Abstract
Background
The role of vitamins and mineral supplementation in the prevention of diabetes mellitus is not well elucidated.
Objective
The effect of prior administration of magnesium on alloxan induced diabetes was assessed in rats.
Methods
36 Male albino rats were used for this study. The animals were divided into 6 groups of 6 animals each; group 1 was healthy control; groups 2 served as diabetic control. Animals in group 3 received magnesium (100 mg/kg) i.p one hour prior to alloxan (120 mg/kg) administration, group 4 were also received magnesium (150 mg/kg) i.p one hour prior to alloxan administration. Animals in group 5 received magnesium (100 mg/kg) i.p only; group 6 animals received magnesium (150 mg/kg) i.p only. Blood samples were obtained from all animals and plasma glucose levels were determined on Day 0 (prior to treatment), Day 2, Day 5, Day 7 and Day 10 after the commencement of treatment.
Results
There was significant increase (P<0.001) in plasma glucose values in the alloxan treated group when compared with the control values. There was also a significant increase (P<0.01) in plasma glucose levels in the magnesium-pretreated (100 mg/kg and 150 mg/kg) diabetic groups when compared with healthy controls whereas there was a significant reduction (P<0.01) in plasma glucose level when compared with the diabetic control.
Conclusion
This study has shown that magnesium pretreatment may delay the onset and subsequently cause a reduction in hyperglycemia in alloxan induced diabetes. This effect of magnesium may be attributed to its role as a scavenger of highly reactive hydroxyl radicals generated through alloxan reactions, its potentiation of glutathione antioxidant production and its role as a calcium blocker.
Keywords: Magnesium, Hyperglycemia, Alloxan
Introduction
Diabetes is a chronic disease characterized by elevated blood glucose level and disturbances in carbohydrate, fat and protein metabolism. There are two main categories of this disease, Type 1 diabetes mellitus also called insulin-dependent diabetes mellitus (IDDM) and Type 2, the non-insulin dependent diabetes mellitus (NIDDM).
IDDM represents a heterogenous and polygenic disorder, with a number of non-HLA loci contributing to the disease susceptibility1. There is yet no identified agent substantially capable of preventing this type of disease2.
NIDDM is far more common and results from a combination of defects in insulin secretion and action. Treatment of Type 2 diabetes is complicated by several factors inherent to the disease process, typically, insulin resistance, hyperinsulinemia, impaired insulin secretion, reduced insulin-mediated glucose uptake and utilization3.
Vitamins and minerals play diverse roles in the body. They most commonly function as essential coenzymes and cofactors for metabolic reactions and thus help support basic cellular reactions (i.e., glycolysis, the citric acid cycle, lipid and amino acid metabolism) required to maintain energy production and life4,5 .They are also important in the regulation of metabolism, gene expression and may influence the development and progression of many chronic diseases.
A number of studies have reported an association between diabetes mellitus (DM) and alterations in the metabolism of several minerals4,5.
Impaired insulin release, insulin resistance and glucose intolerance in experimental animals and humans with DM have been linked to a compromised status of chromium, magnesium, selenium, vanadium and zinc6. Some of these minerals (e.g., zinc, chromium, magnesium) are excreted at higher than normal rates in patients with DM, often leading to excessive urinary mineral wasting4,5.
Magnesium functions as an essential cofactor for more than 300 enzymes. It is essential for all energy-dependent transport systems, glycolysis, oxidative energy metabolism, biosynthetic reactions, normal bone metabolism, neuromuscular activity, electrolyte balance, and cell membrane stabilization6. Poor control of diabetes has often been associated with low serum magnesium7. Magnesium deficiency has also been found to be common in children with type I diabetes8. The reduced concentrations of magnesium seen in diabetics appears to result in part from increased urinary magnesium excretion7
Although a reduction in insulin release has been reported in individuals with compromised magnesium status, most of the focus on magnesium supplementation in DM is directed at preventing long-term complications. There is however a dearth of information regarding the prevention of the onset of diabetes by magnesium. It was previously reported that pre-administration of Magnesium may delay the onset of hyperglycemia in alloxan diabetes9. However, whether this delay was temporary, prolonged or permanent was not elucidated. This study therefore examined the effect of pre-administration of magnesium on alloxan diabetes over a 10 day period.
Methods
Chemicals and Reagents
Magnesium sulphate (Mg2SO4) and alloxan was purchased from Sigma Aldrich Chemical Co. (St. Louis, Missouri). 100 mg of Mg2+ (as Mg2SO4) was dissolved in 1ml of distilled water to give a stock solution of 100 mg/ml and this was thereafter administered to the experimental animals at single doses of 100 mg/kg and 150 mg/kg10 respectively intraperitoneally.
100 mg of alloxan granules were dissolved in 1ml normal saline to make a stock solution of 100 mg/ml and this administered after an overnight fast at a single dose of 120 mg/kg body weight intraperitoneally11.
Animals
Thirty-six male albino rats weighing between 160g – 250g were used in this study. All the animals were acclimatized in the Animal House of the Physiology Department, College of Medicine, University of Ibadan for 14 days prior to any procedure. Animals were house in well aerated cages, fed on standards rat pellets obtained from Ladokun feeds and allowed free access to drinking water.
Animal Grouping
Animals were divided into six (6) groups of six animals each as follows:
Healthy control group
Diabetic control group
Magnesium 100 mg/kg pretreated group
Magnesium 150 mg/kg pretreated group
Magnesium 100 mg/kg only group
Magnesium 150 mg/kg only group
Induction of Diabetes Mellitus
Diabetes was induced with a single intraperitoneal dose of alloxan (120 mg/kg body weight) 11 after an 18 hour overnight fast. Thereafter, the animals were allowed free access to food and water.
Plasma glucose levels were determined using the glucose oxidase method12. The level of blood glucose considered to be normal in rattus novergicus ranges from 50 mg/dl – 135 mg/dl11. In this study, rats with glucose levels above 250 mg/dl were considered as having severe diabetes.
Experimental protocol
The experimental protocol followed is as described by Heikkila and Cabbot13. Male albino rats weighing between 160 – 250g were obtained from the College of Medicine animal house for this study. The rats were kept in the departmental animal house for 2 weeks to stabilize. They were then fasted for 18hours after which basal blood glucose levels were determined using blood from the tail vein of each rat.
The 36 rats were divided into 6 groups of 6 rats each. Animals in group 1 were normal and given single doses of 0.2 ml of 0.9% NaCl intraperitoneally. They served as healthy control. Animals in group 2 were made diabetic with alloxan (120 mg/kg) and did not receive any pretreatment pre or post diabetes, these served as diabetic control. Animals in group 3 were pretreated with magnesium (100 mg/kg) intraperitoneally one hour prior to alloxan (120 mg/kg) administration. Animals in group 4 were also pretreated with magnesium (150 mg/kg) intraperitoneally one hour prior to alloxan (120 mg/kg) administration. Animals in group 5 were given intraperitoneal injection of magnesium (100 mg/kg) only, while animals in group 6 were treated with 150 mg/kg magnesium only.
The blood glucose levels from all groups were monitored for a ten day period with blood samples obtained from the tail vein on day 0, day 2, day 5, day 7 and day 10.
Statistical analysis
Results were expressed as the mean ± SEM. Statistical comparisons were made using Student's t Test. Level of significance were evaluated at P= 0.05, 0.01 and 0.001 and differences were regarded as statistically significant when P values were 0.05 or less.
Results
The results of all the experiments carried out are shown in tables 1 – 2 and figures 1
Table 1.
Body weight changes in control and experimental animals
| Group/Day | Day 0 weight (g) | Day 2 weight (g) | Day 5 weight (g) | Day 7 weight (g) | Day 10 weight (g) |
| Healthy control | 170.00±3.65 | 172.00±4.28 | 175.17±2.01 | 178.83±3.74 | 178.00±4.47 |
| Diabetic control | 188.33±5.43 | 166.67±4.94 | 148.33±7.03 | 131.67±5.43 | 120.00±3.65** |
| Magnesium (100mg/kg) pretreated diabetic group |
216.67±6.15 | 191.67±4.01 | 160±0.00 | 160±0.00 | 148.33±4.77** |
| Magnesium (150mg/kg) pretreated diabetic group |
175.00±3.42 | 151.67±3.07 | 136.67±8.03 | 116.67±4.22 | 115.00±3.42** |
| Magnesium (100mg/kg) only | 230.00±7.30 | 205.00±7.19 | 220.00±5.16 | 228.33±9.10 | 238.33±7.93 |
| Magnesium (150mg/kg) only | 203.33±4.03 | 180.00±3.83 | 193.33±5.89 | 198.33±7.46 | 206.66±5.85 |
Values are mean ± SEM.
P ≤ 0.01 significant difference between day 0 values and day 10 values within the group
Table 2.
Percentage change in Blood glucose levels in control and experimental animals on day 10 when compared with day 0
| Group | Day 10 |
| Healthy Control group | 0% |
| Diabetic Control group | 408.33% ± 11.36%*** |
| Magnesium (100mg/kg) pretreated diabetic group | 351.94% ± 4.36%***## |
| Magnesium (150mg/kg) pretreated group | 236.46% ± 11.03%***## |
| Magnesium (100mg/kg) Only Group | 22.27% ± 2.27% |
| Magnesium (150mg/kg) Only Group | 7.95% ± 1.61% |
Values are percentages differences between day 0 and day 10 values.
= P≤ 0.001 Significant difference between healthy control values and experimental values
= P<0.01 significant difference between magnesium pretreated diabetic group and diabetic control
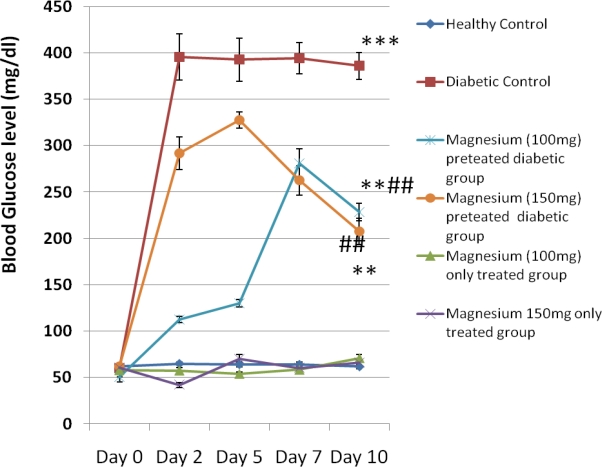
Figure 1.
Blood glucose level in control and experimental animals
Blood Glucose Levels
Figure 1 shows the blood glucose level in control and experimental animals from day 0 to day 10. There was significant increase (P<0.001) in fasting plasma glucose values in the diabetic untreated group on Day 2 ( 395.5 ± 24.78 mg/dl), Day 5 (392.5 ± 23.05 mg/dl), Day 7 (394.17±16.65 mg/dl) and Day 10 (385.83 ± 14.57 mg/dl) when compared with the control values. There was also a significant increase (P<0.01) in plasma glucose levels in the magnesium-pretreated (100 mg /kg and 150 mg/kg) diabetic groups when compared with the healthy control group. However, there was a significant reduction (P<0.01) in blood glucose level in the magnesium pre-treated diabetic groups on Day 2, Day 5, Day 7 and Day 10 when compared with the diabetic controls. The values obtained in the magnesium 100 mg/kg pretreated alloxan group on the days above are as follows 112.5 ± 3.1 mg/dl, 130 ± 3.87 mg/dl, 281.17 ± 15.1 mg/dl, 228.33 ± 9.5 mg/dl while values obtained in the magnesium 150 mg/kg pretreated alloxan group are as follows 291.83 ± 17.79mg/dl, 327.5 ± 8.92mg/dl, 262.5 ± 15.8 mg/dl, 207.5 ± 14.13 mg/dl.
It may be observed that plasma glucose level in the magnesium 150 mg/kg pretreated group declined after day 5 while that of the magnesium 100 mg/kg treated group declined after day 7.
Body weight changes
Table 1 shows the body weight changes in control and experimental from day 0 to day 10. It may be observed that there was an insignificant increase in the body weight in the control group by day 10 when compared with day 0 values, while in the diabetic group there was a steady significant decline (p <0.05) in the body weight of the diabetic abimals. There was a 31.6% and 34.3% reduction in the body weights of magnesium pretreated diabetic animals which was not significantly different 36.3% decrease in the diabetic group.
In the magnesium only groups (100 mg/kg and 150 mg/kg) there was an increase in the body weight on day 10 when compared with day 0 values. This increase in body weight was however statistically insignificant.
Discussion
In this study it was observed that prior administration of magnesium appeared to delay the onset of hyperglycemia as observed in alloxan diabetes and thereafter caused a gradual decline in hyperglycemia in the experimental animals. It was also observed that there was decrease in body weights of the rats in the diabetic group as well as in the magnesium pretreated diabetic groups and this appears to be consistent with the previous report of World Health Organization, that diabetes mellitus is often characterized by rapid and significant weight loss leading to fatigue which is not easily reversed14.
Alloxan administration has been reported to lead to necrosis of pancreatic beta cells. Alloxan and the product of its reduction, dialuric acid, establish a redox cycle with the formation of superoxide radicals with subsequent dismutation to hydrogen peroxide. This leads to formation of highly reactive hydroxyl radicals by the Fenton reaction. One of the targets of the reactive oxygen species is DNA of pancreatic islets15. DNA damage by the reactive oxygen species lead to cell death by necrosis.
Magnesium ions are essential to the basic nucleic acid chemistry of life, and thus are essential to all cells of all known living organisms. Plants have an additional use for magnesium in that chlorophylls are magnesium-centered porphyrins. Many enzymes require the presence of magnesium ions for their catalytic action, especially enzymes utilizing ATP or those which use other nucleotides to synthesize DNA and RNA. Recently there has been growing interest in magnesium deficiency and its correlation with coronary artery disease, chronic complications of diabetes mellitus and antioxidant enzyme activity16. Magnesium has also been reported to be an obligatory cofactor in GSH synthesis and in all biosynthetic reactions involving ATP17. In animals with reduced glutathione levels it has been reported that Mg2+ pretreatment was efficient in restoring renal and testis GSH levels in tissues of mice exposed to acute and subacute cadmium intoxication18.
It has been reported that GSH is the main intracellular free radical scavenger and is known to provide protection against free radicals19. It has been reported to divert hydrogen peroxide from the pathway leading to the formation of hydroxyl radicals20. In addition to this Sakurai and Ogiso observed that the in vitro generation of hydroxyl radicals in the presence of alloxan strongly depends on GSH concentration12.
The reduction in plasma glucose level in the magnesium pretreated group when compared with the diabetic group may be due to the ability of magnesium to increase the activity of GSH within the pancreatic beta cells. The increased GSH activity could have caused an increase in the scavenging of free radicals produced by alloxan administration.
It has also been proposed that disturbances in intracellular calcium homeostasis constitute an important step in the diabetogenic action of alloxan. This concept was confirmed by in vitro and in vivo experiments demonstrating that alloxan elevates cytosolic free Ca ion concentration in pancreatic B cells22,23.
The effect of alloxan on intracellular calcium concentration seems to be mediated, at least partially, by hydrogen peroxide since it has been reported that hydrogen peroxide exerts a similar effect on calcium concentration in B cells23. The exaggerated concentration of calcium ion leads to supraphysiological insulin release and, together with reactive oxygen species, causes damage of pancreatic B cells11.
Magnesium has also been shown to be a physiologic calcium blocker24. It has been reported to inhibit calcium overload through inhibition of calcium transport across most calcium channels25. Hence calcium overload caused by alloxan administration may have been prevented, reduced or delayed as a result of the effects of magnesium as a calcium channel blocker.
Conclusion
It may be deduced from this study that pretreatment with magnesium may cause a delay in the onset and subsequent decline in hyperglycemia as observed in alloxan diabetes. This effect of magnesium may be due to the free radical scavenging ability of magnesium, its ability to potentiate antioxidant glutathione production or it physiological action as a calcium channel blocker.
It is suggested that further studies be carried out in order to evaluate the effect of magnesium intake on the blood glutathione level as well on the plasma level of other free radical scavengers.
References
- 1.Lernmark A, Ott J. Sometimes it's hot, sometimes it's not. Nature Genetics. 1998;19(3):213–214. doi: 10.1038/881. [DOI] [PubMed] [Google Scholar]
- 2.Tiwari AK, Rao JM. Diabetes Mellitus and Multiple Therapeutic Approaches of Phytochemicals: Present Status and Future Prospects. Current Science. 2002;83(1):30–38. [Google Scholar]
- 3.De fronzo RA. Pathogenesis of Type 2 Diabetes. Metabolic and Molecular Implications for Identifying Diabetic Genes. Diabetes Reviews. 1997;5:177–267. [Google Scholar]
- 4.O'Connell BS. Select Vitamins and Minerals in the Management of Diabetes. Diabetes Spectrum. 2001;14(3):133–148. [Google Scholar]
- 5.Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic Review of Herbs and Dietary Supplements for Glycemic Control in Diabetes. Diabetes Care. 2003;26:1277–1294. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- 6.De Valk H. Magnesium in Diabetes Mellitus. Netherlands Journal of Medicine. 1999;54:139–146. doi: 10.1016/s0300-2977(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 7.Sjogren A, Floren CH, Nilsson A. Magnesium Deficiency in IDDM related to level of Glycosylated Hemoglobin. Diabetes. 1986;35:459–463. doi: 10.2337/diab.35.4.459. [DOI] [PubMed] [Google Scholar]
- 8.Fort P, Lifshitz F. Magnesium status in Children with Insulin Dependent Diabetes Mellitus. Journal of the American College of Nutrition. 1986;5:69–78. doi: 10.1080/07315724.1986.10720114. [DOI] [PubMed] [Google Scholar]
- 9.Ige AO, Adewoye EO, Olaleye SB, Salami AT. Pretreatment Effect of Magnesium on Alloxan induced hyperglycemia in rats; Conference proceedings, 1st University of Ibadan Conference of Biomedical Research.2008. [Google Scholar]
- 10.Van Elstraete AC, Sitbon P, Mazoit J, Conti M, Benhamou D. Protective effect of prior administration of magnesium on delayed hyperalgesia induced by fentanyl in rats. Canadian Journal of Anesthesia. 2006;53(12):1180–1185. doi: 10.1007/BF03021578. [DOI] [PubMed] [Google Scholar]
- 11.Szkudelski T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiological Research. 2001;50:536–546. [PubMed] [Google Scholar]
- 12.Trinder P. Determination of Blood Glucose Using an Oxidase Peroxidase System with a Non - Carcinogenic Chromogen. Journal of Clinical Pathology. 1969;22:158–161. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heikkila RE, Cabbat FS. The Prevention of Alloxan-induced Diabetes by Amygdalin. Life Science. 1980;27(8):659–662. doi: 10.1016/0024-3205(80)90006-5. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization, author. WHO technical report series. Geneva: 1985. Diabetes mellitus; Report of a WHO study group; p. 727. [PubMed] [Google Scholar]
- 15.Sakurai K, Ogiso T. Effect of Ferritin on ëDNA strand breaks in the Reaction System of Alloxan plus NADPH-cytochrome P450 reductase: Ferritin's role in Diabetogenic action of Alloxan. Biological and Pharmaceutical Bulletin. 1995;18:262–266. doi: 10.1248/bpb.18.262. [DOI] [PubMed] [Google Scholar]
- 16.Soltania N, Keshavarza M, Sohanakia H, Dehpcourb AR, Aslc SZ. Oral Magnesium Administration Prevents Vascular Complications in STZ-diabetic rats. Life Sciences. 2005;76(13):1455–1464. doi: 10.1016/j.lfs.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Mills BJ, Lindeman RD, Lang CA. Magnesium Deficiency Inhibits Biosynthesis of Blood Glutathione and Tumor Growth in the Rat. Proceedings of the Society for Experimental Biology and Medicine. 1986;181:326–332. doi: 10.3181/00379727-181-42260. [DOI] [PubMed] [Google Scholar]
- 18.Djukic-Cosic D, Djukic-Æosic M, Ninkovic M, Ninkovic Z, Malicevic Z, Malicevic V, Matovic V, Matovic D, Soldatovic D. Effect of Magnesium Pretreatment on Reduced Glutathione Levels in Tissues of Mice Exposed to Acute and Subacute Cadmium Intoxication: A Time Course Study. Magnesium Research. 2007;20(3):177–186. [PubMed] [Google Scholar]
- 19.Donnini D, Zambito AM, Perrella G, Ambesi-Impiombato FS, Curcio F. Glucose May Induce Cell Death Through a Free Radical-Mediated Mechanism. Biochemical and Biophysical Research Communications. 1996;15:412–417. doi: 10.1006/bbrc.1996.0247. [DOI] [PubMed] [Google Scholar]
- 20.Pipeleers DG, Van De Winkel M. Pancreatic B-Cells Possess Defence Mechanism against Cell-specific Toxicity. Proceedings of the National Academy of Sciences. 1986;83:5267–5271. doi: 10.1073/pnas.83.14.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai K, Ogiso T. Inhibitory Effect of Glutathione on the Generation of Hydroxyl Radicals in the Reaction System of Glutathione-Alloxan. Chemical and Pharmaceutical Bulletin. 1991;39:737–742. [Google Scholar]
- 22.Kim HR, Rho HW, Park JW, Kim JS, Kim UH, Chung MY. Role of Ca2+ in Alloxan-induced Pancreatic Beta-cell Damage. Biochimica et Biophysica Acta. 1994;1227:87–91. doi: 10.1016/0925-4439(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 23.Park BH, Rho HW, Park JW, Cho CG, Kim JS, Chung HT, Kim HR. Protective Mechanism of Glucose against Alloxan-induced Pancreatic Beta-cell Damage. Biochemical and Biophysical Research Communications. 1995;210:1–6. doi: 10.1006/bbrc.1995.1619. [DOI] [PubMed] [Google Scholar]
- 24.Woods KL. Possible Pharmacological Actions of Magnesium in Acute Myocardial Infarction. British Journal of Clinical Pharmacology. 1991;32:3–10. doi: 10.1111/j.1365-2125.1991.tb05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao-kui W, Quigley JF, Hanlon SU, O'Rourke B, Haigney MCP. Cytosolic Free Magnesium Modulates Na/Ca Exchange Currents in Pig Myocytes. Cardiovascular Research. 2002;53(2):334–340. doi: 10.1016/s0008-6363(01)00501-6. [DOI] [PubMed] [Google Scholar]