH. Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res 2005;65:6034-41 [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S, et al. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol 2003;200:640-6 [DOI] [PubMed] [Google Scholar]
- 5. Afonja O, Juste D, Das S, Matsuhashi S, Samuels HH. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells: evidence for a role in apoptosis. Oncogene 2004;23:8135-45 [DOI] [PubMed] [Google Scholar]
- 6. Zhang H, Ozaki I, Mizuta T, Hamajima H, Yasutake T, Eguchi Y, et al. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene 2006;25:6101-12 [DOI] [PubMed] [Google Scholar]
- 7. Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer 2007;110:1697-707 [DOI] [PubMed] [Google Scholar]
- 8. Wang Q, Sun Z, Yang H-S. Downregulation of tumour suppressor Pdcd4 promotes invasion and activates both β-catenin/TCF and AP-1-dependent transcription in colon carcinoma cells. Oncogene 2008;27:1527-35 [DOI] [PubMed] [Google Scholar]
- 9. Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem 2008;283:1026-33 [DOI] [PubMed] [Google Scholar]
- 10. Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 2008;27:4373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008;27:2128-36 [DOI] [PubMed] [Google Scholar]
- 12. Dorello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 2006;314:467-71 [DOI] [PubMed] [Google Scholar]
- 13. Schmid T, Jansen AP, Baker AR, Hegamyer G, Hagan JP, Colburn NH. Translation inhibitor Pdcd4 is targeted for degradation during tumor promotion. Cancer Res 2008;68:1254-60 [DOI] [PubMed] [Google Scholar]
- 14. Yang HS, Matthews CP, Clair T, Wang Q, Baker AR, Li CC, et al. Tumorigenesis suppressor Pdcd4 down-regulates mitogen-activated proteinkinase kinase kinase kinase 1 expression to suppress colon carcinoma cell invasion. Mol Cell Biol 2006;26:1297-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leupold JH, Yang HS, Colburn NH, Asangani I, Post S, Allgayer H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene 2007;26:4550-62 [DOI] [PubMed] [Google Scholar]
- 16. Bitomsky N, Wethkamp N, Marikkannu R, Klempnauer K-H. siRNA-mediated knock-down of Pdcd4 expression causes up-regulation of p21(Waf1/Cip1) expression. Oncogene 2008;27:4820-9 [DOI] [PubMed] [Google Scholar]
- 17. Singh P, Marikkannu R, Bitomsky N, Klempnauer K-H. Disruption of the Pdcd4 tumor suppressor gene in chicken DT40 cells reveals its role in the DNA-damage response. Oncogene 2009;28:3758-64 [DOI] [PubMed] [Google Scholar]
- 18. Böhm M, Sawicka K, Siebrasse JP, Brehmer-Fastnacht A, Peters R, Klempnauer K-H. The transformation suppressor protein Pdcd4 shuttles between nucleus and cytoplasm and binds RNA. Oncogene 2003;22:4905-10 [DOI] [PubMed] [Google Scholar]
- 19. Palamarchuk A, Efanov A, Maximov V, Aqeilan RI, Croce CM, Pekarsky Y. Akt phosphorylates and regulates Pdcd4 tumor suppressor protein. Cancer Res 2005;65:11282-6 [DOI] [PubMed] [Google Scholar]
- 20. Yang HS, Knies JL, Stark C, Colburn NH. Pdcd4 suppresses tumor phenotype in JB6 cells by inhibiting AP-1 transactivation. Oncogene 2003;22:3712-20 [DOI] [PubMed] [Google Scholar]
- 21. Bitomsky N, Böhm M, Klempnauer K-H. Transformation suppressor protein Pdcd4 interferes with JNK-mediated phosphorylation of c-Jun and recruitment of the coactivator p300 by c-Jun. Oncogene 2004;23:7484-93 [DOI] [PubMed] [Google Scholar]
- 22. Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol 2003;23:26-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang HS, Cho MH, Zacowicz H, Hegamyer G, Sonenberg N, Colburn N. A novel function of the MA-3 domains in transformation and translation suppressor Pdcd4 is essential for its binding to eukaryotic translation initiation factor 4A. Mol Cell Biol 2004;24:3894-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LaRonde-LeBlanc N, Santhanam AN, Baker AR, Wlodawer A, Colburn NH. Structural basis for inhibition of translation by the tumor suppressor Pdcd4. Mol Cell Biol 2007;27:147-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waters LC, Veverka V, Böhm M, Schmedt T, Choong PT, Muskett FW, et al. Structure of the C-terminal MA-3 domain of the tumour suppressor protein Pdcd4 and characterization of its interaction with eIF4A. Oncogene 2007;26:4941-50 [DOI] [PubMed] [Google Scholar]
- 26. Suzuki C, Garces RG, Edmonds KA, Hiller S, Hyberts SG, Marintchev A, et al. PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains. Proc Natl Acad Sci USA 2008;105:3274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang JH, Cho YH, Sohn SY, Choi JM, Kim A, Kim YC, et al. Crystal structure of the eIF4A-PDCD4 complex. Proc Natl Acad Sci USA 2009;106:3148-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loh PG, Yang HS, Walsh MA, Wang Q, Wang X, Cheng Z, et al. Structural basis for translational inhibition by the tumour suppressor Pdcd4. EMBO J 2009;28:274-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hershey JWB, Merrick WC. Pathway and mechanism of initiation of protein synthesis. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational control of gene expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. p 33-88 [Google Scholar]
- 30. Kang M-J, Ahn H-S, Lee J-Y, Matsuhashi S, Park W-Y. Up-regulation of Pdcd4 in senescent human diploid fibroblasts. Biochem Biophys Res Commun 2002;293:617-21 [DOI] [PubMed] [Google Scholar]
- 31. Burk O, Mink S, Ringwald M, Klempnauer K-H. Synergistic activation of the chicken mim-1 gene by v-myb and C/EBP transcription factors. EMBO J 1993;12:2027-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schubert S, Horstmann S, Bartusel T, Klempnauer K-H. The co-operation of B-Myb with the coactivator p300 is orchestrated by cyclins A and D1. Oncogene 2004;23:1392-404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


