Abstract
Introduction
The burden of both community and hospital acquired adverse drug reactions (ADRs) are some of the important issues in pharmacotherapy. At the time of this study there was very scanty literature in this area from Africa.
Objective
This study was done to determine the frequency and characteristics of ADRs in patients admitted on medical wards in public hospitals.
Methods
This was a longitudinal observational study on 728 adult patients on medical wards in one regional and one district hospitals. Community and hospital acquired ADRs were assessed.
Results
Thirty three patients (4.5%) were admitted with suspected ADR, and an ADR was the reason for hospitalization in 1.5%. Most ADRs were due to antiparasitic products, mainly quinine (61%). Community acquired ADRs prolonged hospital stay, 5.6 days vs 4.0 days (p-value < 0.001). During hospitalization ADRs occurred in 49.5% of the patients. Antiparasitic products, predominantly quinine, were the commonest drugs class associated with ADRs (85.9%). Hospital acquired ADRs did not affect hospital stay, 4.2 days vs 3.9 (p-value 0.129).
Conclusion
ADRs are an important cause of morbidity in patients, both in the community and in hospitals, and the majority are associated with the commonly used drugs.
Keywords: Adverse drug reactions, medical wards, hospitalization
Introduction
Adverse drug reactions have become one of the major burdens in the healthcare system. Other than leading to increased morbidity and mortality, they lead to excess healthcare costs1,2. Studies have shown that 3 to 14% of all hospital admissions on medical wards are related to ADRs3,7. Furthermore, 5 to 20% of all hospitalized patients develop ADRs during hospitalization1,2,7–11. At the time of conducting the current study the majority of the available studies had been conducted in the high-income countries. The results of the only available study from Africa7, had not been published. The high prevalence of HIV/AIDS, especially in sub-Saharan Africa, and the introduction of complex and relatively new therapies make detection of ADRs in these countries more crucial than before.
The aim of this study was to create awareness regarding the burden of ADRs, both at admission and during hospitalization, in Uganda. The objectives were to determine the frequency and characterize the ADRs. Ethical clearance was obtained from Makerere University College of Health Sciences Research Committee and the Uganda National Council for Science and Technology. Informed consent was obtained from the participating patients, or guardians in the case of patients aged below 18 years.
Methods
Study design, site and population
This was a longitudinal observational study, conducted in Kabale Regional Referral and Itojo District Hospitals. The study was conducted from July to December 2005. The study population consisted of patients aged 13 years and above, admitted on the medical wards of the two hospitals during the study period.
Identification of suspected ADRs
Adverse drug reactions were defined according to the World Health Organization (WHO) definition12. ADR identification was based on history and physical examination as the laboratory and other investigational facilities in the two hospitals were limited.
The data was collected by a doctor and a pharmacist supervised by the principal author and the attending physician. On admission, for every patient who fulfilled the inclusion criteria, information on demographic data, reported prehospital drug exposure within one week preceding admission and diagnosis was recorded. The drug exposure was obtained by asking the patient to recall names and dosages of both prescribed and over the counter drugs. Also the patient was asked to show any available prescriptions. Herbal and homeopathic medicines were excluded from the analysis.
At admission a detailed history was obtained and physical examination was done to identify suspected community-acquired ADRs. Patients with a suspected ADR were monitored daily during hospitalization to assess the progress of the reaction. To identify the hospital-acquired ADRs all patients were reviewed daily until discharge. Medical records were reviewed daily and drugs taken were recorded. Any patient who developed a suspected ADR during hospitalization monitored daily to assess the progress of the reaction. For any ADR description of signs and symptoms, duration, suspected drug, and any drugs used to treat the reaction, were recorded.
Those admitted with complaint of reduced hearing suspected to be associated with an ototoxic drug had hearing levels measured using a pure tone field audiometer (Micro Audiometrics Corporation, 1999) before receiving any other treatment. Also patients who were prescribed any ototoxic drug during hospitalization had their hearing levels measured before starting on the drug. This was done for 500, 1000, 2000, 4000, and 6000 Hz. For those on ototoxic drugs assessment was repeated every day until discharge.
The causality assessment and categorization of a suspected ADR was based on Naranjo ADR Probability Scale13, while the severity grading was based on that used by Dormann, et al14. The assessment was done by a committee composed of a physician in charge of the internal medicine ward, a pharmacist and the principal investigator, who met at the end of everyday to ascertain the probability of the suspected ADRs identified during that day. However, if the suspected ADR was considered to require immediate stopping, change of the drug or giving an antidote, the physician was contacted immediately.
The diagnosis was based on International Statistical Classification of Diseases and Related Health Problems (ICD) Version 1015. The ADRs were classified as Type A or Type B16. The classification of symptoms and signs of ADRs was based on WHO Adverse Reaction Terminology17, while Anatomical Therapeutic Chemical Classification (ATC) 18 was used for classification of drugs. Determination of preventability of ADR was based on the criteria defined by Schumock19.
Statistical analysis
The data was entered using Microsoft Access, cleaned and then transferred to SPSS 10.0 for windows for analysis. Differences between groups were tested by independent samples t-test for age, chi-square-test for counts and the Fisher's Exact test for independent samples, where appropriate. The change in hearing levels for patients who received ototoxic drugs was calculated as mean hearing loss.
Results
During the study period 594 and 527 patients were admitted in the regional and district hospitals respectively. Recruited in the study were 366 patients in the regional and 362 in district hospitals, the rest were excluded because either they refused to consent or were too ill to cooperate. The females contributed 57% and 55% in the regional and district hospitals respectively. The mean age of the patients in the regional hospital was 37 years (Std. Dev. 17.2), median 32 years. The corresponding figures in the district hospital were 35 year (Std. Dev. 16.8), median 30 years (p-value 0.055). The commonest diagnosis at discharge in both hospitals was infections and parasitic disease (63.5%) of which the bulk was malaria, followed by diseases of the respiratory system.
Suspected adverse drug reactions at admission
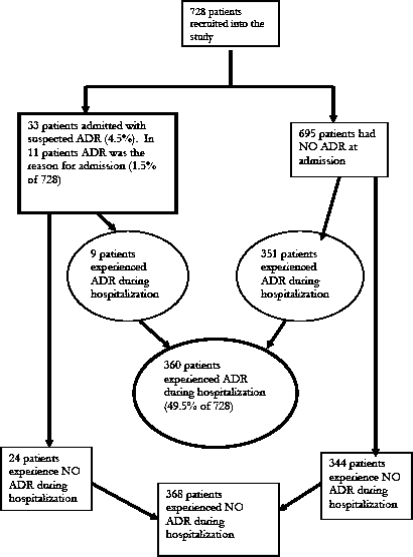
Thirty three patients (4.5%), 18 and 15 from regional and district hospitals respectively, were admitted with a suspected ADR (Figure 1), and in 11 patients, (1.5%), ADRs was the cause for hospitalization. Each patient had one suspected ADR. There were more females than males (64%). Age was not associated with ADRs (Table 1).
Figure 1.
Distribution of patients experiencing an ADR either before or during hospitalization
Table 1.
Factors associated with ADRs
| At admission | During hospitalization | |||||
| No ADR | ADR | p-value | No ADR | ADR | p-value | |
| All hospitals | ||||||
| Mean Age in years (95% CI) | 33.4(28.9 – 37.8) | 36.4(35.1 – 37.6) | 0.332 | 35.0(33.3 – 36.6) | 37.5(35.6 – 39.3) | 0.047 |
| Mean # of drugs at admission (95% CI) |
2.0(1.6 – 2.4) | 0.9(0.8 – 1.0) | <0.001 | 1.0(0.9 – 1.1) | 0.9(0.8 – 1.0) | 0.160 |
| Mean # of drugs during hospitalization (95% CI) |
3.3(3.2 – 3.5) | 3.4(3.3 – 3.6) | 0.288 | |||
| Mean #of days of hospital stay (95% CI) |
5.6(4.0 – 7.2) | 4.0(3.8 – 4.2) | <0.001 | 4.2(3.9 – 4.5) | 3.9(3.6 – 4.1) | 0.129 |
| Regional hospital | ||||||
| Mean Age in years (95% CI) | 34.8(29.6 – 40.0) | 37.6(35.8 – 39.4) | 0.502 | 36.4(34.1 – 38.7) | 38.7(36.0 – 41.4) | 0.206 |
| Mean # of drugs at admission (95% CI) |
2.2(1.6 – 2.7) | 0.9(0.8 – 1.0) | <0.001 | 0.9(0.8 – 1.1) | 0.9(0.8 – 1.1) | 0.956 |
| Mean # of drugs during hospitalization (95% CI) |
3.7(3.6 – 3.9) | 3.9(3.6 – 4.1) | 0.449 | |||
| Mean # of days of hospital stay (95% CI) |
6.7(4.1 – 9.3) | 4.5(4.2 – 4.8) | 0.005 | 4.9(4.4 – 5.3) | 4.3(3.9 – 4.8) | 0.102 |
| District hospital | ||||||
| Mean Age in years (95% CI) | 31.5(23.1 – 39.9) | 35.1(33.3 – 36.9) | 0.433 | 33.3(31.0 – 35.6) | 36.4(33.8 – 38.9) | 0.077 |
| Mean # of drugs at admission (95% CI) |
1.8(1.2 – 2.3) | 0.9(0.8 – 1.0) | <0.001 | 1.1(0.9 – 1.2) | 0.9(0.7 – 1.0) | 0.027 |
| Mean # of drugs during hospitalization (95% CI) |
2.8(2.6 – 3.0) | 3.0(2.9 – 3.2) | 0.062 | |||
| Mean # of days of hospital stay (95% CI) |
4.2(2.9 – 5.5) | 3.4(3.3 – 3.6) | 0.079 | 3.4(3.2 – 3.6) | 3.5(3.3 – 3.8) | 0.456 |
Clinically there were 33 suspected ADRs. However, when classified based on the World Health Organization Adverse Reaction Terminology classification17, which considers individual symptoms and signs, there were 72 ADRs (Table 2). The commonest suspected ADRs were hearing and vestibular disorders (38.9%), followed by gastrointestinal disorders (37.5%).
Table 2.
Clinical presentation of community and hospital acquired ADRs based on WHO Adverse Reaction Terminology classification
| System organ | At admission (n=72) | During hospitalization (n=858) | ||
| Frequency | % | Frequency | % | |
| Skin and appendages disorders | 8 | 11 | 16 | 1.9 |
| Musculo-skeletal system disorders | 0 | 0 | 6 | 0.7 |
| Central & peripheral nervous system disorders | 3 | 4.2 | 11 | 1.3 |
| Vision disorders | 3 | 4.2 | 54 | 6.3 |
| Hearing and vestibular disorders | 28 | 38.9 | 626 | 73.0 |
| Gastro-intestinal system disorders | 27 | 37.5 | 118 | 13.7 |
| Metabolic and nutritional disorders | 0 | 0 | 5 | 0.6 |
| Heart rate and rhythm disorders | 0 | 0 | 15 | 1.7 |
| Respiratory system disorders | 1 | 1.4 | 0 | 0 |
| Body as a whole (General disorders) | 2 | 2.8 | 7 | 0.8 |
| Total | 72 | 100 | 859 | 100 |
Antiparasitic products were the commonest drug class associated with ADRs (n = 20), followed by anti-infectives (n = 12), nine of which were associated with ARVs. Majority of the suspected ADRs (57%), were associated with quinine, followed by a combination of stavudine, lamivudine and nevirapine (triomune, 27%). Patients admitted with ADRs had taken more drugs than those who did not have an ADR.
The type, severity, causality assessment and the state at discharge of the suspected ADRs that occurred before hospitalization are shown in Table 3. Most of the ADRs were Type A, mild and were assessed as possible. Those due to hypersensitivity included two skin reactions due to a triomune, and one associated with co-trimoxazole. ADRs were not associated with prolonged hospital stay and majority had not fully recovered from the ADRs at the time of discharge (Table 3).
Table 3.
Type, severity, causality assessment and the outcome of the suspected ADR, as determined at discharge
| Variable | Patients admitted with an ADR | Patients with ADR during hospitalization | |||
| Frequency (total = 33) | (%) | Frequency (total = 437) | (%) | ||
| Type of ADR | Type A | 30 | 91 | 435 | 99.5 |
| Type B | 3 | 9 | 2 | 0.5 | |
| Grading of severity | Mild | 22 | 67 | 434 | 99.3 |
| Moderate | 11 | 33 | 3 | 0.7 | |
| Causality assessment | Probable | 11 | 33 | 180 | 41.2 |
| Possible | 22 | 67 | 257 | 58.8 | |
| Outcome of the ADR at discharge |
Not fully recovered | 24 | 73 | 380 | 87.0 |
| Fully recovered | 9 | 27 | 56 | 12.8 | |
| Died before evaluation | 0 | 0 | 1 | 0.2 | |
Adverse drug reactions during hospitalization
Out of 728 patients 360 (49.5%) experienced suspected ADR during hospitalization, 57% of these being from regional hospital. The total number of ADRs was 437. In the regional hospital 52.7% of the patients developed a suspected ADR, and in the district hospital the figure was 46.1%. In the regional hospital 66% were females, while in the district hospital the corresponding figure was 56%. ADRs were not associated with age and the number of drugs taken during hospitalization.
Clinically there were 437 suspected ADRs. However, based on the Word Health Organization Adverse Reaction Terminology Classification (17), there was a total of 858 ADRs. The most involved organ system was hearing and vestibular (73%), followed by gastro-intestinal (13.7%). The commonest ADR was reduced hearing (43%), most of which was associated with quinine. In 16 out of the 437 ADRs (3.7%) the reaction warranted stopping the suspected drug.
Almost all the ADRs were Type A (99.5%) of which 99.3% were mild and most were regarded as possible (58.8%). Those due to hypersensitivity were two reactions to penicillins. Those graded as moderate, included two due to penicillins and one due to amphotericin B. Majority of the ADRs were still present at the time of discharge (87%).
Based on the ATC classification the commonest drugs were anti-parasitic products, namely quinine and chloroquine (85.9%) followed by anti-infectives for systemic use (10.7%) the majority of which were antibacterials. In only 18 ADRs (4%) was the reaction regarded as preventable. These included six suspected ADRs to metronidazole and doxycycline each where the dispensing nurse did not give adequate instructions. Others were one each to indomethacin, ibuprofen, prednisone and acetyl salicylic acid where in each case there was a history of peptic ulcer disease. The other two were due to crystalline penicillin and ampicillin where the patient had a history of reacting to penicillin.
Length of hospital stay for most of the patients (50.3%) was 1 – 3 days. ADRs did not significantly affect duration of hospital stay in both hospitals.
Discussion
The findings in this study demonstrate that ADRs are an important contribution to patient morbidity and hospitalization in Uganda. Thus they increase the cost of providing care to patients in an already overstretched healthcare system. In present study the rate of ADRs at admission was lower than that reported in previous studies, 8 to 22%1, 2, 7, 10. In this study the identification of ADRs was based only on clinical assessment and also there was incomplete history on drugs taken before hospitalization in some patients. Therefore, it is likely that some ADRs may have been missed.
The rate of ADRs as a cause of admission on medical wards have been reported to be 3 to 8%1, 2, 7, 9, 10. Though in our study the rate (1.5%) was found to be lower many of the reactions were of moderate severity. Therefore, these required immediate treatment. In a country where access to health care is very limited, especially in rural areas, this poses problems to the life of the patient.
The rate of hospital acquired ADRs is much higher than the 4 to 17% previously reported1, 2, 7, 9, 10. The variation may be partly explained by the differences in the disease pattern. Even at national level it is difficult to generalize our results to tertiary health facilities where the disease pattern is much broader.
Similar to results from previous studies age was not an important fact for community acquired ADRs 1,5,11. However, patients who developed ADRs during hospitalization were younger than those without. This is in agreement with results of a previous study9. However, the role of age cannot be ruled out as the majority of the patients were below 50 years. The predominance of females among patients with ADRs both at admission and during hospitalization is similar to results in a previous study9. However, this cannot be entirely explained by the fact that females are more at risk of developing ADRs since there were more females than males in the study population.
Result of several studies have shown association of the number of drugs with both community and hospital acquired ADRs1,9,11. In contrast in this study there was no association with the number of drugs taken during hospitalization. The ADRs were associated with individual drugs rather than being a result of drug-drug interactions. This highlights the need for intense monitoring of patients receiving medications that are associated with high risk of ADRs.
The commonest systems reported are central nervous, gastrointestinal, metabolic and renal1, 7, 11. Our results vary from this with hearing and vestibular system being the commonest. This may be explained by the difference in the disease patterns and therefore the prescription profile in these countries compared to Uganda. In the current study the commonest reason for hospitalization was complicated malaria and thus the use of antiparastic drugs, namely quinine and chloroquine which are linked to hearing and vestibular disorders.
Similar to results from previous studies most of the ADRs were Type A7, 9, 11. From both patient and healthcare perspectives serious pharmacological type A reactions are of great importance as they are more frequent and theoretically preventable6. In addition, some Type A reactions which may not be graded as severe, affects the quality of life and therefore may have an impact on the clinical outcome. Because we depended only on clinical symptoms and signs to identify the ADRs, some reactions which may have been graded as certain could have been missed. However, similar to the results of previous studies2,10 most ADRs were graded as possible. Nevertheless, this study has shown that without laboratory facilities many ADRs can still be identified based on only clinical symptoms and signs.
Though most of ADRs, both community and hospital acquired, were mild, all the moderate community-acquired ADRs were severe enough to be the reason for hospitalization. This indicates the need for sensitizing both the public and the healthcare professionals about awareness of ADRs and provision of appropriate drug information on dispensing drugs.
Unlike in a previous study2, we found that community acquired ADRs were not associated with prolonged hospital stay. Similarly, unlike in other studies where ADRs occurring during hospital stay have been found to prolong hospitalization1,2,9, we found no association between ADRs and prolonged hospital stay. In our case, quite often patients requested for discharge when they feel slight improvement because of social obligations. However, we did not adjust for these factors in the analysis.
Unlike in previous studies where 33 to 59% of hospital acquired ADRs were preventable7,10, there were very few potentially preventable ADRs. All the preventable reactions in our study were a result of irrational prescribing and dispensing. Therefore, the results of this study emphasize the need for interventions to improve rational prescribing and dispensing in Uganda as one of the strategies for reducing the risk of ADRs.
Study limitations
Information on pre-hospital drugs use was mainly obtained from the patients and/or attendants as documentation was scanty. Therefore, this was a source of bias. Identification of an ADR was based only on clinical assessment. Thus the findings may be an underestimate of the number ADRs. For ethical reasons, where we found that the ADRs were preventable, we informed the staffs and further gave instructions on appropriate prescribing and dispensing. Therefore, this may have further led to reduction of subsequent number of preventable ADRs.
Conclusion
The study has shown that ADRs are an important cause of morbidity in patients, both in the community and in hospitals. This highlights the importance of strengthening strategies to improve rational prescribing and dispensing, as well as therapeutic monitoring. One such intervention is provision of a readily accessible source of up-to-date unbiased drug information to the health care professionals and the public.
Figure 2.
Age distribution of the patients who experienced suspected ADR during hospitalization
Acknowledgement
We are grateful to SIDA/SAREC through the Makerere University-Karolinska Institutet Collaboration, for funding the study.
References
- 1.Lagnaoui R, Moore N, Fach J, Longy-Boursier M, Begaud B. Adverse drug reactions in a department of systemic diseases-oriented internal medicine: prevalence, incidence, direct costs and avoidability. Eur J Clin Pharmacol. 2000 Jun;56(2):181–186. doi: 10.1007/s002280050738. [DOI] [PubMed] [Google Scholar]
- 2.Dormann H, Neubert A, Criegee-Rieck M, Egger T, Radespiel-Troger M, Azaz-Livshits T, et al. Readmissions and adverse drug reactions in internal medicine: the economic impact. J Intern Med. 2004 Jun;255(6):653–663. doi: 10.1111/j.1365-2796.2004.01326.x. [DOI] [PubMed] [Google Scholar]
- 3.Hallas J, Gram LF, Grodum E, Damsbo N, Brosen K, Haghfelt T, et al. Drug related admissions to medical wards: a population based survey. Br J Clin Pharmacol. 1992;33(1):61–68. doi: 10.1111/j.1365-2125.1992.tb04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pouyanne P, Haramburu F, Imbs JL, Begaud B. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. French Pharmacovigilance Centres. BMJ. 2000 Apr 15;320(7241):1036. doi: 10.1136/bmj.320.7241.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mjorndal T, Boman MD, Hagg S, Backstrom M, Wiholm BE, Wahlin A, et al. Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf. 2002 Jan–Feb;11(1):65–72. doi: 10.1002/pds.667. [DOI] [PubMed] [Google Scholar]
- 6.von Euler M, Eliasson E, Ohlen G, Bergman U. Adverse drug reactions causing hospitalization can be monitored from computerized medical records and thereby indicate the quality of drug utilization. Pharmacoepidemiol Drug Saf. 2006 Mar;15(3):179–184. doi: 10.1002/pds.1154. [DOI] [PubMed] [Google Scholar]
- 7.Mehta U, Durrheim DN, Blockman M, Kredo T, Gounden R, Barnes KI. Adverse drug reactions in adult medical inpatients in a South African hospital serving a community with a high HIV/AIDS prevalence: prospective observational study. Br J Clin Pharmacol. 65(3):396–406. doi: 10.1111/j.1365-2125.2007.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a metaanalysis of prospective studies. JAMA. 1998 Apr 15;279(15):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 9.Moore N, Lecointre D, Noblet C, Mabille M. Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol. 1998 Mar;45(3):301–308. doi: 10.1046/j.1365-2125.1998.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gholami K, Shalviri G. Factors associated with preventability, predictability, and severity of adverse drug reactions. Ann Pharmacother. 1999 Feb;33(2):236–240. doi: 10.1345/aph.17440. [DOI] [PubMed] [Google Scholar]
- 11.Camargo AL, Cardoso Ferreira MB, Heineck I. Adverse drug reactions: a cohort study in internal medicine units at a university hospital. Eur J Clin Pharmacol. 2006 Feb;62(2):143–149. doi: 10.1007/s00228-005-0086-7. [DOI] [PubMed] [Google Scholar]
- 12.Edwards IR, Biriell C. Harmonisation in pharmacovigilance. Drug Saf. 1994;10:93–102. doi: 10.2165/00002018-199410020-00001. [DOI] [PubMed] [Google Scholar]
- 13.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 14.Dormann H, Muth-Selbach U, Krebs S, Criegee-Rieck M, Tegeder I, Schneider HT, et al. Incidence and costs of adverse drug reactions during hospitalisation: computerised monitoring versus stimulated spontaneous reporting. Drug Saf. 2000 Feb;22(2):161–168. doi: 10.2165/00002018-200022020-00007. [DOI] [PubMed] [Google Scholar]
- 15.WHO, author. International Classification of Diseases, Version 10. 2007a. [Google Scholar]
- 16.Meyboom RH, Lindquist M, Egberts AC. An ABC of drug-related problems. Drug Saf. 2000 Jun;22(6):415–423. doi: 10.2165/00002018-200022060-00001. [DOI] [PubMed] [Google Scholar]
- 17.WHO, author. Adverse Reaction Terminology, December 2006. The Uppsala Monitoring Centre. World Health Organization; 2006. [Google Scholar]
- 18.WHO, author. Guidelines for ACT Classification and DDD assignment. 2007b. [Google Scholar]
- 19.Schumock GT, Thornton J P. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]