Abstract
Ligands and their tyrosine kinase (TK) receptors regulate a variety of biological systems in animals. Vascular endothelial growth factor (VEGF) and its receptor (Flt/VEGFR family) system play a crucial role not only in physiological but also in most parts of pathological angiogenesis including cancer. Flt-1/VEGFR-1 and KDR/VEGFR-2 bind VEGF-A but have different functions on angiogenesis at early embryogenesis: Flt-1 has a negative role by trapping ligands, whereas KDR (Flk1 in mice) exerts a strong positive signal, resulting in a balance in blood vessel formation. At adult stages, however, both VEGFRs contribute to pathological angiogenesis either directly or through stimulation of migration/activation of macrophage lineage cells and stimulate tumor growth, metastasis, and inflammation. VEGFRs activate downstream signaling of the phospholipase Cγ–protein kinase C–MAP kinase pathway but not Ras pathway for cell proliferation. The VEGF-C/D and Flt-4/VEGFR-3 system regulates lymphangiogenesis. Thus, VEGFs as well as these receptor TKs are attractive targets for suppressing pathological angiogenesis.
Keywords: vascular endothelial growth factor, tyrosine kinase receptor, phospholipase C, tumor angiogenesis
Introduction
Receptor-type as well as nonreceptor-type TKs are major signal transducers in various systems in vertebrates including angiogenesis. Historically, researches on TKs had started in 1980.1 In 1980, a viral Src protein, designated v-src, was identified as the first tyrosine kinase in animals. After that, nonreceptor TKs such as v-fps and v-yes as well as receptor-type TKs such as v-erbB (epidermal growth factor receptor [EGFR]) were isolated as viral oncogenes. At this time, no structurally mutated or activated TK gene had yet been found in human tumors. We found that glioblastomas often carry a mutated form of the EGFR gene: about one-third of the ligand-binding domain was deleted at the genomic DNA level, and this mutant EGFR (now known as EGFRvIII) was constitutively active without the ligand EGF.2 Several groups including our own demonstrated that the Shc-Grb2/Sos-Ras pathway is essential for the downstream signaling of EGFR towards cell proliferation and transformation. During these studies, we learned how the Ras pathway plays a major role in intracellular signaling in typical TKs with oncogenic potential.
The question is whether any nonreceptor-type TK/receptor-type TK is not associated with oncogenic potential nor with the Ras pathway but promotes biological systems such as tumor angiogenesis. As described later, the VEGFR family appears to be an example.
Isolation of flt-1 cDNA and Its Unique Expression Pattern
In 1987, we obtained a genomic DNA that carries an exon-like structure possibly encoding a portion of receptor-type TK (TKR).3 Since TKR is thought to be pro-oncogenic, a higher gene expression and mutations of this new gene are expected in human tumors. However, most of the tumor cells examined so far did not express it at detectable levels. We thought it might be a pseudogene, but finally, its expression was detected in placenta, and a full-length cDNA of about 8 kb was isolated for this new TK.4 A few years later, several groups including our own found that this gene is specifically expressed in vascular endothelial cells.5-8
Unique Structure of the New TKR, Flt-1
The amino acid sequence encoded in the cDNA was unique: it carried 7 immunoglobulin (Ig) domains in the extracellular domain, a transmembrane domain, and a TK domain with a kinase insert (KI) about 70 amino acids long (Fig. 1). The entire structure indicates that it is a new type of TKR, distantly related to the family of Fms, Kit, and PDGFR, which contain 5 Ig domains in the extracellular domain, known as the PDGFR family/ 5-Ig-TKR family. Since, at that time, the Fms tyrosine kinase oncogene was the most famous of the 5-Ig-TKRs, we designated this new gene flt-1 (fms-like tyrosine kinase-1) in 1990.4,9-11
Figure 1.
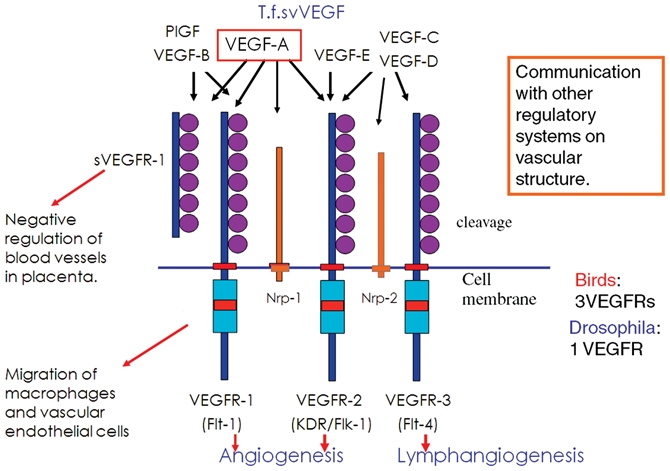
The VEGF-VEGFR system in mammals. VEGF-A and 3 VEGFR genes are essential for normal angiogenesis during embryogenesis. Knockout mice of these genes are embryonic lethal due to abnormality of blood vessel formation. In the case of the VEGF-A gene, even the heterozygote mice die in embryogenesis because of the dysfunction of blood vessels.
Another interesting feature of Flt-1 is that, although the 5-Ig-TKRs have a tyrosine (Y)-x-x-methionine (M) motif in the KI region critical for PI3 kinase/Akt activation and for a strong mitotic signal, the Flt-1 KI region did not have this motif.4 A second member of the Flt-1 family/7-Ig-TKR family, KDR (Flk-1 in mice), isolated in 1991, did not have this motif either, strongly suggesting that the intracellular signaling pathway of the Flt family/7-Ig-TKR family is quite different from that of the 5-Ig-TKRs.12,13
In 1992, de Vries et al. showed that vascular endothelial growth factor (VEGF) binds Flt-1 with high affinity and activates its kinase activity.5 It is well known that pathological angiogenesis is crucial to the development of various diseases including cancer.14 The VEGF-VEGFR (Flt family) system could play a central role in tumor angiogenesis. Therefore, several groups decided to focus on the molecular biology of angiogenesis, particularly on VEGF-VEGFRs.15 A few years later, Alitalo’s group isolated a third member of the Flt family, Flt-4 (VEGFR-3), and a new ligand, VEGF-C, which specifically binds VEGFR-3. The VEGF-C/D and VEGFR3 system mostly regulates lymphangiogenesis.16
Characteristics of the Signaling of VEGFRs
The novel structure of VEGFRs, lacking the PI3K activation motif (Y-x-x-M) in the KI region, suggests a unique signaling system in vascular endothelial cells.6 We found that, unlike typical TKRs such as EGFR, neither VEGFR-1 nor VEGFR-2 showed any cell-transforming activity toward NIH3T3 fibroblasts. These observations further support that the intracellular signaling from VEGFR-1/VEGFR-2 is different from that for typical TKs.
VEGFR-2 has TK activity about one order of magnitude stronger than that of VEGFR-1, and the gene knockout of VEGFR-2/flk-1 in mice indicated that it is the major positive signal transducer in angiogenesis.17,18 We found that the signaling of VEGFR-2 is unique among TKs, with almost no activation of Ras but strong activation of a phospholipase Cγ (PLCγ)–PKC pathway towards activation of MAP kinase and proliferation of vascular endothelial cells in response to VEGF.19
Furthermore, we identified that a single amino acid residue, Y-1175, in human VEGFR-2 (Y at 1173 in mice) is a major autophosphorylation site for the stimulation of the PLCγ-PKC pathway in vitro.20 To show that this pathway is really used in animals, we made a knockin mouse with a single amino acid mutation at 1173 from Y to phenylalanine (F). Homozygotes with the 1173F/1173F mutation demonstrated severe angiogenic defects, resulting in embryonic death at E9.0, although VEGFR-2 was expressed and TK activity was maintained.21 These results indicate that the VEGFR2-Y1175-PLCγ-PKC pathway is crucial for vasculogenesis and angiogenesis in vivo. Lawson et al. also supported the importance of this pathway in angiogenesis in zebrafish.22
Involvement of VEGFR-1 in Carcinogenesis
VEGFR-1 (Flt-1) has very high affinity for VEGF (Kd = 1-10 × 10−12 M) but about 10-fold less kinase activity than VEGFR-2. Fong et al. showed that flt-1 –/– mice die in the embryonic stage due to the overgrowth and disorganization of blood vessels.23 This indicates that Flt-1 has a negative role in proangiogenic signaling early in embryogenesis and achieves an appropriate balance in terms of this signaling. We examined whether a high-affinity ligand-binding domain or a weak TK is important for this negative role of Flt-1 by making Flt-1 TK domain–deficient mice. The Flt-1 TK–/– mice lacked the TK domain but had the ligand-binding domain and TM domain. Surprisingly, they were basically healthy, and their angiogenesis was almost normal. This indicates the major role of Flt-1 in early embryogenesis to be the trapping of the VEGF molecule with a Flt-1 ligand-binding domain.24
Since Flt-1 TK–/– mice are missing the signaling from Flt-1 kinase, they are useful for analyzing the involvement of Flt-1 signaling in various diseases. By using normal mice and Flt-1 TK–/– mice, Flt-1 kinase was shown to be important for promotion of the lung-oriented metastasis of 3LL-LLC (a highly metastatic variant of Lewis lung carcinoma) via premetastatic induction of matrix metalloproteinase 9 (MMP9) in the lung25 (Fig. 2). In a similar experimental model, Kaplan et al. demonstrated that VEGFR1-positive hematopoietic bone marrow progenitors initiate the premetastatic niche in the lung and other tissues, stimulating tissue-oriented tumor metastasis.26 We have recently found that the signaling of Flt-1 kinase stimulates melanoma and uterine tumor growth at the primary site in mice via recruitment of bone marrow–derived monocyte/macrophages into tumor tissues.27,28 Furthermore, Tammela et al. reported that Flt-4/VEGFR-3 promotes not only lymphangiogenesis but also tumor angiogenesis.29 On the other hand, the contribution of a ligand of Flt-1, placenta growth factor (PlGF), to tumor growth appears context dependent; thus, whether the PlGF-neutralizing antibody has a strong tumor-suppressive effect in vivo is not clarified yet.30,31
Figure 2.
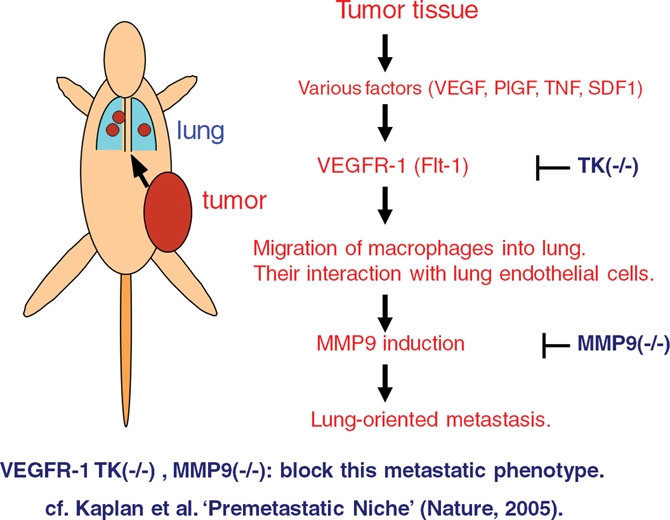
Premetastatic activation in the lung via VEGFR-1 (Flt-1) signaling. Transplanted tumors in mice induce macrophage lineage cell migration and MMP9 expression in the lung before metastasis. VEGFR-1 signal–deficient mice (flt-1 TK–/– mice) show less migration of macrophages and MMP9 expression as well as decreased metastasis, indicating these processes to be partly dependent on VEGFR-1 TK signaling.
Based on these researches, not only VEGFR-2 but also VEGFR-1 as well as VEGFR-3 were shown to be an attractive target for suppression of tumor angiogenesis. VEGF-A–neutralizing monoclonal antibody (bevacizumab) and multikinase inhibitors of small molecules (sorafenib and sunitinib) were developed and have been widely used in clinics for the treatment of colorectal, breast, lung (nonepithelial), hepatic, and renal cancers as well as glioblastomas.
Involvement of Flt-1/VEGFR-1 in Other Diseases
Flt-1 is expressed not only in vascular endothelial cells but also in macrophage lineage cells and regulates VEGF-oriented migration, survival, and angiogenic factor production. Using the wild-type and Flt-1 TK–/– mice, inflammatory responses such as immune cell infiltration, angiogenesis, and edema in the knee joints were found to be significantly weaker in TK–/– mice than in wild-type mice in a rheumatoid arthritis (RA) model.32 Taken together, these results suggest that the TK signaling of Flt-1 is a potential target for treatment of cancer and RA.
Soluble Flt-1 and Pre-eclampsia
In 1990, 2 major transcripts of flt-1 were described: one for the full-length TKR, and another for a short protein without the TK region.4 Later, the structure of soluble Flt-1 was characterized and was found to contain only the ligand-binding domain. We found that soluble Flt-1 is conserved not only in mammals but also in birds. In the placenta in mammals, soluble Flt-1 was reported to be expressed in trophoblasts, which form a border between the maternal and fetal blood vessels. Since soluble Flt-1 has very high affinity for VEGF, it is likely a natural inhibitor of the VEGF system, negatively regulating excess angiogenesis or hyperpermeability in the placenta. Recent studies strongly suggest that an abnormally high level of soluble Flt-1 in the placenta causes maternal pathological symptoms in pre-eclampsia such as hypertension and renal dysfunction.33-35 It is of interest that an anti-VEGF-A–neutralizing antibody, bevacizumab (Avastin, Genentech Inc., South San Francisco, CA), now widely used in clinics for the treatment of various solid tumors, has side effects such as hypertension and proteinuria. Thus, excess trapping of VEGF-A in the body by the soluble Flt-1 produced during pre-eclampsia appears to be the major cause of the maternal symptoms of this disease, suggesting suppression of the function of soluble Flt-1 to be a good strategy for treatment.
Other VEGF-Like Molecules
In addition to the studies on angiogenesis described above, VEGF-E encoded in a parapoxvirus genome was found to activate only VEGFR-2, stimulating less inflammatory and well-organized blood vessels.36 On the other hand, another VEGF-like protein (T.f.svVEGF) specifically expressed in the venom of the habu snake increases vascular permeability, most likely for the rapid distribution of toxins in prey.37 These results further support that VEGF-VEGFR is a crucial system for regulating angiogenesis and vascular permeability and is widely used not only in animals but also in viruses.
Conclusion
The benefits of the anti-VEGF and anti-VEGFR medicines for cancer patients are clear; however, they are still limited. In the Delta-Notch system, a neutralizing antibody against Dll4 (Delta-like 4) was shown to be effective for suppression of tumor growth in mice.38,39 However, more recently, Yan et al. reported that anti-Dll4 antibody treatment often induced a tumor, angioma, in mice, suggesting a risk in the host animals.40 We found that double-deprivation stress with hypoxia and low nutrition, which mimics a condition under antiangiogenic therapy, induces a resistant phenotype in tumor cells.41 Thus, further studies are necessary to elucidate the mechanism of tumor resistance against antiangiogenic therapy and to find a new strategy to overcome this resistance.
Acknowledgments
Starting in Saburo’s laboratory, I have had a wonderful period of research on TKs lasting more than 30 years. I dedicate this review to the memory of Saburo and Teruko Hanafusa. I also thank all the alumni and staff of Hanafusa’s laboratory and colleagues in Japan as well as all over the world.
Footnotes
The author declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
This work was supported by Grant-in-aid Special Project Research on Cancer-Bioscience 17014020 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Jove R, Hanafusa H. Cell transformation by the viral src oncogene. Annu Rev Cell Biol. 1987;3:31-56 [DOI] [PubMed] [Google Scholar]
- 2. Yamazaki H, Fukui Y, Ueyama Y, et al. Amplification of the structurally and functionally altered epidermal growth factor receptor gene (c-erbB) in human brain tumors. Mol Cell Biol. 1988;8:1816-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsushime H, Yoshida MC, Sasaki M, Shibuya M. A possible new member of tyrosine kinase family, human frt sequence is highly conserved in vertebrates and located on human chromosome 13. Jpn J Cancer Res (Gann). 1987;78:655-61 [PubMed] [Google Scholar]
- 4. Shibuya M, Yamaguchi S, Yamane A, et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519-24 [PubMed] [Google Scholar]
- 5. De Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989-91 [DOI] [PubMed] [Google Scholar]
- 6. Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988-95 [PubMed] [Google Scholar]
- 7. Yamane A, Seetharam L, Yamaguchi S, et al. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1). Oncogene. 1994;9:2683-90 [PubMed] [Google Scholar]
- 8. Kaipainen A, Korhonen J, Pajusola K, et al. The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med. 1993;178:2077-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shibuya M. Role of VEGF-Flt receptor system in normal and tumor angiogenesis. Adv Cancer Res. 1995;67:281-316 [DOI] [PubMed] [Google Scholar]
- 10. Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549-60 [DOI] [PubMed] [Google Scholar]
- 11. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967-74 [DOI] [PubMed] [Google Scholar]
- 12. Terman BI, Carrion ME, Kovacs E, et al. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991; 6:1677-83 [PubMed] [Google Scholar]
- 13. Matthews W, Jordan CT, Gavin M, Jenkins NA, Copeland NG, Lemischka IR. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci U S A. 1991;88:9026-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353-64 [DOI] [PubMed] [Google Scholar]
- 15. Shibuya M. Vascular permeability/vascular endothelial growth factor. In: Figg W, Folkman J, editors. Angiogenesis: an integrative approach from science to medicine. New York: Springer; 2008. p. 89-98 [Google Scholar]
- 16. Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219-27 [DOI] [PubMed] [Google Scholar]
- 17. Sawano A, Takahashi T, Yamaguchi S, Aonuma T, Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor (PlGF), which is related to vascular endothelial growth factor (VEGF). Cell Growth Diff. 1996;7:213-21 [PubMed] [Google Scholar]
- 18. Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62-6 [DOI] [PubMed] [Google Scholar]
- 19. Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221-30 [DOI] [PubMed] [Google Scholar]
- 20. Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20:2768-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (vascular endothelial growth factor receptor-2) tyrosine residue-1173 in vasculogenesis in mice. Proc Natl Acad Sci U S A. 2005;102:1076-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawson ND, Mugford JW, Diamond BA, Weinstein BM. Phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;7:1346-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fong GH, Rossant J, Gertsentein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66-70 [DOI] [PubMed] [Google Scholar]
- 24. Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hiratsuka S, Nakamura K, Iwai S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung specific metastasis. Cancer Cell. 2002;2:289-300 [DOI] [PubMed] [Google Scholar]
- 26. Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerber M, Reiss Y, Wickersheim A, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68:7342-51 [DOI] [PubMed] [Google Scholar]
- 28. Muramatsu M, Yamamoto S, Osawa T, Shibuya M. VEGF-1 signaling promotes mobilization of macrophage-lineage cells from bone marrow and stimulateolid tumor growth. Cancer Res. In press [DOI] [PubMed] [Google Scholar]
- 29. Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656-60 [DOI] [PubMed] [Google Scholar]
- 30. Bais C, Wu X, Yao J, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141:166-77 [DOI] [PubMed] [Google Scholar]
- 31. Van de Veire S, Stalmans I, Heindryckx F, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178-90 [DOI] [PubMed] [Google Scholar]
- 32. Murakami M, Iwai S, Hiratsuka S, et al. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocyte/macrophages. Blood. 2006;108:1849-56 [DOI] [PubMed] [Google Scholar]
- 33. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348-51 [DOI] [PubMed] [Google Scholar]
- 35. Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-83 [DOI] [PubMed] [Google Scholar]
- 36. Kiba A, Sagara H, Hara T, Shibuya M. VEGFR-2-specific ligand VEGF-E induces non-edematous hyper-vascularization in mice. Biochem Biophys Res Commun. 2003;301:371-7 [DOI] [PubMed] [Google Scholar]
- 37. Takahashi H, Hattori S, Iwamatsu A, Takizawa H, Shibuya M. A novel snake venom vascular endothelial growth factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF receptor-1. J Biol Chem. 2004;279:46304-14 [DOI] [PubMed] [Google Scholar]
- 38. Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032-7 [DOI] [PubMed] [Google Scholar]
- 39. Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083-7 [DOI] [PubMed] [Google Scholar]
- 40. Yan M, Callahan CA, Beyer JC, et al. Chronic DLL4 blockade induces vascular neoplasms. Nature. 2010;463:E6-7 [DOI] [PubMed] [Google Scholar]
- 41. Osawa T, Muramatsu M, Watanabe M, Shibuya M. Hypoxia and low nutrition double stress induces aggressiveness in a murine model of melanoma. Cancer Sci. 2009;100:844-51 [DOI] [PMC free article] [PubMed] [Google Scholar]


