Abstract
Cancer cells depend on signals that promote cell cycle progression and prevent programmed cell death that would otherwise result from cumulative, aberrant stress. These activities require the temporally controlled destruction of specific intracellular proteins by the ubiquitin-proteasome system (UPS). To a large extent, the control points in this process include a family of E3 ubiquitin ligases called cullin-RING ligases (CRLs). The ligase activity of these multicomponent complexes requires modification of the cullin protein situated at their core with a ubiquitin-like protein called NEDD8. Neddylation results in conformational rearrangements within the CRL, which are necessary for ubiquitin transfer to a substrate. The NEDD8 pathway thus has a critical role in mediating the ubiquitination of numerous CRL substrate proteins involved in cell cycle progression and survival including the DNA replication licensing factor Cdt-1, the NF-κB transcription factor inhibitor pIκBα, and the cell cycle regulators cyclin E and p27. The initial step required for attachment of NEDD8 to a cullin is catalyzed by the E1, NEDD8-activating enzyme (NAE). The first-in-class inhibitor of NAE, MLN4924, has been shown to block the activity of NAE and prevent the subsequent neddylation of cullins. Preclinical studies have demonstrated antitumor activity in various solid tumors and hematological malignancies, and preliminary clinical data have shown the anticipated pharmacodynamic effects in humans. Here, we review the NEDD8 pathway, its importance in cancer, and the therapeutic potential of NAE inhibition.
Keywords: NEDD8, ubiquitin, cullin-RING ligase, DNA rereplication
Introduction
The ubiquitin-proteasome system (UPS) is responsible for the bulk of protein turnover in eukaryotic cells and plays a key role in maintaining cellular protein homeostasis.1-5 Among UPS substrates are proteins involved in cell cycle regulation, cellular growth and proliferation, intracellular signaling, DNA repair, membrane receptor regulation, and proapoptotic and antiapoptotic signaling.1-5 Dysregulation of the UPS, through constitutive activation of particular signaling pathways or loss of specific functions, for example, can result in uncontrolled proliferation or sustained prosurvival signaling and potentially result in the development of cancer and other diseases.1-4 Indeed, protein turnover and UPS signaling pathways are upregulated in some cancer cells and may explain why these cells are also susceptible to the apoptotic effects of inhibition of the UPS.1 The therapeutic potential of UPS inhibition has been validated with the first-in-class proteasome inhibitor, bortezomib (VELCADE, Millennium Pharmaceuticals Inc., Cambridge, MA), which is approved for the treatment of multiple myeloma6,7 and the treatment of relapsed mantle cell lymphoma.8
Proteins destined for degradation by the proteasome are usually first marked by covalent attachment of a polyubiquitin chain.9 Ubiquitination of a substrate results from a multistep enzymatic cascade (Fig. 1), initiated by an E1 ubiquitin–activating enzyme (primarily UAE, although another E1, Uba6, may also be important) in an ATP-dependent process. Ubiquitin from the E1 is then passed via a transthiolation reaction to one of multiple different E2 ubiquitin–conjugating enzymes. The ubiquitin-charged E2 can then form a complex with an E3 ubiquitin ligase, and ultimately, ubiquitin is transferred to a lysine residue on the substrate.10 There are several hundred different E3 ligases,11 which belong to 3 different classes defined according to the domain found in their core protein: HECT (homologous to E6-AP carboxy terminus), RING (Really Interesting New Gene) finger, and U-box E3s.12 Within the RING finger E3s are 2 subclasses. The simple RING finger E3s contain the E2-binding RING domain and substrate-binding domain within the same polypeptide. In contrast, the cullin-RING ligases (CRLs) are multicomponent complexes based upon a cullin protein core tightly associated with the RING finger domain– containing protein, RBX1/2, and various interchangeable adaptor and receptor components for substrate recognition (Fig. 2).12
Figure 1.
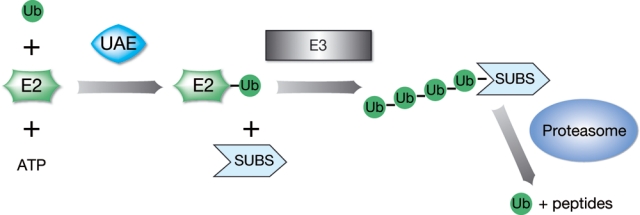
Protein degradation by the UPS is a highly regulated process. Proteasomes degrade proteins that are tagged with polyubiquitin chains. The formation of the polyubiquitin chain is catalyzed by a series of enzymes. In the first step, the E1-activating enzyme, UAE, activates ubiquitin and transfers it via a transthiolation reaction to one of many E2 enzymes. The ubiquitin-charged E2 then collaborates with specific E3 ligases to catalyze the formation of the polyubiquitin chain on the substrate protein recruited by that ligase. There are 3 broad subclasses of E3 ligases: HECT domain, RING finger domain, and U-box E3s (for review, see Nalepa et al.125).
Figure 2.

CRLs represent the largest subfamily of RING finger domain E3 ligases. These multisubunit ligases consist of a core cullin protein tightly associated with the RING finger domain–containing protein RBX1/2. Various interchangeable adaptor and receptor components facilitate diverse substrate recognition. The cullin protein must be modified by NEDD8 to activate holoenzyme ubiquitin ligase activity. Neddylation, similar to ubiquitination, is initiated by a specific E1 for NEDD8, NAE. NAE utilizes ATP to form NEDD8 adenylate; the NEDD8 is subsequently transferred to a specific cysteine within NAE, forming a NAE-NEDD8 thioester before transthiolation to the active site cysteine of UBC12 or UBE2F, E2s specific for the NEDD8 pathway. Conjugation of NEDD8 to cullin activates the ubiquitin ligase activity of the CRL, and removal of NEDD8 by the COP9 signalosome facilitates dissociation of CRL components. Unneddylated cullin-RING complexes bind to CAND1 until recruited to form a new CRL. The NAE-specific inhibitor MLN4924 forms a covalent adduct with NEDD8 in a reaction catalyzed by NAE, locking the enzyme in an inactive state.
While the UAE-ubiquitination pathway is the best characterized, a number of homologous enzymatic cascades have been described for another 8 different classes of ubiquitin-like proteins (UBLs).10 These UBLs are structurally related to ubiquitin and similarly form conjugates with a broad range of different substrates through discrete E1-E2 (and sometimes E3) cascades. Each cascade is initiated by a unique E1; UBLs and their E1s include NEDD8 (NEDD8-activating enzyme, NAE), SUMO-1, -2, -3 (SUMO-activating enzyme, SAE), ATG8, ATG12 (both ATG7), ISG15 (Uba7), Urm1 (Uba4), Ufm1 (Uba5), and FAT10 (Uba6).9,10 While each UBL pathway shares similar structural and mechanistic features with the ubiquitin pathway, the biological consequences of each are somewhat different with respect to cellular physiology, with each pathway associated with different functions.10,13,14 For example, the NEDD8 pathway plays a critical role in the activation of the ubiquitin E3 ligase activity of CRL E3s via the covalent attachment of NEDD8 to the core cullin protein of these enzyme complexes.12 This process of neddylation has been shown to be essential for the E3 ligase activity of CRLs. CRLs are a large superfamily of E3s that are responsible for the ubiquitination of multiple substrate proteins, including several that are involved in the regulation of normal cellular function as well as some that have been shown to be associated with cancer (Table 1). Here, we review the NEDD8 conjugation pathway, its importance in cancer biology, and the potential for targeting it as a novel therapeutic approach.
Table 1.
Substrate Proteins of the Cullin-RING Ligases (CRLs) and Their Associations with Cancer
| Substrate | Role | CRL | Association with Cancer |
|---|---|---|---|
| Cdt-176,78,79,110 | DNA replication licensing factor | CRL1Skp2/CRL4cdt2 | Dysregulated expression in human tumors; overexpression linked to genomic instability |
| p2748,67,69,81,94 | Cyclin-dependent kinase inhibitor | CRL1Skp2/CRL4 | Reduced levels due to increased degradation; seen in multiple cancers, associated with poor prognosis |
| pIκBα46,74,75,109 | Inhibitor of NF-κB | CRL1βTrCP1 | Constitutive IKK activity results in pIκBα degradation and constitutive NF-κB activity, demonstrated in human cancers including ABC-DLBCLNF-κB signaling associated with chemoresistance in various tumor types |
| NRF270,111 | Stress-response transcription factor | CRL3Keap1 | Overexpressed in multiple human cancers; may also be associated with acquired chemoresistance |
| HIF-1α71-73,87 | Stress (hypoxia)–response transcription factor | CRL2VHL | Aberrant expression of VHL tumor suppressor protein results in development of a variety of tumors; HIF-1α important for tumor survival |
| Cyclin E66,67,112-115 | Cell cycle regulator | CRL1Fbw7 | Aberrant cyclin E expression associated with tumor development and progression in breast cancer and with various other cancers |
| c-Jun116 | AP1 transcription factor | CRL1Fbw7 | c-Jun identified as an oncoprotein |
| β-catenin75 | Transcription factor | CRL1βTrCP | Upregulated in various cancers including colon and prostate cancer and melanoma |
| Cdc25A117-119 | Activator of cyclin-dependent kinase complexes CDK2/cyclin A and CDK2/cyclin E | CRL1βTrCP | Upregulated in various cancers |
| Emi168,120 | APCCdc20 inhibitor | CRL1βTrCP/Slimb | Possible involvement in development of ovarian clear cell carcinoma |
| c-Myc121 | Cell proliferation regulator | CRL1Fbw7 | c-Myc identified as an oncoprotein |
| mTOR122,123 | Cell growth and proliferation signaling | CRL1Fbw7 | Dysregulated mTOR signaling in various human tumors |
| BimEL124 | Tumor suppressor; proapoptotic BH3-only protein | CRL1βTrcP | Levels decreased in transformed cells through enhanced degradation |
Biochemistry of Neddylation
NEDD8 is an 81–amino acid protein with 9 kDa relative molecular mass and is 60% identical and 80% homologous to ubiquitin.15,16 NEDD8 has a dedicated E1-activating enzyme (AppBp1/UBA3, or NAE)17 and E2-conjugating enzymes (UBC12, UBE2F)18 and is essential for the enzymatic activity of the CRL family of E3 ligases,12 through conjugation to the cullin scaffold. Other components of the neddylation pathway include DEN1,19 which processes NEDD8 to its mature, 76-amino acid form, and the COP9 signalosome complex, which is responsible for removing NEDD8 from cullin proteins.20,21 CAND1 (cullin-associated and neddylation-dissociated) is an additional component that regulates CRL complex assembly by binding to the cullin in the absence of NEDD8 activation.22 In this section, we will review our understanding of each of these important steps in the neddylation pathway and the key role of NEDD8 in CRL-mediated ubiquitinating activity.
The Neddylation Enzymatic Cascade
NEDD8 maturation and activation by NAE
The initial step in the NEDD8 cascade is the maturation of NEDD8. Human deneddylase 1 (DEN1),19 also known as NEDD8-specific protease (NEDP1) or SENP8,23 is responsible for processing the precursor form of NEDD8 into the mature form by removing 5 amino acid residues from its C-terminal tail.24-26 This processing step reveals the C-terminal residues of mature NEDD8, an essential step for the activation of NEDD8 and its downstream functions. DEN1 is highly specific for the NEDD8 precursor27 and does not process other UBL precursors, including SUMO or the highly homologous ubiquitin.28
NAE is a heterodimer consisting of the proteins AppBp1 and Uba317,29,30 and is structurally and mechanistically similar to UAE and the other UBL-activating enzymes. Like all E1s, NAE is highly selective for its cognate UBL, which in this case is NEDD8.31-33 In the first step in NEDD8 activation, MgATP and NEDD8 bind to NAE and form an acyl adenylate intermediate, NEDD8-AMP.18 NEDD8-AMP subsequently reacts with the active thiol site of the enzyme to form a NEDD8-NAE thioester, coupled with the release of AMP.29 A second round of MgATP and NEDD8 binding results in the formation of a second NEDD8-AMP, yielding a ternary complex capable of transferring NEDD8 to 1 of its 2 E2s (see below and Fig. 2). This enzymatic mechanism of NEDD8 activation mirrors that by which ubiquitin is activated by UAE.29
Conjugating NEDD8: the E2s UBC12 and UBE2F and CRL neddylation
NEDD8 is transferred from the NAE ternary complex to 1 of 2 NEDD8-specific E2s, UBC12 and UBE2F,34 via a transthiolation reaction. The specificity of the NEDD8 pathway is mediated by a unique interaction between NEDD8-charged NAE and its cognate E2s that promotes NEDD8-E2 thioester formation35; E2 docking occurs in a Uba3-specific groove that is not seen in other E1s, thus preventing cross-reactivity with other UBL pathways.35 NAE is able to recognize its 2 distinct E2s through a combination of the pliability of this specific hydrophobic interaction and conformational flexibility, and as a consequence, the neddylation cascade incorporates further specificity with regards to cullin modification, as UBC12 and UBE2F have been shown to neddylate different cullins.34 UBC12 specifically pairs with RING box protein 1 (RBX1), which associates with cullins 1 to 4 in CRL complexes, while UBE2F pairs with RBX2, to mediate cullin 5 neddylation.34
For this latter stage of the neddylation process, the transfer of NEDD8 from UBC12 or UBE2F to the respective cullin, there are some suggestions that ligase-like activity may be required, with Dcn1 (SCCRO) and related proteins suggested to function as facilitators of specific cullin neddylation.36-39 Similarly, it has been suggested that RBX1 has E3-type ligase activity for cullin-1 neddylation40 and regulates poly-NEDD8 chain formation.41
NEDD8 Modification of CRLs: An Essential Component of Enzymatic Activity
NEDD8 has been shown to modify all members of the cullin family via covalent attachment, and it is well established that NEDD8 modification of cullins is required for CRL-dependent ubiquitination.42-44 For example, ubiquitination of IκBα by CRL1βTrCP1 is activated by neddylation of cullin-1,45-47 and similarly, neddylation of cullin-1 is essential for the ubiquitination of p27kip1 by CRL1Skp2.48,49 Neddylation also increases the likelihood of polyubiquitination of β-catenin by CRL1βTrCP,50 while neddyla-tion of cullin-2 in the ECV (Elongin B/C, cullin-2, VHL) CRL is essential for ubiquitination of HIF1α, with the process driven by recognition of the HIF1α substrate by the von Hippel-Lindau (VHL) protein.51,52
Recent studies have helped elucidate the mechanistic importance of cullin neddylation to enhanced CRL substrate ubiquitination. One important aspect is that neddylation of the cullin scaffold has been shown to result in conformational rearrangements in the CRL complex structure,50,53,54 with the attachment of NEDD8 changing the CRL from a closed conformation in its unmodified state to an open one.53 These rearrangements are essential to CRL ligase activity, providing appropriate catalytic geometries for an associated E2 to transfer ubiquitin to a bound substrate molecule.53 The presence of NEDD8 has also been shown to eliminate the CAND1-binding site from the CRL, as described below.53 In addition, neddylation has been shown to accelerate the formation of the E2-E3 complex required for ubiquitination of substrates,45,50 and the presence of NEDD8 on the cullin scaffold of CRLs has been suggested to enhance E2 ubiquitin binding.55,56
Deneddylation and Cullin Binding: The Role of the COP9 Signalosome and CAND1
Deneddylation of CRLs is mediated via the COP9 signalosome (or CSN), a protein complex comprising 8 subunits.20,21 NEDD8 is removed by the metalloprotease activity of the JAMM motif in the CSN5 subunit,20,21 thereby deactivating the CRLs.57 Although deneddylation by CSN switches off the ubiquitinating activity of CRLs in vitro, CSN has been shown to be essential for CRL activity in vivo.57 This may be due to its protective effects against autoubiquitination and subsequent degradation of substrate-receptor F-box proteins in the CRL in the absence of substrate.58 In addition to COP9, cullins are deneddylated by DEN1 in vitro24-26,59; however, in vivo, COP9 appears solely responsible for cullin deneddylation, whereas DEN1 is responsible for deneddylation of other proteins, and thus, their activities are nonoverlapping.24
CAND1 is a protein that binds deneddylated cullin-RING complexes but not the neddylated forms.22 CAND1 binding to cullins blocks the binding sites for adaptor proteins and NEDD8 and thus acts as an inhibitor of CRL assembly and activation. However, as with COP9/CSN, CAND1 has also been shown to be required for efficient CRL activity,60 possibly by exerting some degree of control on the recruitment of different F-box proteins and thus ensuring CRL ubiquitinating activity against a full range of substrate proteins.22,61 The balance between neddylation and deneddylation may be influenced by the availability of adaptor proteins and substrates. In the case of CRL1Skp2, increased levels of Skp2 and p27 substrate were shown to increase levels of neddylated cullin-1 protein, with increased Skp-2 levels overcoming the inhibitory influence of CAND1 and elevated p27 inhibiting COP9-mediated deneddylation.62 Similarly, neddylation of cullin-1 has been shown to depend upon the binding of adaptor proteins and subunits involved in substrate recognition, although this regulation is independent of CAND1 and COP963; it has been suggested that E3 ligase activity may thus be regulated through the availability of substrate recognition subunits and substrates, which promote subsequent neddylation and CRL activity.63
Therapeutic Rationale for Targeting the NEDD8 Pathway
The Importance of the NEDD8 Pathway in Normal and Malignant Cells
The NEDD8 pathway has been shown to be essential for cellular function,64,65 through its critical role in mediating the ubiquitination by CRLs of numerous proteins involved in cell cycle progression and cell growth and survival. Table 1 highlights some key substrate proteins of NEDD8-regulated CRLs whose dysregulation is often associated with cancer. These proteins include various cell cycle regulators (cyclin E,66,67 Emi1,68 and p2748,67,69), transcription factors (NRF2,70 HIF-1α71-73), and inhibitors of transcription (pIκBα46,74,75), as well as regulators of DNA replication (Cdt-176).
Given the importance of the NEDD8 pathway and CRL substrates in cell cycle regulation, it is not surprising that disruption of normal NEDD8 processes adversely affects normal cell cycle progression. The ts41 Chinese hamster ovary cell line contains a temperature-sensitive mutation in the NEDD8 conjugation pathway. At the nonpermissive temperature, cells pass through successive S phases without intervening mitosis and accumulate DNA through multiple replications.77 This mutation could be rescued by transfection with vectors expressing AppBp1, one component of the NAE heterodimer, which subsequently restores functional neddylation.30 Interestingly, the levels of the replication licensing factor Cdt-1 must be precisely controlled via NEDD8-regulated CRL4aDDB1-cdt2 and CRL1Skp2-mediated ubiquitination and degradation to ensure appropriate DNA replication only once per cell cycle. Overriding this control mechanism by either overexpression of Cdt-1 or knockdown of geminin, the endogenous inhibitor of Cdt-1, has been shown to lead to rereplication and genomic instability, a similar phenotype to that observed in the ts41 cell line. Cdt-1 is also reported to be targeted for ubiquitination by CRL4aDDB1-cdt2 in response to UV-induced DNA damage.78,79
Examples of upregulated CRL activity have been described in the cancer setting. Recently, Merlin was described as an inhibitor of CRLDCAF1. Mutations in the tumor suppressor NF2, which encodes for Merlin, are associated with a number of tumor types.80 Mutations in NF2 disrupt the inhibitory activity of Merlin, leading to enhanced proliferation through uncontrolled ubiquitination of substrate proteins by CRL4DCAF1. Similarly, overexpression of the F-box protein Skp2 is associated with tumorigenesis, and inactivation of Skp2 and consequently the activity of its associated CRL trigger cellular senescence and tumor regression.81 Moreover, cullin-1 overexpression has been demonstrated in 40% of lung tumors.82 While it is unclear whether alteration in the neddylation pathway is a contributing factor in such settings, inhibition of neddylation may reverse these effects.
Although the best characterized role of NEDD8 is the activation of CRLs via modification of cullin proteins, various studies have suggested that NEDD8 plays additional roles in other cellular processes. Numerous other proteins have been reported to be associated with or modified by NEDD8,43 including p5383 and Mdm2,84,85 epidermal growth factor receptor,86 VHL tumor suppressor protein,87,88 L11,89 and other ribosomal proteins90 (see Rabut and Peter91 and Xirodimas92 for reviews). While it is not clear what role NEDD8 modification plays in many of these proteins, in the case of pVHL, it has been suggested to be functionally important in both HIF1α ubiquitination and fibronectin matrix assembly. In renal cell carcinoma cells, expression of pVHL that could not be neddylated resulted in a lack of fibronectin matrix assembly and in tumor formation in vivo, suggesting the importance of NEDD8 modification to pVHL’s tumor suppression function.88
Therapeutic Potential of NEDD8 Pathway Inhibition
CRL substrates and their importance in cancer
In a recent study of NEDD8 pathway inhibition, it was estimated that CRLs are responsible for ubiquitinating approximately 20% of all intracellular proteins that are marked for proteasomal degradation.93 A number of these proteins have been identified as having associations with cancer, as detailed in Table 1.
For example, dysregulation of p27 expression occurs in various cancers,94 and low levels of p27 are associated with poor outcomes in mantle cell lymphoma,95 prostate cancer,96 non–small cell lung cancer,97 and colorectal carcinoma.98 One E3 ligase for p27 is CRL1Skp2,81 and Skp2 overexpression is reported in a number of human cancers.81,99-101 Such overexpression potentially drives the enhanced proteasomal degradation of p27. In addition, CRL4 has been shown to promote the ubiquitination and degradation of p27 independent of SCFSkp2 in mammary tumors, associated with induction of cullin 4a and 4b by active Wnt signaling.94
Another important example is the constitutive NF-κB signaling that occurs in activated B-cell–like (ABC) diffuse large B-cell lymphoma (DLBCL).102 This constitutive activity arises due to upregulated IKK activity, which makes the NF-κB inhibitor, IκBα, a constant substrate for CRL1βTrcP-mediated degradation.
MLN4924: a specific inhibitor of NAE
MLN4924 (Millennium Pharmaceuticals Inc.) was recently reported as a potent and selective inhibitor of NAE.93 MLN4924 (Fig. 3), an adenosine sulfamate analog,93 is a mechanism-based inhibitor of NAE, in that NAE catalyzes the formation of a NEDD8-MLN4924 inhibitor adduct in situ (Fig. 2). This adduct resembles adenylated NEDD8, binds tightly to the adenylation site, but cannot be used to form the thioester, and thus locks the enzyme in an inactive state.103
Figure 3.

MLN4924, a first-in-class inhibitor of NAE.
Preclinically, MLN4924 demonstrated the anticipated effects of NAE inhibition. Levels of numerous CRL substrates were shown to increase in cultured cells and in human tumor xenografts following compound treatment. Tumor growth inhibition was also demonstrated with MLN4924 in HCT-116 xenografts and in lung tumor xenografts.93 Although MLN4924 was shown to block the turnover of multiple CRL substrates, surprisingly, the most common consequence of NAE inhibition observed in cells was DNA rereplication. This phenotype is associated with the inhibition of Cdt-1 degradation (Milhollen et al., manuscript in preparation).93 As mentioned above, Cdt-1 is a critical licensing factor for DNA replication, and its overexpression has been shown to result in rereplication, DNA damage, and consequent apoptosis.76,78,104,105 In HCT-116 cells and other human tumor–derived cell lines, MLN4924 caused cells to accumulate in S phase and to increase their DNA content without transitioning into mitosis,93 with the resulting DNA damage leading to eventual apoptosis. Indicators of DNA damage were also observed in the HCT-116 tumor xenograft following MLN4924 treatment.93
Although DNA rereplication was the most frequent phenotype observed in cells following NAE inhibition, a distinct phenotype was observed in models of ABC-DLBCL, which depend on NF-κB signaling for survival.106 In NF-κB–dependent DLBCL, apoptosis resulted from blocking NF-κB signaling, a consequence of inhibiting CRL-mediated turnover of pIκBα.106 In NF-κB–independent germinal center B-cell–like (GCB) DLBCL cells, the rereplication phenotype was observed. In in vivo studies, MLN4924 resulted in tumor growth inhibition in both ABC- and GCB-DLBCL mouse xenograft models, with evidence for the alternative mechanism of action of NF-κB inhibition in the ABC-DLBCL model.106 These results suggest that MLN4924 may have activity against tumors that are dependent on NF-κB signaling for survival. Recent preclinical studies with acute myeloid leukemia cell lines and mouse xenograft models have shown that MLN4924 results in CRL substrate accumulation, DNA damage (as evidenced by increased pCHK1 levels), and decreased expression of NF-κB targets as well as reduced DNA binding activity of NF-κB–p65.107 Finally, MLN4924 has also been shown to result in cellular senescence in PC3 prostate cancer cells and growth suppression of PC3 tumors in vivo,81 possibly associated with inhibition of Skp2 CRL activity.
Recently, preliminary findings have been reported from a phase 1 study of MLN4924 in patients with relapsed and/or refractory multiple myeloma or non-Hodgkin lymphoma.108 Analysis of NEDD8-cullin and pIκBα levels in peripheral blood mononuclear cells, plus Cdt-1 and NRF2 levels in skin, demonstrated that the expected pharmacodynamic effects of NAE inhibition were seen in humans.108 This phase 1 study is ongoing, and additional phase 1 studies are evaluating MLN4924 in patients with melanoma, other nonhematological malignancies, and acute myeloid leukemia.
Conclusions
Our emerging understanding of the UPS and its specific components is resulting in an increasing number of potential therapeutic targets for the modulation of specific cellular pathways of importance. In addition, our understanding of the importance of the neddylation pathway in various cellular processes has emerged over the past decade, as well as the recognition that its specific function of regulating CRL activity provides an opportunity for targeting this group of enzymes within the UPS. The CRLs are responsible for the degradation of numerous proteins known to be of importance in various tumor types, thus demonstrating the relevance of the neddylation pathway in cancer. Furthermore, the example of NAE inhibition with MLN4924 may provide a blueprint for similar targeting of other UBL pathways of relevance in cancer and other human diseases.
NEDD8 pathway inhibition appears to be a potentially important anticancer strategy, as evidenced by preliminary studies with the first-in-class NAE inhibitor MLN4924; however, only the results of clinical studies will determine the development of this approach in different tumors. NEDD8 pathway inhibition may prove effective in a number of indications, possibly including tumors associated with Skp2 overexpression and other upregulated CRL activity, such as Wnt signaling–mediated CRL4 activity, and potentially including tumors dependent on NF-κB signaling. Given the phenotypes associated with NAE inhibition by MLN4924, combination strategies may prove effective for enhancing the antitumor effects of NEDD8 pathway inhibition; for example, in tumors in which the rereplication phenotype is observed, combined treatment with other agents active in S phase may prove a promising approach, while in NF-κB–dependent tumors, the combination of MLN4924 with other agents targeted at this pathway may result in enhanced activity. Of note, NF-κB has been associated with mechanisms of resistance to other chemotherapeutic agents,109 and thus, MLN4924 may represent an important combination partner for such agents. Further studies may identify other cellular pathways, the targeting of which results in synergistic antitumor activity with NAE inhibition.
Acknowledgments
The authors thank Steve Hill of FireKite for writing assistance in the development of this paper, which was supported by funding from Millennium Pharmaceuticals Inc.
Footnotes
The authors are employees of Millennium Pharmaceuticals Inc.
References
- 1. Reinstein E, Ciechanover A. Narrative review. Protein degradation and human diseases: the ubiquitin connection. Ann Intern Med. 2006;145:676-84 [DOI] [PubMed] [Google Scholar]
- 2. Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ciechanover A, Schwartz AL. The ubiquitin-proteasome pathway: the complexity and myriad functions of proteins death. Proc Natl Acad Sci U S A. 1998;95:2727-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ. 2005;12:1178-90 [DOI] [PubMed] [Google Scholar]
- 5. Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005;12:1191-7 [DOI] [PubMed] [Google Scholar]
- 6. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487-98 [DOI] [PubMed] [Google Scholar]
- 7. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906-17 [DOI] [PubMed] [Google Scholar]
- 8. Kane RC, Dagher R, Farrell A, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291-4 [DOI] [PubMed] [Google Scholar]
- 9. Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature. 2009;458:422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schulman BA, Harper JW. Ubiquitin-like protein activation by E1 enzymes: the apex for downstream signalling pathways. Nat Rev Mol Cell Biol. 2009;10:319-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petroski MD. The ubiquitin system, disease, and drug discovery. BMC Biochem. 2008;9 Suppl 1:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9-20 [DOI] [PubMed] [Google Scholar]
- 13. Ye Y, Rape M. Building ubiquitin chains: E2 enzymes at work. Nat Rev Mol Cell Biol. 2009;10:755-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeh ET, Gong L, Kamitani T. Ubiquitin-like proteins: new wines in new bottles. Gene. 2000;248:1-14 [DOI] [PubMed] [Google Scholar]
- 15. Kumar S, Yoshida Y, Noda M. Cloning of a cDNA which encodes a novel ubiquitin-like protein. Biochem Biophys Res Commun. 1993;195:393-9 [DOI] [PubMed] [Google Scholar]
- 16. Kamitani T, Kito K, Nguyen HP, Yeh ET. Characterization of NEDD8, a developmentally down-regulated ubiquitin-like protein. J Biol Chem. 1997;272:28557-62 [DOI] [PubMed] [Google Scholar]
- 17. Huang DT, Schulman BA. Expression, purification, and characterization of the E1 for human NEDD8, the heterodimeric APPBP1-UBA3 complex. Methods Enzymol. 2005;398:9-20 [DOI] [PubMed] [Google Scholar]
- 18. Gong L, Yeh ET. Identification of the activating and conjugating enzymes of the NEDD8 conjugation pathway. J Biol Chem. 1999;274:12036-42 [DOI] [PubMed] [Google Scholar]
- 19. Gan-Erdene T, Nagamalleswari K, Yin L, Wu K, Pan ZQ, Wilkinson KD. Identification and characterization of DEN1, a deneddylase of the ULP family. J Biol Chem. 2003;278:28892-900 [DOI] [PubMed] [Google Scholar]
- 20. Kato JY, Yoneda-Kato N. Mammalian COP9 signalosome. Genes Cells. 2009;14:1209-25 [DOI] [PubMed] [Google Scholar]
- 21. Wei N, Serino G, Deng XW. The COP9 signalosome: more than a protease. Trends Biochem Sci. 2008;33:592-600 [DOI] [PubMed] [Google Scholar]
- 22. Liu J, Furukawa M, Matsumoto T, Xiong Y. NEDD8 modification of CUL1 dissociates p120(CAND1), an inhibitor of CUL1-SKP1 binding and SCF ligases. Mol Cell. 2002;10:1511-8 [DOI] [PubMed] [Google Scholar]
- 23. Hemelaar J, Borodovsky A, Kessler BM, et al. Specific and covalent targeting of conjugating and deconjugating enzymes of ubiquitin-like proteins. Mol Cell Biol. 2004;24:84-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chan Y, Yoon J, Wu JT, et al. DEN1 deneddylates non-cullin proteins in vivo. J Cell Sci. 2008;121:3218-23 [DOI] [PubMed] [Google Scholar]
- 25. Mendoza HM, Shen LN, Botting C, et al. NEDP1, a highly conserved cysteine protease that deNEDDylates Cullins. J Biol Chem. 2003;278:25637-43 [DOI] [PubMed] [Google Scholar]
- 26. Wu K, Yamoah K, Dolios G, et al. DEN1 is a dual function protease capable of processing the C terminus of Nedd8 and deconjugating hyper-neddylated CUL1. J Biol Chem. 2003;278:28882-91 [DOI] [PubMed] [Google Scholar]
- 27. Shen LN, Liu H, Dong C, Xirodimas D, Naismith JH, Hay RT. Structural basis of NEDD8 ubiquitin discrimination by the deNEDDylating enzyme NEDP1. EMBO J. 2005;24:1341-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reverter D, Wu K, Erdene TG, Pan ZQ, Wilkinson KD, Lima CD. Structure of a complex between Nedd8 and the Ulp/Senp protease family member Den1. J Mol Biol. 2005;345:141-51 [DOI] [PubMed] [Google Scholar]
- 29. Bohnsack RN, Haas AL. Conservation in the mechanism of Nedd8 activation by the human AppBp1-Uba3 heterodimer. J Biol Chem. 2003;278:26823-30 [DOI] [PubMed] [Google Scholar]
- 30. Chen Y, McPhie DL, Hirschberg J, Neve RL. The amyloid precursor protein-binding protein APP-BP1 drives the cell cycle through the S-M checkpoint and causes apoptosis in neurons. J Biol Chem. 2000;275:8929-35 [DOI] [PubMed] [Google Scholar]
- 31. Walden H, Podgorski MS, Huang DT, et al. The structure of the APPBP1-UBA3-NEDD8-ATP complex reveals the basis for selective ubiquitin-like protein activation by an E1. Mol Cell. 2003;12:1427-37 [DOI] [PubMed] [Google Scholar]
- 32. Souphron J, Waddell MB, Paydar A, Tokgoz-Gromley Z, Roussel MF, Schulman BA. Structural dissection of a gating mechanism preventing misactivation of ubiquitin by NEDD8’s E1. Biochemistry. 2008;47:8961-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Whitby FG, Xia G, Pickart CM, Hill CP. Crystal structure of the human ubiquitin-like protein NEDD8 and interactions with ubiquitin pathway enzymes. J Biol Chem. 1998;273:34983-91 [DOI] [PubMed] [Google Scholar]
- 34. Huang DT, Ayrault O, Hunt HW, et al. E2-RING expansion of the NEDD8 cascade confers specificity to cullin modification. Mol Cell. 2009;33:483-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang DT, Miller DW, Mathew R, et al. A unique E1-E2 interaction required for optimal conjugation of the ubiquitin-like protein NEDD8. Nat Struct Mol Biol. 2004;11:927-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim AY, Bommelje CC, Lee BE, et al. SCCRO (DCUN1D1) is an essential component of the E3 complex for neddylation. J Biol Chem. 2008;283:33211-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurz T, Chou YC, Willems AR, et al. Dcn1 functions as a scaffold-type E3 ligase for cullin neddylation. Mol Cell. 2008;29:23-35 [DOI] [PubMed] [Google Scholar]
- 38. Meyer-Schaller N, Chou YC, Sumara I, et al. The human Dcn1-like protein DCNL3 promotes Cul3 neddylation at membranes. Proc Natl Acad Sci U S A. 2009;106:12365-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang X, Zhou J, Sun L, et al. Structural basis for the function of DCN-1 in protein Neddylation. J Biol Chem. 2007;282:24490-4 [DOI] [PubMed] [Google Scholar]
- 40. Morimoto M, Nishida T, Nagayama Y, Yasuda H. Nedd8-modification of Cul1 is promoted by Roc1 as a Nedd8-E3 ligase and regulates its stability. Biochem Biophys Res Commun. 2003;301:392-8 [DOI] [PubMed] [Google Scholar]
- 41. Ohki Y, Funatsu N, Konishi N, Chiba T. The mechanism of poly-NEDD8 chain formation in vitro. Biochem Biophys Res Commun. 2009;381:443-7 [DOI] [PubMed] [Google Scholar]
- 42. Hori T, Osaka F, Chiba T, et al. Covalent modification of all members of human cullin family proteins by NEDD8. Oncogene. 1999;18:6829-34 [DOI] [PubMed] [Google Scholar]
- 43. Jones J, Wu K, Yang Y, et al. A targeted proteomic analysis of the ubiquitin-like modifier nedd8 and associated proteins. J Proteome Res. 2008;7:1274-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wada H, Yeh ET, Kamitani T. Identification of NEDD8-conjugation site in human cullin-2. Biochem Biophys Res Commun. 1999;257:100-5 [DOI] [PubMed] [Google Scholar]
- 45. Kawakami T, Chiba T, Suzuki T, et al. NEDD8 recruits E2-ubiquitin to SCF E3 ligase. EMBO J. 2001;20:4003-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Read MA, Brownell JE, Gladysheva TB, et al. Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha. Mol Cell Biol. 2000;20:2326-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu K, Chen A, Pan ZQ. Conjugation of Nedd8 to CUL1 enhances the ability of the ROC1-CUL1 complex to promote ubiquitin polymerization. J Biol Chem. 2000;275:32317-24 [DOI] [PubMed] [Google Scholar]
- 48. Podust VN, Brownell JE, Gladysheva TB, et al. A Nedd8 conjugation pathway is essential for proteolytic targeting of p27Kip1 by ubiquitination. Proc Natl Acad Sci U S A. 2000;97:4579-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morimoto M, Nishida T, Honda R, Yasuda H. Modification of cullin-1 by ubiquitin-like protein Nedd8 enhances the activity of SCF(skp2) toward p27(kip1). Biochem Biophys Res Commun. 2000;270:1093-6 [DOI] [PubMed] [Google Scholar]
- 50. Saha A, Deshaies RJ. Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol Cell. 2008;32:21-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sufan RI, Ohh M. Role of the NEDD8 modification of Cul2 in the sequential activation of ECV complex. Neoplasia. 2006;8:956-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wada H, Yeh ET, Kamitani T. The von Hippel-Lindau tumor suppressor gene product promotes, but is not essential for, NEDD8 conjugation to cullin-2. J Biol Chem. 1999;274:36025-9 [DOI] [PubMed] [Google Scholar]
- 53. Duda DM, Borg LA, Scott DC, Hunt HW, Hammel M, Schulman BA. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134:995-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zheng N, Schulman BA, Song L, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703-9 [DOI] [PubMed] [Google Scholar]
- 55. Pan ZQ, Kentsis A, Dias DC, Yamoah K, Wu K. Nedd8 on cullin: building an expressway to protein destruction. Oncogene. 2004;23:1985-97 [DOI] [PubMed] [Google Scholar]
- 56. Sakata E, Yamaguchi Y, Miyauchi Y, et al. Direct interactions between NEDD8 and ubiquitin E2 conjugating enzymes upregulate cullin-based E3 ligase activity. Nat Struct Mol Biol. 2007;14:167-8 [DOI] [PubMed] [Google Scholar]
- 57. Wu JT, Lin HC, Hu YC, Chien CT. Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation. Nat Cell Biol. 2005;7:1014-20 [DOI] [PubMed] [Google Scholar]
- 58. Schmidt MW, McQuary PR, Wee S, Hofmann K, Wolf DA. F-box-directed CRL complex assembly and regulation by the CSN and CAND1. Mol Cell. 2009;35:586-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamoah K, Wu K, Pan ZQ. In vitro cleavage of Nedd8 from cullin 1 by COP9 signalosome and deneddylase 1. Methods Enzymol. 2005;398:509-22 [DOI] [PubMed] [Google Scholar]
- 60. Bosu DR, Kipreos ET. Cullin-RING ubiquitin ligases: global regulation and activation cycles. Cell Div. 2008;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dubiel W. Resolving the CSN and CAND1 paradoxes. Mol Cell. 2009;35:547-9 [DOI] [PubMed] [Google Scholar]
- 62. Bornstein G, Ganoth D, Hershko A. Regulation of neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate. Proc Natl Acad Sci U S A. 2006;103:11515-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chew EH, Hagen T. Substrate-mediated regulation of cullin neddylation. J Biol Chem. 2007;282:17032-40 [DOI] [PubMed] [Google Scholar]
- 64. Tateishi K, Omata M, Tanaka K, Chiba T. The NEDD8 system is essential for cell cycle progression and morphogenetic pathway in mice. J Cell Biol. 2001;155:571-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ohh M, Kim WY, Moslehi JJ, et al. An intact NEDD8 pathway is required for Cullin-dependent ubiquitylation in mammalian cells. EMBO Rep. 2002;3:177-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369-81 [DOI] [PubMed] [Google Scholar]
- 67. Nakayama K, Nagahama H, Minamishima YA, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Margottin-Goguet F, Hsu JY, Loktev A, Hsieh HM, Reimann JD, Jackson PK. Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev Cell. 2003;4:813-26 [DOI] [PubMed] [Google Scholar]
- 69. Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193-9 [DOI] [PubMed] [Google Scholar]
- 70. Kobayashi A, Kang MI, Okawa H, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci U S A. 2000;97:10430-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koh MY, Spivak-Kroizman TR, Powis G. HIF-1alpha and cancer therapy. Recent Results Cancer Res. 2010;180:15-34 [DOI] [PubMed] [Google Scholar]
- 73. Liakopoulos D, Busgen T, Brychzy A, Jentsch S, Pause A. Conjugation of the ubiquitin-like protein NEDD8 to cullin-2 is linked to von Hippel-Lindau tumor suppressor function. Proc Natl Acad Sci U S A. 1999;96:5510-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tanaka K, Kawakami T, Tateishi K, Yashiroda H, Chiba T. Control of IkappaBalpha proteolysis by the ubiquitin-proteasome pathway. Biochimie. 2001;83:351-6 [DOI] [PubMed] [Google Scholar]
- 75. Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nishitani H, Sugimoto N, Roukos V, et al. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Handeli S, Weintraub H. The ts41 mutation in Chinese hamster cells leads to successive S phases in the absence of intervening G2, M, and G1. Cell. 1992;71:599-611 [DOI] [PubMed] [Google Scholar]
- 78. Hu J, McCall CM, Ohta T, Xiong Y. Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat Cell Biol. 2004;6:1003-9 [DOI] [PubMed] [Google Scholar]
- 79. Kondo T, Kobayashi M, Tanaka J, et al. Rapid degradation of Cdt1 upon UV-induced DNA damage is mediated by SCFSkp2 complex. J Biol Chem. 2004;279:27315-9 [DOI] [PubMed] [Google Scholar]
- 80. Li W, You L, Cooper J, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lin HK, Chen Z, Wang G, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Salon C, Brambilla E, Brambilla C, Lantuejoul S, Gazzeri S, Eymin B. Altered pattern of Cul-1 protein expression and neddylation in human lung tumours: relationships with CAND1 and cyclin E protein levels. J Pathol. 2007;213:303-10 [DOI] [PubMed] [Google Scholar]
- 83. Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell. 2004;118:83-97 [DOI] [PubMed] [Google Scholar]
- 84. Watson IR, Blanch A, Lin DC, Ohh M, Irwin MS. Mdm2-mediated NEDD8 modification of TAp73 regulates its transactivation function. J Biol Chem. 2006;281:34096-103 [DOI] [PubMed] [Google Scholar]
- 85. Watson IR, Li BK, Roche O, Blanch A, Ohh M, Irwin MS. Chemotherapy induces NEDP1-mediated destabilization of MDM2. Oncogene. 2010;29:297-304 [DOI] [PubMed] [Google Scholar]
- 86. Oved S, Mosesson Y, Zwang Y, et al. Conjugation to Nedd8 instigates ubiquitylation and down-regulation of activated receptor tyrosine kinases. J Biol Chem. 2006;281:21640-51 [DOI] [PubMed] [Google Scholar]
- 87. Russell RC, Ohh M. NEDD8 acts as a ‘molecular switch’ defining the functional selectivity of VHL. EMBO Rep. 2008;9:486-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stickle NH, Chung J, Klco JM, Hill RP, Kaelin WG, Jr, Ohh M. pVHL modification by NEDD8 is required for fibronectin matrix assembly and suppression of tumor development. Mol Cell Biol. 2004;24:3251-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sundqvist A, Liu G, Mirsaliotis A, Xirodimas DP. Regulation of nucleolar signalling to p53 through NEDDylation of L11. EMBO Rep. 2009;10:1132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Xirodimas DP, Sundqvist A, Nakamura A, Shen L, Botting C, Hay RT. Ribosomal proteins are targets for the NEDD8 pathway. EMBO Rep. 2008;9:280-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rabut G, Peter M. Function and regulation of protein neddylation. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 2008;9:969-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans. 2008;36:802-6 [DOI] [PubMed] [Google Scholar]
- 93. Soucy TA, Smith PG, Milhollen MA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732-6 [DOI] [PubMed] [Google Scholar]
- 94. Miranda-Carboni GA, Krum SA, Yee K, et al. A functional link between Wnt signaling and SKP2-independent p27 turnover in mammary tumors. Genes Dev. 2008;22:3121-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chiarle R, Budel LM, Skolnik J, et al. Increased proteasome degradation of cyclin-dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood. 2000;95:619-26 [PubMed] [Google Scholar]
- 96. Yang RM, Naitoh J, Murphy M, et al. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol. 1998;159:941-5 [PubMed] [Google Scholar]
- 97. Esposito V, Baldi A, De LA, et al. Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res. 1997;57:3381-5 [PubMed] [Google Scholar]
- 98. Loda M, Cukor B, Tam SW, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231-4 [DOI] [PubMed] [Google Scholar]
- 99. Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol. 2003;13:41-7 [DOI] [PubMed] [Google Scholar]
- 100. Chiarle R, Fan Y, Piva R, et al. S-phase kinase-associated protein 2 expression in non-Hodgkin’s lymphoma inversely correlates with p27 expression and defines cells in S phase. Am J Pathol. 2002;160:1457-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nakayama KI, Nakayama K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin Cell Dev Biol. 2005;16:323-33 [DOI] [PubMed] [Google Scholar]
- 102. Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Brownell JE, Sintchak MD, Gavin JM, et al. Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol Cell. 2010;37:102-11 [DOI] [PubMed] [Google Scholar]
- 104. Fujita M. Cdt1 revisited: complex and tight regulation during the cell cycle and consequences of deregulation in mammalian cells. Cell Div. 2006;1:22-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Vaziri C, Saxena S, Jeon Y, et al. A p53-dependent checkpoint pathway prevents rereplication. Mol Cell. 2003;11:997-1008 [DOI] [PubMed] [Google Scholar]
- 106. Milhollen MA, Traore T, Adams-Duffy J, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010. June 4 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 107. Swords RT, Kelly KR, Smith PG, et al. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood. 2010;115:3796-800 [DOI] [PubMed] [Google Scholar]
- 108. Shah JJ, Jakubowiak AJ, O’Connor OA, et al. Phase 1 dose-escalation study of MLN4924, a novel NAE inhibitor, in patients with multiple myeloma and non-Hodgkin lymphoma. Blood. 2009;114:735a-6a (abstract 1854). [Google Scholar]
- 109. Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14:45-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Petropoulou C, Kotantaki P, Karamitros D, Taraviras S. Cdt1 and Geminin in cancer: markers or triggers of malignant transformation? Front Biosci. 2008;13:4485-94 [DOI] [PubMed] [Google Scholar]
- 111. Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ye X, Nalepa G, Welcker M, et al. Recognition of phosphodegron motifs in human cyclin E by the SCF(Fbw7) ubiquitin ligase. J Biol Chem. 2004;279:50110-9 [DOI] [PubMed] [Google Scholar]
- 113. Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83-93 [DOI] [PubMed] [Google Scholar]
- 114. Wingate H, Puskas A, Duong M, et al. Low molecular weight cyclin E is specific in breast cancer and is associated with mechanisms of tumor progression. Cell Cycle. 2009;8:1062-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Minella AC, Welcker M, Clurman BE. Ras activity regulates cyclin E degradation by the Fbw7 pathway. Proc Natl Acad Sci U S A. 2005;102:9649-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nateri AS, Riera-Sans L, Da CC, Behrens A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science. 2004;303:1374-8 [DOI] [PubMed] [Google Scholar]
- 117. Donzelli M, Squatrito M, Ganoth D, Hershko A, Pagano M, Draetta GF. Dual mode of degradation of Cdc25 A phosphatase. EMBO J. 2002;21:4875-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Fernandez-Vidal A, Mazars A, Manenti S. CDC25A: a rebel within the CDC25 phosphatases family? Anticancer Agents Med Chem. 2008;8:825-31 [DOI] [PubMed] [Google Scholar]
- 119. Kanemori Y, Uto K, Sagata N. Beta-TrCP recognizes a previously undescribed nonphosphorylated destruction motif in Cdc25A and Cdc25B phosphatases. Proc Natl Acad Sci U S A. 2005;102:6279-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kobayashi H, Kajiwara H, Kanayama S, et al. Molecular pathogenesis of endometriosis-associated clear cell carcinoma of the ovary [review]. Oncol Rep. 2009;22:233-40 [PubMed] [Google Scholar]
- 121. Yada M, Hatakeyama S, Kamura T, et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23:2116-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mao JH, Kim IJ, Wu D, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27 Suppl 2:S43-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Dehan E, Bassermann F, Guardavaccaro D, et al. betaTrCP- and Rsk1/2-mediated degradation of BimEL inhibits apoptosis. Mol Cell. 2009;33:109-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596-613 [DOI] [PubMed] [Google Scholar]


