Abstract
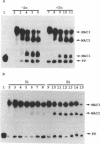
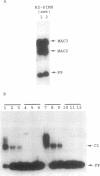
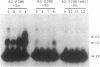
The smt locus of Synechococcus PCC 7942 contains a metal-regulated gene (smtA), which encodes a class II metallothionein, and a divergently transcribed gene, smtB, which encodes a repressor of smtA transcription. Regions containing cis-acting elements required for efficient induction, and required for smtB-dependent repression, of the smtA operator-promoter were identified. Specific interactions between proteins extracted from Synechococcus PCC 7942 and defined regions surrounding the smtA operator-promoter were detected by electrophoretic mobility shift assays. Three metallothionein operator-promoter associated complexes were identified, one of which (MAC1) showed Zn-dependent dissociation and involved a region of DNA immediately upstream of smtA. Treatment with Zn-chelators facilitated re-association of MAC1 in vitro. MAC1 was not observed in extracts from smt deficient mutants but was restored in extracts from mutants complemented with a plasmid borne smtB. SmtB is thus required for the formation of a Zn-responsive complex with the smt operator-promoter and based upon the predicted structure of SmtB we propose direct SmtB-DNA interaction exerting metal-ion inducible negative control.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd I. B., Egan J. B. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990 Sep 11;18(17):5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborough K. M., West S. C. Specific binding of cruciform DNA structures by a protein from human extracts. Nucleic Acids Res. 1988 May 11;16(9):3603–3616. doi: 10.1093/nar/16.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P., Hu S., Hackett R., Hamer D. Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell. 1988 Nov 18;55(4):705–717. doi: 10.1016/0092-8674(88)90229-2. [DOI] [PubMed] [Google Scholar]
- Ivey D. M., Guffanti A. A., Shen Z., Kudyan N., Krulwich T. A. The cadC gene product of alkaliphilic Bacillus firmus OF4 partially restores Na+ resistance to an Escherichia coli strain lacking an Na+/H+ antiporter (NhaA). J Bacteriol. 1992 Aug;174(15):4878–4884. doi: 10.1128/jb.174.15.4878-4884.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G., Silver S. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J Bacteriol. 1992 Jun;174(11):3684–3694. doi: 10.1128/jb.174.11.3684-3694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondorosi E., Pierre M., Cren M., Haumann U., Buiré M., Hoffmann B., Schell J., Kondorosi A. Identification of NolR, a negative transacting factor controlling the nod regulon in Rhizobium meliloti. J Mol Biol. 1991 Dec 20;222(4):885–896. doi: 10.1016/0022-2836(91)90583-r. [DOI] [PubMed] [Google Scholar]
- Olafson R. W., McCubbin W. D., Kay C. M. Primary- and secondary-structural analysis of a unique prokaryotic metallothionein from a Synechococcus sp. cyanobacterium. Biochem J. 1988 May 1;251(3):691–699. doi: 10.1042/bj2510691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. J., Gupta A., Fordham-Skelton A. P., Croy R. R., Whitton B. A., Huckle J. W. Prokaryotic metallothionein gene characterication and expression: chromosome crawling by ligation-mediated PCR. Proc Biol Sci. 1990 Dec 22;242(1305):241–247. doi: 10.1098/rspb.1990.0130. [DOI] [PubMed] [Google Scholar]
- San Francisco M. J., Hope C. L., Owolabi J. B., Tisa L. S., Rosen B. P. Identification of the metalloregulatory element of the plasmid-encoded arsenical resistance operon. Nucleic Acids Res. 1990 Feb 11;18(3):619–624. doi: 10.1093/nar/18.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan D. J., Bloye S. A., Mann N. H., Hodgson D. A., Carr N. G. Construction of lacZ promoter probe vectors for use in Synechococcus: application to the identification of CO2-regulated promoters. Gene. 1990 May 31;90(1):43–49. doi: 10.1016/0378-1119(90)90437-v. [DOI] [PubMed] [Google Scholar]
- Shi J., Lindsay W. P., Huckle J. W., Morby A. P., Robinson N. J. Cyanobacterial metallothionein gene expressed in Escherichia coli. Metal-binding properties of the expressed protein. FEBS Lett. 1992 Jun 1;303(2-3):159–163. doi: 10.1016/0014-5793(92)80509-f. [DOI] [PubMed] [Google Scholar]
- Thiele D. J. Metal-regulated transcription in eukaryotes. Nucleic Acids Res. 1992 Mar 25;20(6):1183–1191. doi: 10.1093/nar/20.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Coleman J. E., Auld D. S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K. P., Silver S. A second gene in the Staphylococcus aureus cadA cadmium resistance determinant of plasmid pI258. J Bacteriol. 1991 Dec;173(23):7636–7642. doi: 10.1128/jb.173.23.7636-7642.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas J., Hegeman H., de Vrieze G., Tuyl M., Borrias M., Weisbeek P. Genomic integration system based on pBR322 sequences for the cyanobacterium Synechococcus sp. PCC7942: transfer of genes encoding plastocyanin and ferredoxin. Gene. 1990 Oct 30;95(1):39–48. doi: 10.1016/0378-1119(90)90411-j. [DOI] [PubMed] [Google Scholar]