Abstract
The Hippo tumor suppressor pathway regulates the size of organs by controlling 2 opposing processes: proliferation and apoptosis. The pathway was originally defined in Drosophila, but it is well conserved in mammals. One of the unique features of Hippo signaling is the unusually wide occurrence of WW domains and its cognate PPxY ligand motifs within components of this pathway. Recently, it was proposed that the prevalence of WW domain–mediated complexes in the Hippo signaling pathway should facilitate its molecular analysis and help in the identification of new components of the Hippo-centered network. Indeed, several new members of the Hippo pathway, which form functional complexes with WW domains of YAP and TAZ effectors, were recently described. We focus here on 2 families of such proteins, angiomotins and SMADs, plus 1 regulatory factor, WBP-2, which together shed new light on the rapidly expanding Hippo network. Since the Hippo pathway acts as a tumor suppressor pathway, the complexes described here, which assemble on WW domains of YAP and TAZ, represent potential targets of cancer therapy.
Keywords: YAP and TAZ oncogenes, WW module, angiomotins, SMADs
Introduction
In metazoans, there are several pathways that regulate organ size and ultimately that of the entire organism. One such pathway is the Hippo pathway, which plays a key role in controlling a balance between cell proliferation and apoptosis.1,2 The name of the pathway is derived from the Hippo tumor suppressor gene described in the Drosophila melanogaster fly.1 The gene encodes a serine/threonine protein kinase, which when mutated gives rise to a large fly with folds of overgrown tissues that resemble the body folds of a hippopotamus. The mammalian ortholog of Hippo is encoded by 2 paralogs, MST1 and MST2 (mammalian ste20-like protein kinases), which are central to the Hippo pathway. The upstream signals that activate MST1/2 kinases lead to the activation of 2 downstream serine/threonine kinases called LATS1 and LATS2, which in turn negatively regulate 2 transcriptional coactivators, YAP (Yes kinase-associated protein)3,4 and TAZ (transcriptional co-activator with PDZ binding domain).5 LATS (large tumor suppressor kinase)–phosphorylated YAP and TAZ are anchored in the cytoplasm via 14-3-3 proteins and cannot access their target genes involved in cell proliferation and survival. Therefore, the activation of the Hippo pathway results in growth inhibitory or proapoptotic signals. Upstream of MST kinases, there is a WW (tryptophan-tryptophan) domain–containing adapter KIBRA (kidney and brain expressed protein) and a tumor suppressor NF2 (neurofibromatosis 2, also known as Merlin), which causes neurofibromatosis. Somehow, KIBRA and NF2 convey signals from membrane receptors to the core kinase components of the Hippo pathway.6-8 The membrane receptor(s) and their ligands, which activate the pathway, are well defined in Drosophila and include Crumbs, Fat, and Fat ligand called Dachsous, but it is not clear yet that their orthologs act in a similar manner in the mammalian Hippo pathway.2
One of the unique features of the Hippo pathway is that many of its components contain either WW domain or its PPxY-containing ligand (where P is proline, Y is tyrosine, and x is any amino acid).9,10 To illustrate this in the scheme of the mammalian Hippo pathway (Fig. 1), those components that contain either WW domain or its cognate PPxY motif are colored in orange. In this scheme, we also included AMOT (angiomotin), SMAD (protein related to a worm and fly proteins: SMA and MAD [mothers against decapentaplegic polypeptide]), and WBP-2 (WW domain binding protein 2) as proteins that have been functionally implicated in the Hippo pathway and are the subject of this short review.11-17
Figure 1.
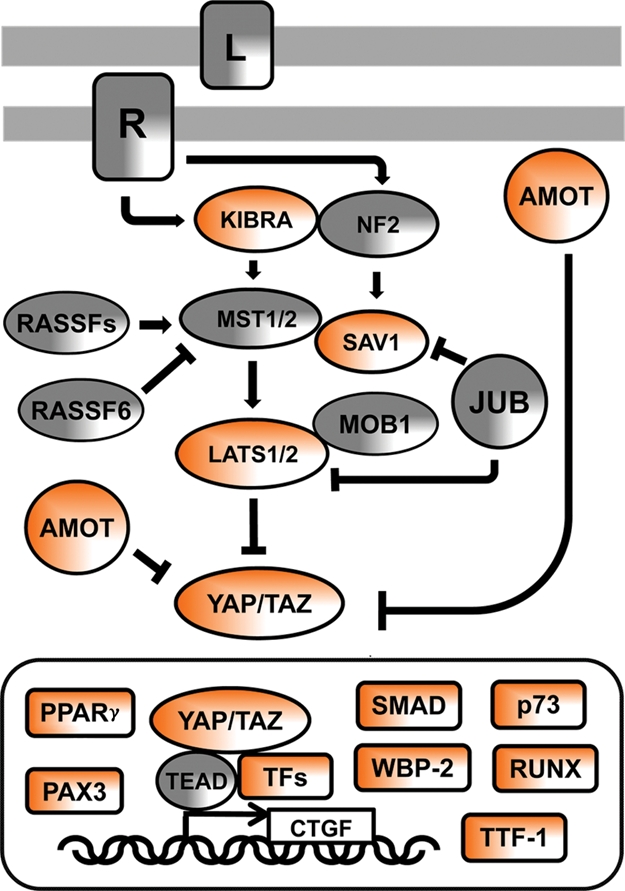
The Hippo signaling network in mammals. In the signaling diagram, the proteins that contain WW domains or PPxY ligand motif are in orange. “L” denotes an unknown ligand, and “R” denotes its cognate but still unknown receptor. L and R are engaged by cell-to-cell contacts and activate the pathway. JUB, also known as AJUBA, transduces signals from sites of cell adhesion. JUB has an inhibitory function on SAV1 (SALVADOR 1) adapter protein and LATS kinases. RASSF is a family of Ras association domain–containing proteins that stimulate or inhibit MST kinases. MOB1 is the Mps One Binder kinase activator-like 1B protein, whose binding to LATS activates its kinase activity. TFs = transcription factors, examples of which are as follows: TEAD = TEA domain proteins that have a conserved DNA binding domain and can function as repressors or activators of gene transcription; PPARγ = peroxisome proliferator-activated receptor γ; PAX3 = paired box 3 protein; p73 = a member of the p53 family of proteins implicated in apoptotic signaling; RUNX = runt-related transcription factor; TTF-1 = transcription termination factor-1; CTGF = connective tissue growth factor, one of the genes induced by YAP/TEAD nuclear signaling. Other abbreviations are described in the text.
Briefly regarding the WW domain and its cognate PPxY motif, the WW domain is a small protein module that is composed of 38 amino acids that fold as a meander of 3 β strands, which forms a binding pocket for proline-rich ligands.9,18 There are several classes of WW domains based on the ligand preference, but the largest class binds ligands that contain PPxY motif.19 The tyrosine in the motif must be in the nonphospho state, and when the Y is phosphorylated, it negatively regulates WW domain binding. Therefore, the WW domain resembles both the SH2 (Src homology 2) and SH3 (Src homology 3) domain in terms of regulation by tyrosine phosphorylation and the requirement of proline-rich motif in the ligand. There are approximately 100 WW domains in the human proteome and more than 1,890 PPxY motifs scattered within human proteins.20 Most recently, several WW domains, including WW domains of Hippo pathway proteins, were shown to dimerize, and at least in one case, it was documented that an additional β strand at the carboxy-terminal region was required for the dimmer formation.21 The molecular and functional plasticity of WW domain complexes is quite diverse (Fig. 2), and the Hippo pathway takes advantage of these molecular inventions.
Figure 2.

Modes of signaling by the WW domain. The WW domain recognizes PPxY-containing ligand (A), with the Y in a nonphospho state. Phosphorylation of the signature Y in PPxY abrogates the complex (B). In some instances, 2 WW domains mediate interaction with a single PPxY-containing ligand (C), as discussed in the text for the YAP (isoform 2) and SMAD1 complex. WW domains were also shown to homodimerize (D) and heterodimerize (E). In one instance, an additional β strand at the carboxy-terminal region of the WW domain was required for dimer formation (F). See the text for references.
Angiomotins as New Tumor Suppressors in the Hippo Pathway
Angiomotin (AMOT) was originally identified as a partner of angiostatin, a protein that regulates endothelial cell migration. In addition, AMOT was shown to be a part of the actin cytoskeleton and cell-to-cell junction complexes. AMOT is a member of a family of proteins composed of AMOT and 2 paralogs, AMOTL1 (angiomotin-like 1) and AMOTL2 (angiomotin-like 2).22
Three recent reports suggest that AMOT, AMOTL1, and AMOTL2 interact with YAP and TAZ effectors via WW domain– and PPxY-mediated complexes.11-13 YAP and TAZ WW domains and PPxY motifs of AMOTs were shown to be critical for the binding. Using different approaches, these 3 studies documented that the AMOT/YAP-TAZ complexes have inhibitory function on YAP’s and TAZ’s ability to promote growth and oncogenic transformation. The main mechanism of this regulation is the sequestration of YAP and TAZ proteins by AMOTs in the cytoplasm and in cell junction complexes, therefore preventing YAP and TAZ from transcriptional coactivator function in the nucleus. There is also a possibility that by increasing the phosphorylation of YAP/TAZ on phosphodegron, AMOTs down-regulate the level of YAP/TAZ proteins in cells and therefore have an inhibitory function. In general, AMOTs play a role similar to the 14-3-3 anchor, but AMOT-mediated exclusion of YAP/TAZ from the nuclear compartment is not dependent on LATS-mediated phosphorylation and thus may provide a signaling link between cell contact inhibition and the core of the Hippo pathway.11-13
YAP Signals with SMAD1 in BMP Pathway
The TGF-β (transforming growth factor) belongs to a family of cytokines that are key regulators of metazoan development and tissue homeostasis.23,24 In the canonical pathway, the TGF-β and the BMP (bone morphogenic protein) bind to heteromeric serine/theronine kinase receptor complexes, which in turn phosphorylate SMAD transcription factors at their carboxy-terminal tails. The carboxy-terminally modified SMAD1, SAMD5, and SMAD8 in the BMP pathway and SMAD2 and SMAD3 in the TGF-β pathway concentrate in the nucleus and assemble transcriptional complexes that regulate ligand-specific repertoires of target genes.
Recently, YAP was implicated in the BMP pathway as a cotranscriptional partner of SMAD1, with which YAP WW domain 1 and 2 form a complex via PPxY motifs present in SMAD1.15 YAP and SMAD1 in concert regulate expression of BMP target genes, as also documented by the YAP knockdown that down-regulated the expression of the BMP target genes. Interestingly, this complex is regulated by 2 kinases, CDK (cyclin-dependent kinase)–8 and CDK-9, which are known components of the transcriptional mediator and elongation complexes. CDK-8/9 phosphorylate SMAD1 on serine residues that are located within a linker region that is upstream of the PPxY motif.15 These phosphorylation events increase the strength of binding between SMAD1 and YAP. It is possible that for the purpose of fine signaling regulation, the PPxY motifs on SMADs evolved as low affinity ligands to WW domains of YAP by selecting specific amino acids that are juxtaposed to the very motif. However, the WW domain of YAP evolved to recognize serine-phosphorylated linkers on SMADs as switches for tight binding. It would be interesting to solve the crystal structure of the YAP-SMAD1 complex to identify molecular contacts of this interaction.
In contrast to YAP, TAZ was not shown to form WW domain–mediated complexes with SMAD1 perhaps because the interaction requires 2 tandem WW domains, as in YAP, while TAZ has only a single WW domain. However, TAZ is able to form functional complexes with SMADs via a different mode. TAZ regulates nucleocytoplasmic shuttling of SMAD2/3-4 heteromers and their transcriptional activity.25 The complex between TAZ and SMAD2/4 is mediated by a coil-coil domain of TAZ and is helped by the PDZ binding motif (bm) that is located at the carboxy-terminus of TAZ, as the mutant with the deletion of coil-coil and PDZ bm rendered the TAZ-SMAD2/4 complex inactive.25
It should be noted that TAZ uses its WW domain and PDZ bm to cross-talk with signaling proteins of other pathways. TAZ associates strongly with DVL (disheveled) of the Wnt/β-catenin pathway (Wnt, a pathway in which Wingless and Int morphogenic proteins play a role). The TAZ-DVL complex inhibits the CK1 (casein kinase 1)–mediated phosphorylation of DVL, thereby inhibiting the Wnt/β-catenin signaling.26
WBP-2 Estrogen Receptor and the Hippo Pathway
WBP-2 was originally identified and cloned as a ligand of WW domain of YAP.9 The repeated PPxY motif within WBP-2, and within another similar ligand protein called WBP-1, helped to identify the first ligand of WW domain with the PPxY consensus and established the WW domain as a bona fide modular domain that mediates protein-protein interactions.9,27
Most recently, WBP-2 emerged as an enhancer of TAZ-mediated transformation of MCF10A and NIH3T3 cells, and its knockdown by RNAi technique reduced the transforming potential of TAZ. The interaction between TAZ and WBP-2 involved WW domains of TAZ and the PPxY-containing carboxy-terminal region of WBP-2.17
It is possible that the WBP-2 enhances TAZ-mediated (and most likely, YAP-mediated) transformation and their oncogenic activity by linking the Hippo pathway with the estrogen receptor (ER) pathway. Study from the Nawaz laboratory described that YAP and WBP-2 are a part of the E6-AP (E6-associated protein) and ER complex and are required for estrogen-mediated gene expression (Fig. 3).16 If the nuclear function of this multicomponent complex is further documented, WBP2 could be an important target of cancer therapy. Inhibition of its expression or interfering with the complexes it forms with YAP, TAZ, E6AP, and ER may attenuate oncogenic signaling by YAP and TAZ and provide strategies to develop drugs that would control cancer. In particular, such new drugs could be effective in treating patients with estrogen-positive breast cancer. Perhaps WBP-2–based drugs would synergize with tamoxifen to effectively inhibit proliferation of tumor cells.
Figure 3.

Schematic of nuclear signaling by the YAP/TAZ and estrogen receptor complex. YAP and its ligand, WBP-2, were shown to be a part of the E6-AP and ER-E (estrogen receptor - estrogen) complex and are required for estrogen-mediated gene expression. The nuclear signaling of this multicomponent complex may identify targets for the development of cancer drugs. Other abbreviations are described in the text.
Concluding Remarks
The study of the Hippo tumor suppressor pathway represents a dynamic branch of current basic cancer research. Only 6 years ago, YAP was characterized as the main effector of the Hippo core cassette,4 and TAZ, as the closest ortholog, was implicated soon after. YAP and TAZ are inactivated by the activation of the Hippo pathway, but YAP and TAZ are activated as nuclear effectors and oncogenes, when the pathway is inhibited or its core upstream components are mutated. At the 2 recent Hippo workshops held in Rome, Italy, in 2009 and 2010, where most of the Hippo researchers gathered to discuss new developments, the community realized that the fast progress in this field is largely due to an active dialog between Drosophila fly geneticists and mammalian signalers. It is hoped that this friendly and collaborative dialog will continue and small molecules that regulate this pathway will soon be discovered and tested in clinical trials of cancer therapy. Most likely successes in targeting the Hippo pathway by cancer drugs will be first seen in the treatment of liver cancer, an organ where the pathway robustly controls hepatocyte proliferation.28
Acknowledgments
Special thanks to the participants of Hippo I and Hippo II workshops in Rome for stimulating research discussions that precipitated some of the thoughts discussed here. This minireview is not meant to cover all relevant publications, and the author apologizes for omission of many pertinent publications. The late Hidesaburo Hanafusa, the author’s past mentor, is remembered with respect and warmth.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
This work was supported by the PA Breast Cancer Coalition (grants #60707 and #9200903); and the Geisinger Clinic.
References
- 1. Harvey K, Tapon N. The Salvador-Warts-Hippo pathway: an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182-91 [DOI] [PubMed] [Google Scholar]
- 2. Pan D. The Hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491-505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sudol M, Bork P, Einbond A, et al. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem. 1995;270:14733-41 [DOI] [PubMed] [Google Scholar]
- 4. Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 2005;122:421-34 [DOI] [PubMed] [Google Scholar]
- 5. Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumgartner R, Poembacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309-16 [DOI] [PubMed] [Google Scholar]
- 7. Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/ Warts/Hippo signaling network. Dev Cell. 2010; 18:300-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen HI, Sudol M. The WW domain of Yes-associated protein binds a novel proline-rich ligand that differs from the consensus established for SH3-binding modules. Proc Natl Acad Sci U S A. 1995;92:7819-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sudol M, Harvey K. The modularity in the Hippo signaling pathway. TiBS. 2010;35:627-33 [DOI] [PubMed] [Google Scholar]
- 11. Weng W, Huang J, Chen J. Angiomotin-like protein associate and negatively regulate YAP1. J Biol Chem. Epub 2010 Dec 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao B, Li L, Lu Q, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan SW, Lim CJ, Chong YF, et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. Epub 2011 Jan 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferrigno O, Lallemand F, Verrecchia F, et al. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879-84 [DOI] [PubMed] [Google Scholar]
- 15. Alarcon C, Zaromytidou AI, Xi Q, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGEF-beta pathways. Cell. 2009;139:757-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dhananjayan SC, Ramamoorthy S, Khan OY, et al. WW domain binding protein-2, an E6-assoiated protein interacting protein, acts as a coactivator of estrogen and progesterone receptors. Mol Endocrinol. 2006;20:2343-54 [DOI] [PubMed] [Google Scholar]
- 17. Chan SW, Lim CJ, Huang C, et al. WW domain-mediated interaction with Wbp2 is important for the oncogenic property of TAZ. Oncogene. Epub 2010 Oct 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bork P, Sudol M. The WW domain: a signalling site in dystrophin? Trends Biochem Sci. 1996;19:531-3 [DOI] [PubMed] [Google Scholar]
- 19. Sudol M, Hunter T. NeW Wrinkles for an old domain. Cell. 2000;103:1001-4 [DOI] [PubMed] [Google Scholar]
- 20. Tapia VE, Nicolaescu E, McDonald CB, et al. The Y65C missense mutation in the WW domain of the Golabi-Ito-Hall syndrome protein PQBP1 affects its binding activity and deregulates pre-mRNA splicing. J Biol Chem. 2010;285:19391-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Senturia R, Faller M, Yin S, et al. Structure of the dimerization domain of Di George Critical Region 8. Protein Sci. 2010;19:1354-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bratt A, Wilson WJ, Troyanovsky B, et al. Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene. 2002;298:69-77 [DOI] [PubMed] [Google Scholar]
- 23. Massague J. TGF-beta signal transduction. Ann Rev Biochem. 1998;67:753-91 [DOI] [PubMed] [Google Scholar]
- 24. Mauviel A. Transforming growth factor-beta signaling in skin: stromal to epithelial cross-talk. J Invest Dermatol. 2009;129:7-9 [DOI] [PubMed] [Google Scholar]
- 25. Varelas X, Sakuma R, Samavarchi P, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nature Cell Biol. 2008;10:837-48 [DOI] [PubMed] [Google Scholar]
- 26. Varelas X, Miller BW, Sopko R, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579-91 [DOI] [PubMed] [Google Scholar]
- 27. Chen HI, Einbond A, Kwak SJ, et al. Characterization of the WW domain of human Yes-associated protein and its polyproline-containing ligands. J Biol Chem. 1997;272:17070-7 [DOI] [PubMed] [Google Scholar]
- 28. Dong J, Feldman G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120-33 [DOI] [PMC free article] [PubMed] [Google Scholar]


