Abstract
Background
Highly active antiretroviral therapy (HAART) for people living with HIV/AIDS (PLWHA) has been generally accepted as the gold standard for the management of HIV patients but conflicting reports about the ability of HAART to improve upon the quality of life of HIV patients has cast doubts over the efficacy and the need for therapy.
Objective
This study was conducted to assess the efficacy and ability of HAART to resolve immunological and haematological abnormalities in HIV infected patients, existent sex variations in immunological and haematological parameters and CD4 predictive ability of the study parameters.
Methods
A total of 442 PLWHA consisting of 166 patients on HAART (28 males and 138 females) and 276 HAART-naïve patients (76 males and 200 females) were recruited for this study. Complete haemogram, immunological analysis (CD4 & CD3) and weight were measured for all the patients.
Results
HAART patients were older and heavier than their naïve counterparts. The incidence of anaemia (Hb less or equal to 10.5 (63%) and PCV < 30% (37.6%)) and lymphopoenia (16.7%) in HAART-naïve patients was significantly higher compared to their counterparts on HAART (46%, 15.2% and 5.3%) respectively. 70% of HAART-naïve females had anaemia in comparison to 44% in HAART-naïve males (P = 0.0001). The likelihood of developing microcytic hypochromic anaemia in HAART-naïve patients was 5 times more compared to those on HAART (P = 0.0002). Total lymphocyte count, haemoglobin, lymphocyte count and weight were significant predictors of CD4 counts and TLC values between 1.0 – 2.0 k µL−1 was a significant predictor of CD4 <200 cells mm−3.
Conclusion
HAART has the capability of reducing the incidence of anaemia and lymphopoenia which are associated with disease progression and death in HIV infected patients. Total lymphocyte count, haemoglobin and weight could also serve as useful predictive tools in the management and monitoring of HIV infected patients in resource limited settings.
Keywords: Antiretroviral, Lymphocyte, TLC, CD4, WHO/ACTG
Introduction
Acquired immune deficiency syndrome (AIDS) is caused by the human immunodeficiency virus (HIV) and is characterized by progressive damage to the body's immune system which results in a number of opportunistic infections, immunological and haematological complications1. The main immunological complication and hallmark of HIV infection is cellular CD4 T-lymphocyte depletion for which various mechanisms: HIV induced cytolysis; dysregulation of cytokines; cytotoxic T-lymphocyte responses and HIV induced autoimmune reactions 2–3 which are not mutually exclusive have been suggested. Haematological complications have been documented to be the second most common cause of morbidity and mortality in HIV patients4–6 and are generally marked with cytopoenias such as anaemia, neutropoenia, lymphopoenia and thrombocytopenia 7. The incidence and severity of the cytopoenia generally correlate to the stage of the disease with anaemia being the most commonly encountered haematologic abnormality and a significant predictor of progression to AIDS or death 8–9.
The introduction of highly active antiretroviral therapy (HAART) for treatment of HIV infection has generally been accepted as the gold standard in the management of HIV patients 9. Gea-Banacloche and Lane, 10 and Odunukwe et al.,9 reported improvements in haematocrit and haemoglobin values resulting in reduction in morbidity and mortality of HIV patients and Omoregie et al.,11 reported no improvement in haematocrit values of HAART treated HIV patients compared with HAART-naïve patients in his study.
Against the background of such conflicting reports which cast doubts on the efficacy and the need for HAART in this HAART era, this study was conducted to assess the impact of HAART in resolving immunological and haematological complications in HIV patients by comparatively analysing the results (immunological and haematological) of HAART-naïve patients and those on HAART so as to establish the continual need of HAART in the management of HIV patients. Existent sex variations in the study parameters and the ability of the study parameters to predict CD4 count and whether they can be used as surrogates for CD4 in resource poor settings where assay methods for viral loads and CD4 count are not available were assessed.
Methods
Study area and design
This cross-sectional study was carried out at the antiretroviral (ART) clinic in the Regional Hospital, Bolgatanga which is located in the Upper East Region of Ghana from September 2008 to September 2009.
Study population
A total of 442 people living with HIV/AIDS (PLWHA) and confirmed to be positive for HIV (HIV-1, HIV-2 or both) visiting the ART clinic were recruited for this study upon approval by the Clinical Coordination and Research Development Board of the hospital and obtaining written informed consent from the patients. The study population consisted of 276 (76 males and 200 females) highly active antiretroviral therapy (HAART) naïve patients and 166 (28 males and 138 females) patients who were on HAART for a period of three (3) months or more. HAART use was defined as receipt of two nucleoside reverse transcriptase inhibitors (NRTI) and one non-nucleoside reverse transcriptase inhibitor (NNRTI) or one protease inhibitor (PI). Patients who were pregnant and HAART-naïve patients who were on medication (antibiotics, vitamin supplements and tuberculosis treatment) at the time of sampling were excluded from the study.
Sample collection and preparation
A volume of 5 ml of venous blood was collected from each patient and dispensed into two vacutainer ethylene diamine tetraacetic acid (EDTA) tubes (2 ml and 3 ml) respectively. The 2 ml sample was used for immunological analysis (CD4/CD3 estimation) and the 3 ml sample used for haematological analysis.
Immunological Assay
Absolute counts of CD4 and CD3 lymphocytes were assayed using the BD FACSCOUNT system (Becton Dickenson and Company, California, USA).
Haematological Assay
Haematological parameters: haemoglobin (Hb), haematocrit (PCV), mean cell volume (MCV), mean cell haemoglobin (MCH), mean cell haemoglobin concentration (MCHC), red blood cell count (RBC), red cell distribution width (RDW), white blood cell count (WBC), lymphocyte count (LC), total lymphocyte count (TLC), platelet count (PLT) and mean platelet volume (MPV) were determined using the automated blood analyzer Cell-Dyn 1800 (Abott Laboratories Diagnostics Division, USA).
Weight
Body weight was determined to the nearest 0.1kg in light clothing with a bathroom scale (Zhongshan Camry Electronics Co. Ltd. Guangdong, China).
Statistical Analysis
Results are presented as Means ± SEM. Unpaired t-test was used to compare the means of all continuous variables. Categorical data were analyzed using Fisher's exact test or x2 for trend. Linear regression was used to test for the degree of association between test parameters. A p-value of < 0.05 was considered to be statistically significant. All statistical analyses were done using GraphPad Prism version 5.0 for windows (GraphPad software, San Diego California USA, www.graphpad.com)
Results
Demographic characteristics and weight
Out of the 442 HIV/AIDS patients categorized into HAART-naïve and on HAART patients, majority of the HAART-naïve patients (88%) and those on HAART (83%) were within the age brackets of 20 – 49 years. Of the 276 HAART-naïve patients, 200 (72.5%) were females and 76 (27.5%) were males giving a female to male ratio of 3:1. Out of the 166 patients on HAART, 138 (83.1%) were females and 28 (16.9%) were males giving a female to male ratio of 5:1 (Table 4B). Patients on HAART were however older (36.91 ± 0.77 years, P ≤ 0.01) and heavier (54.92 ± 3.61 kg, P ≤ 0.05) than their HAART-naïve counterparts (33.42 ± 0.88 years; 48.93 ± 1.24 kg). Females on HAART were significantly older (36.10 ± 0.85 years, P ≤ 0.01) when compared to those off HAART (32.17 ± 0.89 years) but there was no significant difference in the mean ages of male patients on HAART and those who were HAART-naïve (P > 0.05) (Table 1). Furthermore, male patients on HAART were significantly heavier (75.50 ± 2.50 kg, P ≤ 0.01) than their HAART-naïve counterparts who were also heavier (54.08 ± 2.44 kg, P ≤ 0.01) when consequently compared with HAART-naïve females (47.00 ± 1.34 kg).
Table 4B.
Age and sex distribution of the study population
| HIV Patients | ||
| HAART-Naïve | On HAART | |
| Age (years) | N = 276; (n/N) | N = 166; (n/N) |
| 0–9 | 10(3.6) | 0(0.0) |
| 10–19 | 5(1.8) | 2(1.2) |
| 20–29 | 78(28.3) | 28(16.0) |
| 30–39 | 108(39.1) | 76(44.5) |
| 40–49 | 57(20.7) | 40(22.8) |
| 50–59 | 10(3.6) | 18(10.3) |
| 60–69 | 5(1.8) | 2(1.2) |
| >70 | 3(1.1) | 0(0.0) |
| Sex | ||
| Male | 76(27.5) | 28(16.9) |
| Female | 200(72.5) | 138(83.1) |
Table 1.
General characteristic of the study population stratified by drug and gender
| Parameters | HIV Patients | Male Patients | Female Patients | |||
| HAART naïve | On HAART | HAART naïve | On HAART | HAART naïve | On HAART | |
| Age (yrs) | 33.42 ± 0.88 | 36.91 ± 0.77** | 36.68 ± 2.15 | 39.63 ± 1.68 | 32.17 ± 0.89‡ | 36.10 ± 0.85§§ |
| WT (kg) | 48.93 ± 1.24 | 54.92 ± 3.61* | 54.08 ± 2.44 | 75.50 ± 2.50†† | 47.00 ± 1.34‡‡ | 51.18 ± 3.04## |
| CD4 (cell mm-3) | 272.60 ± 13.24 | 315.30 ± 13.92* | 251.60 ± 26.40 | 277.70 ± 29.85 | 295.50 ± 17.33 | 326.30±15.78 |
| CD3 (cell mm-3) | 1243.00 ± 43.55 | 1216.00 ± 45.19 | 1251.00 ± 89.33 | 1148.00 ± 92.36 | 1276.00 ± 56.32 | 1238.00 ± 52.08 |
| TWBC (x 103 µl−1) | 5.19 ± 0.18 | 4.39 ± 0.12** | 5.53 ± 0.41 | 4.32 ± 0.24† | 5.14 ± 0.23 | 4.41 ± 0.14§ |
| TLC (x 103 µl−1) | 2.81 ± 0.09 | 2.73 ± 0.08 | 2.86± 0.21 | 2.48 ± 0.18 | 2.79 ± 0.12 | 2.79 ± 0.09 |
| LC (%) | 55.48 ± 1.03 | 62.49 ± 1.00*** | 53.79 ± 2.18 | 57.72 ± 2.57 | 55.97 ± 1.38 | 63.77 ± 1.05§§§,# |
| RBC (x 106 µl−1) | 3.55 ± 0.04 | 3.31 ± 0.05*** | 3.67 ± 0.11 | 3.51 ± 0.09 | 3.51 ± 0.05 | 3.25 ± 0.05§§§,# |
| HB (g dl−1) | 9.80 ± 0.12 | 10.59 ± 0.15 | 10.59 ± 0.29 | 11.54 ± 0.33† | 9.45 ± 0.13‡‡‡ | 10.33 ± 0.16§§§,### |
| PCV (%) | 32.64 ± 0.48 | 35.68 ± 0.55*** | 35.19 ± 1.19 | 38.00 ± 0.10 | 31.33 ± 0.52‡‡‡ | 35.06 ± 0.63§§§,### |
| MCV (fL) | 93.02 ± 0.94 | 110.50 ± 1.33*** | 96.32 ± 2.30 | 112.5 ± 2.79††† | 90.99 ± 1.11‡ | 109.90± 1.51§§§ |
| MCH (pg) | 27.92 ± 0.27 | 32.60 ± 0.50*** | 29.61 ± 0.66 | 33.51 ± 0.81††† | 27.19 ± 0.31‡‡‡ | 32.35 ± 0.59§§§ |
| MCHC (g dl−1) | 30.30 ± 0.25 | 29.66 ± 0.40 | 31.10 ± 0.65 | 29.95 ± 0.49 | 30.17 ± 0.31 | 29.58 ± 0.48 |
| RDW (%) | 20.88 ± 0.50 | 18.64 ± 0.52 | 21.91 ± 1.39 | 17.26 ± 0.59† | 20.73 ± 0.59 | 18.98 ± 0.63§ |
| PLT (x 103 µl−1) | 174.40 ± 7.20 | 148.80 ± 6.07* | 178.5 ± 18.82 | 163.1 ± 14.09 | 178.10 ± 8.29 | 144.90 ± 6.69§§ |
| MPV (fL) | 11.25 ± 0.22 | 11.88 ± 0.20* | 11.30 ± 0.48 | 11.80 ± 0.32 | 10.99 ± 0.27 | 11.90 ± 0.25§ |
Results are presented as means ± SEM. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001 indicates the level of significance when the HAART naïve were compared to those on HAART (unpaired t-test); †P ≤ 0.05, ††P ≤ 0.01, †††P ≤ 0.001 indicates the level of significance when the male HAART naïve were compared to the male on HAART; §P ≤ 0.05, §§P ≤ 0.01, §§§P ≤ 0.001 indicates the level of significance when the female HAART naïve were compared to the female on HAART; ‡P ≤ 0.05, ‡‡P ≤ 0.01, ‡‡‡P ≤ 0.001 indicates the level of significance when the male HAART naïve were compared to the female HAART naïve; #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001 indicates the level of significance when the male on HAART were compared to the female on HAART.
Anaemia, Packed Cell Volume (PCV) and Haemoglobin
Using packed cell volume (PCV) less than 30% as an indicator for anaemia, 37.6% (88/234) of the HAART-naïve patients were 3 times at risk of having reduced PCV compared to 15.2% (28/151) of the patients on HAART (P < 0.0001). The odds of PCV being reduced in both male and female patients on HAART compared to those off HAART was however not significant (P > 0.05) (Table 4A) and the calculated mean PCV (35.68 ± 0.55%, P ≤ 0.001) in patients on HAART was significantly higher than that of patients who were HAART-naïve (32.64 ± 0.48%)(Table 1).
Table 4A.
Cytopoenic tendency in the study population
| HIV Patients | Male Patients | |||||
| Parameters | HAART | On | OR | HAART | On | OR |
| Naïve | HAART | (95% CI) | Naïve | HAART | (95%CI) | |
| TWBC (< 2.5 k/µL) | 18(7.7) | 9(6.0) | 1.3(0.6–3.0) | 4(6.2) | 1(3.1) | 2.0(0.2–19.0) |
| LYM (< 40%) | 39(16.7) | 8(5.3) *** | 3.6(1.6–7.9) | 13(20.0) | 4(12.5) | 1.8(0.5–5.9) |
| Neut (< 60%) | 225(96.2) | 150(99.3) | 0.2(0.02–1.3) | 61(93.9) | 31(96.9) | 0.5(0.05–4.59) |
| TLC (< 1.2 k/µL) | 16(6.8) | 6(4.0) | 1.8(0.8–4.6) | 6(9.2) | 3(9.4) | 0.9(0.2–4.2) |
| PCV (< 30%) | 88(37.6) | 23(15.2)*** | 3.4(2.0–5.6) | 18(27.7) | 4(12.5) | 2.7(0.8–8.7) |
| PLT (< 150 k/µL) | 118(50.4) | 78(51.7) | 1.0(0.6–1.4) | 32(49.2) | 13(40.6) | 1.4(0.6–3.3) |
*P ≤ 0.05, ***P ≤ 0.001 indicates the level of significance when HAART-naïve were compared to those on HAART (unpaired t-test); TWBC = Total white blood cell count; LYM = Lymphocyte; Neut = Neutrophil; TLC = Total lymphocyte count; PCV = Packed cell volume; PLT = Platelet.
From Table 2, a further classification of the study population according to the World Health Organization/Aids Clinical Trial Group (WHO/ACTG) anaemia toxicity grades gave a 63% and 46% calculated incidence of anaemia (Hb ≤ 10.5 gdL−1) in HAART-naïve patients and those on HAART respectively (X2 = 10.68, P = 0.0011) . Additionally, HAART-naïve patients are 3 times at risk of developing Grade 2 (P < 0.0001) and Grade 3 anaemia (P = 0.0005) compared to the patients on HAART. The odds of developing grades 2 and 3 anaemia in HAART-naïve females is 3 times more when compared to their counterparts on HAART (P < 0.0001) and a comparison of males on HAART to their naïve counterparts in developing all four grades of anaemia did not show any significant difference (P > 0.05). The calculated mean haemoglobin of (11.54 ± 0.33 g dL−1, P ≤ 0.05) and (10.33 ± 0.16 g dL−1, P ≤ 0.001) in males and females on HAART respectively were significantly higher when compared to their naïve counterparts (10.59 ± 0.29 g dL−1 and 9.45 ± 0.13g dL−1) but there was however no statistical significance between the mean haemoglobin values of patients on HAART and those who are HAART-naïve (P > 0.05) (Table 1).
Table 2.
Study population stratified by anaemia, type of anaemia, CD4 counts, total lymphocyte count and drug use
| HIV Patients | Male Patients | |||||
| Parameters | HAARTNaïve | On HAART | OR (95% CI) | HAARTNaïve | On HAART | OR (95% CI) |
| WHO/ACTG Grade | ||||||
| Grade 1 (9.5–10.5) | 51(20.1%) | 39(25.8%) | 0.7(0.5–1.2) | 12(16.7%) | 7(21.9%) | 0.7(0.3–2.0) |
| Grade 2 (8.0–9.4) | 68(26.8%) | 16(10.6%) | 3.1(1.7–5.6) | 12(16.7%) | 3(9.4%) | 1.9(0.5–7.4) |
| Grade 3 (6.5–7.9) | 33(13.0%) | 9(6.0%) | 2.4(1.1–5.1) | 6(8.3%) | 1(3.1%) | 2.8(0.3–24.4) |
| Grade 4 (<6.5) | 8(3.1%) | 6(4.0%) | 0.8(0.3–2.3) | 2(2.8%) | 0(0.0%) | 2.3(0.1–49.4) |
| Type of Anaemia | ||||||
| Micro. Hypo. | 34(14.5%) | 5(3.3%) | 5.0(1.9–13.0) | 4(6.2%) | 0(0.0%) | 4.7(0.2–91.1) |
| Macro. Hypo. | 11(4.7%) | 8(5.3%) | 0.9(0.4–2.2) | 2(3.1%) | 1(3.1%) | 1.0(0.1–11.3) |
| Normo. Hypo. | 46(19.7%) | 11(7.3%) | 3.1(1.6–6.2) | 12(18.5%) | 3(9.4%) | 2.2(0.6–8.6) |
| Normo. Normoc. | 50(21.4%) | 11(7.3%) | 3.5(1.7–6.9) | 15(23.1%) | 2(6.3%) | 4.5(1.0–21.1) |
| CD4 (CDC) | ||||||
| 0–199 | 122(44.2%) | 46(27.7%) | 2.1(1.4–3.1) | 39(50.0%) | 14(37.8%) | 1.6(0.7–3.6) |
| 200–499 | 112(40.6%) | 94(56.6%) | 0.5(0.4–2.2) | 28(35.9%) | 19(51.4%) | 0.5(0.2–1.2) |
| 500+ | 42(15.2%) | 26(15.7%) | 1.0(0.6–1.6) | 11(14.1%) | 4(10.8%) | 1.4(0.4–4.6) |
| TLC | ||||||
| <1.0 | 7(3.0%) | 3(2.0%) | 1.5(0.4–6.0) | 4(6.2%) | 1(3.1%) | 2.0(0.2–18.9) |
| 1.0–2.0 | 77(32.9%) | 39(25.8%) | 1.4(0.9–2.2) | 17(26.2%) | 11(34.4%) | 0.7(0.3–1.7) |
| >2.0 | 150(64.1%) | 109(72.2%) | 0.7(0.4–1.1) | 44(67.7%) | 20(62.5%) | 1.3(0.5–3.0) |
*P ≤ 0.05, ***P ≤ 0.001 indicates the level of significance when the HAART naïve were compared to those on HAART (unpaired t-test); Micro = microcytic; Hypo = hypochromic; Macro = macrocytic; Normoc. = normochromic
HAART = Highly Active Antiretroviral Therapy; WHO/ACTG = World Health Organization/Aids Clinical Trial Group; OR = Odds Ratio; CD = Cluster of Differentiation; TLC = Total Lymphocyte count; CI = Confidence Interval.
Type of anaemia
Using the mean cell volume (MCV) range of 80 – 96 fL and mean cell haemoglobin (MCH) range of 27 – 32 pg as pointers in distinguishing between the different types of anaemia where low MCV (<80 fL) is indicative of microcytosis, high MCV (>96 fL) indicates macrocytosis and low MCH (<27 pg) indicates hypochromia, HAART-naïve patients are 5 times at risk of developing microcytic hypochromic anaemia (P = 0.0002) in comparison to their counterparts on HAART and the relative risk of developing normocytic hypochromic (P = 0.0007) and normocytic normochromic anaemia (P = 0.0002) is 3 times more compared to the patients on HAART (Table 2). The same risk pattern, microcytic hypochromic (P = 0.0004), normocytic hypochromic (P = 0.0013) and normocytic normochromic (P = 0.0025) is observed in a comparative analysis of females on HAART to those who are HAART-naïve. A marginally significant risk of developing normocytic normochromic anaemia (P = 0.0485) was seen in male patients who are HAART-naïve compared to those on HAART but no significant differences were observed in the relative risk of developing macrocytic hypochromic anaemia in all the study populations (P > 0.05) (Table 2). From Table 1, calculated MCV values of (110.50 ± 1.33 fL, 112.5 ± 2.79 fL, 109.90 ± 1.51 fL; P ≤ 0.001) and MCH values of (32.60 ± 0.50 pg, 33.51 ± 0.81 pg, 32.35 ± 0.59 pg; P ≤ 0.001) in patients on HAART, males on HAART and females on HAART respectively were significantly higher compared to their naïve counterparts.
Leucopoenia, Lymphopoenia and Neutropoenia
Using a total white blood cell count (<2.5 k µL−1), a lymphocyte count (<40%) and neutrophil count (<60%) as indicators of leucopoenia, lymphopoenia and neutropoenia respectively, there was no significant difference in the relative risk of developing leucopoenia in all three study groups (P > 0.05) (Table 4A). The mean total white cell counts in patients on HAART (4.39 ± 0.12 k µL−1, P ≤ 0.01), males on HAART (4.32 ± 0.24 k µL−1, P ≤0.05) and females on HAART (4.41 ± 0.14 k µL−1, P ≤ 0.05) were however significantly lower when compared to their naïve counterparts (5.19 ± 0.18 k µL−1; 5.33 ± 0.41 k µL−1 and 5.14 ± 0.23 k µL−1) (Table 1).
The calculated incidence of lymphopoenia in HAART-naïve patients and those on HAART was 16.7% and 5.3% respectively and the difference in the proportion was significant (X2 = 11.07, P = 0.0009). The relative risk of developing lymphopoenia is 3 times in HAART-naïve patients (P = 0.0007) and 5 times more in HAART-naïve females (P = 0.0007) when compared to their counterparts on HAART. However, there was no significant difference in the odds of developing lymphopoenia when HAART-naïve males were compared to males on HAART (P > 0.05) (Table 4A). Significant increases in the mean lymphocyte counts of patients on HAART (62.49 ± 1.00%, P ≤ 0.001) and females on HAART (63.77 ± 1.05%, P ≤ 0.001) was observed when compared to their HAART-naïve counterparts. A comparative analysis of the mean lymphocyte value in males on HAART and HAART-naïve males showed no significant difference (P > 0.05) (Table 1).
There was no significant difference in the relative risk of developing neutropoenia in all three study populations (P > 0.05) (Table 4A).
CD4 counts
In Table 2, the Center for Disease Control (CDC) criteria was used to classify the study population into three categories based on CD4 counts: stage 1 (CD4 >500 cells mm−3), stage 2 (CD4 between 200 – 499 cells mm−3) and stage 3 (CD4 <200 cells mm−3). The risk of having CD4 count <200 cells mm−3 is twice in HAART-naïve patients than those on HAART (P = 0.0006) and the chances of having CD4 count between 200 – 499 cells mm−3 is 2 times more in patients on HAART than those who are HAART-naïve (P = 0.0012). HAART-naïve females are 2.5 times at risk of having CD4 count <200 cells mm−3 when compared to their counterparts on HAART (P = 0.001) who have a twice better chance of developing CD4 counts between 200 – 499 cells mm−3 (P = 0.005). No significant difference was observed in the three categories of CD4 counts when males on HAART were compared to HAART-naïve males (P > 0.05) likewise with CD4 counts >500 cells mm−3 when patients on HAART and females on HAART were compared to their naïve counterparts. The mean CD4 count (315.30 ± 13.92 cells mm−3, P > 0.05) in patients on HAART is however greater when compared to that of patients who are HAART-naïve (272.60 ± 13.24 cell mm−3) (Table 1).
CD4 counts and TLC
In a trend analysis of the three categories of CD4 counts and TLC in HAART-naïve patients (Table 3), when the TLC was between 1.0 – 2.0 k µL−1, there was a gradual increase in the proportion of patients within the three CD4 categories from 8.3% in stage 1, 26.1% in stage 2 to a peak percentage of 46.5% within stage 3 (P < 0.0001). With a TLC >2.0 k µL−1, a steady decline in the proportion of patients within the three categories was seen with the highest percentage of 91.7% occurring within stage 1 (P < 0.0001). A gradual increase in the proportion of patients within the CD4 categories was observed with a peak proportion of 4% in stage 3 when the TLC was <1.0 k µL−1 but this was not statistically significant.
Table 3.
Trend analysis of the study population stratified by drug use, TLC, anaemia and CD4 counts
| CD4 counts | |||||
| Variables | Stage 3(0 – 199) | Stage 2(200 – 499) | Stage 1(≥500) | P value | |
| HAART Naïve | |||||
| TLC | |||||
| <1.0 | 4(4.0) | 3(3.1) | 0(0.0) | 0.2713 | |
| 1.0 – 2.0 | 47(46.5) | 25(26.1) | 3(8.3) | <0.0001 | |
| >2.0 | 50(49.5) | 68(70.8) | 33(91.7) | <0.0001 | |
| Anaemia | |||||
| 9.5 – 10.5 | 20(18.2) | 25(24.0) | 6(15.4) | 0.9454 | |
| 8.0 – 9.4 | 32(29.1) | 31(29.8) | 5(12.8) | 0.116 | |
| 6.5 – 7.9 | 21(19.1) | 9(8.7) | 3(7.7) | 0.0224 | |
| <6.5 | 6(5.5) | 2(1.9) | 0(0.0) | 0.0589 | |
| On HAART | |||||
| TLC | |||||
| <1.0 | 3(7.3) | 0(0.0) | 0(0.0) | 0.0193 | |
| 1.0 – 2.0 | 17(41.5) | 20(25.3) | 1(4.0) | 0.0008 | |
| >2.0 | 21(51.2) | 59(74.7) | 24(96.0) | <0.0001 | |
| Anaemia | |||||
| 9.5 – 10.5 | 15(36.6) | 20(25.3) | 2(8.0) | 0.0107 | |
| 8.0 – 9.4 | 4(9.8) | 7(8.9) | 4(16.0) | 0.4977 | |
| 6.5 – 7.9 | 5(12.2) | 3(3.8) | 1(4.0) | 0.12 | |
| <6.5 | 2(4.9) | 4(5.1) | 0(0.0) | 0.4019 | |
CD = Cluster of Differentiation; HAART = Highly Active Antiretroviral; TLC = Total Lymphocyte Count
In patients on HAART, when the TLC was <1.0 kµL−1 and between 1.0 – 2.0 kµL−1, there were gradual increases in the proportion of patients to the peak percentages of 7.3% (P = 0.0193) and 41.5% (P = 0.0008) respectively found in stage 3. However, when the TLC was >2.0 kµL−1, the proportional trend decreased steadily through the CD4 categories from 96.0% (P < 0.0001) in stage 1 through to 51.2% in stage 3.
CD4 counts and anaemia
Comparing the four grades of anaemia to the three categories of CD4 for trend (Table 3), a gradual increase in the proportion of HAART-naïve patients within the three CD4 categories was observed with peak proportion of 19.1% in stage 3 developing grade 3 anaemia (P = 0.0224) and 5.5% developing grade 4 anaemia (P = 0.0589).
36.6% of the patients on HAART within stage 3 CD4 count had grade 1 anaemia (P = 0.0107). No significant trend was however observed between grades 2, 3, 4 anaemia and the CD4 stages (P > 0.05).
Thrombocytopoenia
Using a platelet count (<150 k µL−1) as an indicator for thrombocytopenia in Table 4A, there was no significant difference in the odds of developing thrombocytopenia in all the three study populations (P > 0.05). However the calculated mean platelet counts (148.80 ± 6.07 k µL−1, P ≤ 0.05) in patients on HAART and (144.90 ± 6.69 k µL−1, P ≤ 0.01) in females on HAART were significantly lower compared to their naïve counterparts. Consequently, no significant difference was observed in the mean platelet count when males on HAART were compared to those who are HAART-naïve (P > 0.05).
Predictive parameters for CD4 counts
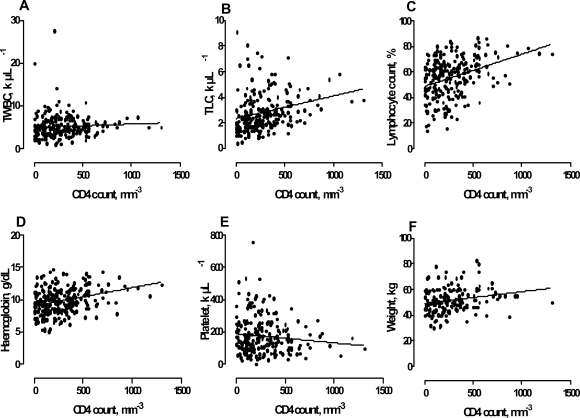
Figure 1 shows linear regression graphs of some selected haematological parameters and weight in HAART-naïve patients against CD4 counts in order to test for their ability to predict CD4 counts. For every cell µL−1 increase in TLC (r2 = 0.07, P < 0.0001), a percent increase in lymphocyte count (r2 = 0.12, P < 0.0001), a g dL−1 increase in haemoglobin (r2 = 0.10, P < 0.0001) and a kg increase in weight (r2 = 0.04, P = 0.0041), there was a mean increase of 0.002, 0.025, 0.003 and 0.009 cells mm−3 in the CD4 count. Platelet count showed a negative relationship with CD4 count (â = −0.057, r2 = 0.013, P = 0.0827) and TWBC showed no significant association with CD4 (â = 0.000, r2 = 0.00 P = 0.3379).
Figure 1.
Regression line graphs between total white blood cell (TWBC), total lymphocyte count (TLC), Lymphocyte count, Haemoglobin, Platelet, Weight and CD4 counts of HAART naïve subjects
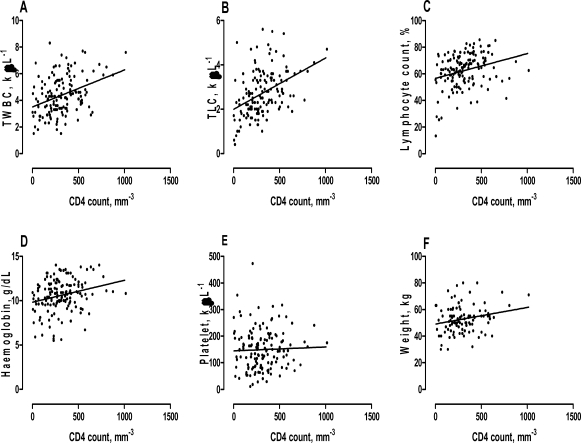
A related regression analysis conducted in patients on HAART with the same study predictors showed that, for every cell µL−1 increases in TWBC (r2 = 0.13, P < 0.0001) and TLC (r2 = 0.18, P < 0.0001), a percent rise in lymphocyte count (r2 = 0.08, P = 0.0007), a g dL−1 rise in haemoglobin (r2 = 0.06, P = 0.0026) and a kg increase weight (r2 = 0.05, P = 0.0314), mean increase of 0.003, 0.002, 0.019, 0.002, and 0.012 cells mm−3 in the CD4 count was observed. There was no significant linear relationship between platelet count and CD4 count (â = 0.015, r2 = 0.00, P = 0.6654) (Figure 2).
Figure 2.
Regression line graphs between total white blood count (TWBC), total lymphocyte count (TLC), Lymphocyte count, Haemoglobin, Platelet, Weight and CD4 counts in subjects on HAART
Discussion
Haematological manifestations have been documented to be the second most common cause of morbidity and mortality in HIV patients 4–6. Amballie et al., 12 conducted a study to identify haematological parameters which could serve as pointers to the diagnosis of HIV/AIDS in resource poor settings and Odunukwe et al., 9 reported the use of haematological response for monitoring patients on highly active antiretroviral therapy (HAART) in resource poor settings in view of the fact that the cost of diagnostic and monitoring techniques for HIV/AIDS are unaffordable to most people in resource poor settings. Due to the general acceptability of HAART as the gold standard in the treatment of HIV patients 13–14, establishing the presence of abnormal haematological manifestations in HAART-naïve HIV patients and those on HAART and performing a comparative analysis within the two study groups will give a clear pointer to the overall impact of the treatment regimen in HIV patients.
Demographics and HIV infection
In this study, about 86% (88% of HAART-naïve patients and 83% of patients on HAART) of the study population fell within the 20 – 49 years age brackets which is known to be the sexually active age group with highest peak percentage observed within the 30 – 39 age groups for both sexes. Amornkul et al., 15 reported a 25 – 29 years peak age for females and 30 – 34 years peak age for males. This means that heterosexual contact may probably contribute to a greater proportion of HIV infections in our study group and this finding is buttressed by the Ghana Aids Commission report 16 which states that heterosexual intercourse accounts for 75 to 80 percent of all HIV/AIDS infections. Amballi et al., 12 in their study on people living with HIV/AIDS reported a percentage of 75 within this age bracket. Females formed about 76% of the study population and a further finding of female to male ratio of 3:1 in HAART-naïve patients and 5:1 in patients on HAART strongly suggests that females are at a greater risk of getting infected with HIV than men and this could happen at an early stage in their lives probably early to mid thirties considering the significant differences in the mean ages of HAART-naïve males to HAART-naïve females (36.68 ± 2.15 and 32.17 ± 0.89; P < 0.05). The World Health Organization (WHO) reported that HIV/AIDS affects females most severely in sub-Saharan Africa and women of reproductive age make up almost 57% of adults living with HIV, accounting for up to 80% of HIV infected women in the world 17–18. The significant difference in age between patients on HAART and HAART-naïve patients further supports the proposition that HIV/AIDS infection may be acquired at an early age and most probably during the reproductive stage of life. The strict eligibility criteria for qualifying for HAART (WHO recommendation of CD4 <350 cells mm−3, WHO stages I, II and III HIV disease with CD4 <200 cells mm−3, WHO stage IV HIV disease (clinical AIDS) irrespective of CD4, WHO stage II HIV disease with TLC less than 1200 cells mm−3) 19 may also explain why most of the patients on HAART appear to be older than their naïve counterparts in that there could be a time lag between the time a patient tests positive for HIV and the time treatment is initiated and treatment once started is continued throughout the life of the patient.
Wood et al., 20 reported inadequate nutrient intake among a large proportion of HIV patients and Oguntibeju et al., 21 reported that there is evidence to show that nutritional intervention assists in maintaining and optimizing nutritional status and immune function, prevents the development of nutritional deficiency, loss of weight and lean body mass, promotes response to medical treatment and increases longevity in HIV patients. The improvement in the weight of patients on HAART, males on HAART and females on HAART over their naïve counterparts observed in this study could be a direct consequence of strict adherence to counselling which insists on good nutritional status alongside drug therapy. Bouic et al., 22 and Oguntibeju et al., 21 reported that nutritional supplementation can decrease viral load and through this intervention therefore, HAART might improve upon the quality of life of an HIV patient.
Anaemia and HAART
From this study, HAART-naïve patients have a high risk of developing reduced PCV compared to their counterparts on HAART. Furthermore, HAART-naïve patients and HAART-naïve females have a higher risk of developing moderate to severe anaemia compared to their counterparts on HAART and the calculated mean haemoglobin values in males and females on HAART were significantly higher compared to their naïve counterparts. As related in the studies conducted by Moore et al., 23, Mocroft et al., 24 and Levine et al., 25, the improvement in haemoglobin levels could be due to increase in the proportion of erythrocytes in relation to total blood volume as a result of elevated PCV. The reduced incidence of anaemia, the reduced risk of developing moderate to severe anaemia, the overall improvement in PCV and haemoglobin concentration when on HAART in this study confirms the effectiveness of HAART in improving the quality of life of HIV patients. Belperio & Rhew 26 and Odunukwe et al., 9 reported improved haematocrit values, increased haemoglobin concentration and decreased prevalence of anaemia in their study which is in agreement with our studies and Abrams et al.,27 reported that small increases in haemoglobin level (up to 2 g dL−1) were associated with a beneficial effect on total quality of life. The calculated incidence of anaemia by sex was 50% in females on HAART and 70% in HAART-naïve females compared to 34% and 44% in males on and off HAART respectively. The difference in proportion of HAART-naïve females to HAART-naïve males was statistically significant (X2 = 14.8, P = 0.0001) showing that HIV infected females in this study are more at risk of developing anaemia compared to infected males. Levine et al., 25 reported a similar finding in their study and attributed it to sex and race whereas Volberding et al., 28 attributed it to menstrual blood loss in women and to the drains on iron stores that occur with pregnancy and delivery. This finding is however in sharp contrast to the findings of a study conducted by Omoregie et al., 29 which reported high prevalence of anaemia in HAART-naïve males compared to their female counterparts. The absence of a significant difference in the proportion of females on HAART to males on HAART (X2 = 2.34, P = 0.1257) further buttresses the ability of HAART to improve upon the severity of anaemia in females.
Type of anaemia and HAART
The relatively high risk of developing microcytic hypochromic anaemia found in HAART-naïve patients and HAART-naïve females compared to those on HAART in this study reflects the overall nutritional deficiencies (malnutrition and malabsorption) associated with HIV patients. Blood loss and drains on iron stores that occur with pregnancy and delivery in HIV infected women could be an added consequence of anaemia in females especially since microcytic anaemia is associated with iron deficiency. The high risk of developing the other types of anaemia (normocytic hypochromic, normocytic normochromic and macrocytic hypochromic) in HAART-naïve patients in this study can be attributed to the multifactorial aetiology of anaemia as related in the study conducted by Volberding et al., (2004) where causes of anaemia were associated to blood loss or decreased red blood cell (RBC) production, increased RBC destruction and ineffective RBC production.
The odds of developing macrocytic hypochromic anaemia was the same in all the study populations and did not differ significantly (Table 2) but the average MCV for patients on HAART, males on HAART and females on HAART (Table 1) were significantly higher compared to their naïve counterparts. Moyle 7 reported that elevated MCV (macrocytosis) is typically associated with vitamin B12 or folate deficiency and in the setting of HIV treatment reflects the use of zidovudine (AZT) or stavudine (d4T). The elevated MCV observed in patients on HAART in this study could therefore be attributed to drug usage since most of them had a combination therapy of either AZT or d4T with lamivudine (3TC) but other factors will come into play considering the fact that HAART-naïve patients had a similar likelihood of developing macrocytosis compared to the patients on HAART. Burkes et al., 30 first reported low levels of vitamin B12 in HIV positive patients and Beach et al.,31 as well as Boudes et al.,32 described folate deficiency in HIV infected patients. Conversely, Hepburn et al.,33 reported that HAART may increase serum B12 levels and patients with low serum B12 in their study did not display characteristic findings of vitamin B12 deficiency, namely macrocytic anaemia and neuropathy.
Remacha et al.,34 suggested that low serum B12 levels are reflective of low levels of B12 transport proteins (transcobalamin I or haptocorrin) which are produced by neutrophils and not a tissue deficiency of B12. A high percentage of neutropoenia was observed in the study population with the percentages in patients, males and females on HAART being slightly higher than their naïve counterparts although the difference was not statistically significant. Low levels of transport proteins associated with neutropoenia could therefore be indirectly implicated in elevated MCV observed in both HAART-naïve patients and those on HAART (AZT induced). We however did not conduct studies into serum B12 levels in all our study populations and its relationship to HAART usage.
Immunological status and HAART
Depletion of lymphocytes, primarily of the CD4 cell subset subsequent to cellular CD4 immunodeficiency has been noted as the hallmark 35–36 of HIV infection with leucopoenia and lymphopoenia being documented in different proportions in HIV patients 12. In testing the ability to readily identify the presence of lymphopoenia by using the differential lymphocyte count and absolute lymphocyte count in this study, it was observed that the differential lymphocyte count gave strong indications of lymphopoenia than the absolute counts when HAART-naïve patients were compared to those on HAART. The absolute lymphocyte counts gave a marginally significant indication of lymphopoenia when HAART-naïve females were compared to their counterparts on HAART whilst the differential count gave a stronger indication. On a gender comparison therefore, HIV infected females will probably have a faster progression in the depletion of lymphocytes than infected males.
A further observation of significant increments in the mean differential lymphocyte counts in patients on HAART and females on HAART over their naïve counterparts gives proof that the observed lymphopoenia may have been corrected and improved upon by an intervention (HAART usage). The mean absolute counts gave no such findings in all the study populations. Differential lymphocyte counts may therefore serve as a useful tool in indicating lymphopoenia and improvements in lymphocyte counts in HIV infected patients than absolute counts. Significant reductions in the total white blood cell counts when the study population on HAART were compared to their naïve counterparts and could be due to the broad myelosuppressive effects of the drug regimen mostly associated with AZT usage.
The mean CD4 count in patients on HAART was higher compared to their naïve counterparts and we consistently found that HAART-naïve patients and females are at greater risk of having CD4 < 200 cells mm−3 in comparison to their counterparts on HAART who are better placed to have greater improvements in their CD4 counts to levels within 200 – 499 cells mm−3. It was further observed that CD4 counts <200 cells mm−3 was strongly associated with severe anaemia (Grades 3 & 4) in HAART-naïve patients and could lead to rapid disease progression and decreased survival as related in the study conducted by Obirikorang & Yeboah 37 and Curkendall et al., 38. When on HAART however, a high proportion of the patients with CD4 <200 cells mm−3 had a better chance of falling within the Grade 1(9.5 – 10.5 g dL−1) anaemia toxicity range. The significant finding of the ability of HAART to improve upon CD4 counts and anaemia in HIV infected patients in this study proves the ability of HAART to reduce morbidity and mortality in HIV infection as related in the study of Gea-Banacloche & Lane, 10. The ability of HAART to improve upon CD4 counts and anaemia observed in this study can be attributed to the effectiveness of HAART in reducing viral replication and viral load as related in the studies of Belperio & Rhew 26 and Odunukwe et al., 9.
Thrombocytopoenia and HAART
Although the relative risk of developing thrombocytopenia in all the study populations was the same, the mean platelet counts of patients on HAART and females on HAART was significantly lower compared to their naïve counterparts meaning that we are likely to find lower platelet counts in patients and females on HAART than in HIV infected patients. HAART though effective in improving on haematocrit and decreasing the prevalence of anaemia could lead to a decrease in platelet counts. This finding is in sharp contrast to that of Attili et al., 39 where thrombocytopenic incidence of 4.8% in HIV patients was reported and their platelet counts increased after antiretroviral therapy. An incidence of about 50% was calculated in both HAART-naïve and patients on HAART which almost agrees with the findings of the study conducted by Pechere et al.,40 who reported a thrombocytopenic incidence of 40%.
Parameters that can predict CD4
A linear regression model was conducted to assess the ability of some selected study parameters to predict CD4 counts in HAART-naïve patients and those on HAART. In HAART-naïve patients, it was observed that for unit increases in CD4 as an independent variable, there were corresponding unit increases in TLC, lymphocyte count, haemoglobin and weight. There was no linear relationship between total white blood cell count and CD4 count and platelet count showed a negative relationship. However in patients on HAART, there was a positive relationship between total white blood cell count and CD4 counts and platelet count gave a non-linear relationship with CD4 counts. TLC, lymphocyte count, haemoglobin and weight still gave positive relationships with CD4. The ability of TLC, lymphocyte count, haemoglobin and weight to predict CD4 count in both HAART-naïve patients and those on HAART suggests that these parameters which are relatively inexpensive and easily available compared to techniques for assaying CD4 and viral load could serve as accurate tools that can be used for monitoring the patients' immune status during therapy in addition to determining when patients should start antiretroviral therapy. Mwamburi et al., 41 reported a similar finding and suggested modification of the models to suit specific needs for use in underserved areas.
TLC and CD4
The value of TLC as a surrogate for CD4 in monitoring HIV disease in the absence of viral loads and CD4 counts has been argued 42–44 but current WHO guidelines only commit to using TLC in conjunction with clinical data as a criterion to initiate HAART in resource poor settings 19. A trend analysis to find the ability of TLC to predict CD4 < 200 cells mm−3 was conducted and it was observed that with a TLC of 1.0 – 2.0 k µL−1, a greater proportion of the HAART-naïve patients had CD4 counts <200 cells mm−3 and TLC >2.0 k µL−1 was associated with a peak proportion of patients with CD4 ≥500 cells mm−3. The same trend was observed in patients on HAART. A range of TLC cut-offs have been used and reported as predictors of CD4 <200 cells mm−3 and these range from 1.0 k µL−1 with a specificity of 98% and a sensitivity of 53% to 1.4 k µL−1 with a sensitivity of 73% and a specificity of 88% 43, 45. Our study shows that a TLC range of 1.0 – 2.0 k µL−1 could predict CD4 <200 cells mm−3 but this will serve a better purpose in the management and monitoring of HIV patients if a calibration cut-off could be established in our settings. The calibration cut-off in addition to clinical data (haemoglobin, weight, lymphocyte count) could serve as useful models in resource poor setting.
Conclusion
This study has shown that HAART usage is effective in decreasing the prevalence of anaemia by improving upon haematocrit and haemoglobin values and this positive impact leads to increased survival in people living with HIV/AIDS. Furthermore there was a positive impact on lymphocyte count and improvements in CD4 counts to values e” 200 cells mm−3 was observed when on HAART. TLC, Lymphocyte count, haemoglobin and weight were good predictors of CD4 counts and as such may serve as useful tools in the monitoring and management of HIV patients in resource poor settings considering the fact that they are easier and cheaper to perform than techniques for assaying CD4 counts and viral load. TLC could further predict CD4 counts ≥ 200 cells mm−3 and this could add to the strength of the study predictors when time insensitive methods are used in the care and management of HIV patients. Necessary efforts should therefore be taken to scale up therapy in this HAART era particularly in resource poor settings. The study further revealed that females in their reproductive age are at a greater risk of getting infected than males and this could perpetuate documented social complications associated with HIV infection. Continued efforts on educating the citizenry (targeted by age group) on social lifestyle and possible risk factors associated with HIV infection should still be emphasized considering the dire implications of new infections on the social fabric.
References
- 1.Okolie MN, Eghafona NO, Omoregie R. Anti-human immunodeficiency virus agents. Journal of Medical Laboratory Science. 2003;12:1–14. [Google Scholar]
- 2.Voth R, Rossol S, Graff E, Laubenstein HP, Schroder HC, Muller WE, Meyer zum Buschenfelde KH, Hess G. Natural killer cell activity as a prognostic parameter in the progression to AIDS. J Infect Dis. 1988;157(4):851–852. doi: 10.1093/infdis/157.4.851. [DOI] [PubMed] [Google Scholar]
- 3.Tersmette M, Schuitemaker H. Virulent HIV strains? AIDS. 1993;7(8):1123–1125. doi: 10.1097/00002030-199308000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Coyle TE. Hematologic complications of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Med Clin North Am. 1997;81(2):449–470. doi: 10.1016/s0025-7125(05)70526-5. [DOI] [PubMed] [Google Scholar]
- 5.Salond E. Haematologic complications of HIV infection. AIDS Rev. 2005;7:187–196. [PubMed] [Google Scholar]
- 6.Cosby CD. Hematologic disorders associated with human immunodeficiency virus and AIDS. J Infus Nurs. 2007;30(1):22–32. doi: 10.1097/00129804-200701000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Moyle G. Anaemia in persons with HIV infection: prognostic marker and contributor tomorbidity. AIDS Rev. 2002;4(1):13–20. [PubMed] [Google Scholar]
- 8.Volberding P. The impact of anemia on quality of life in human immunodeficiency virus-infected patients. J Infect Dis. 2002;185(Suppl 2):S110–S114. doi: 10.1086/340198. [DOI] [PubMed] [Google Scholar]
- 9.Odunukwe N, Idigbe O, Kanki P, Adewole T, Onwujekwe D, Audu R, Onyewuche J. Haematological and biochemical response to treatment of HIV-1 infection with a combination of nevirapine + stavudine + lamivudine in Lagos, Nigeria. Turkish Journal of Haematology. 2005;22:125–131. [PubMed] [Google Scholar]
- 10.Gea-Banacloche JC, Lane HC. Immune reconstitution in HIV-1 infections. AIDS. 1999;13:525–538. [PubMed] [Google Scholar]
- 11.Omoregie R, Egbeobauwaye A, Ogefere H, Omokaro EU, Ekeh CC. Prevalence of Antibodies to HAART Agents among HIV Patients in Benin city, Nigeria. African Journal of Biomedical Research. 2008;11:33–37. [Google Scholar]
- 12.Amballi AA, Ajibola A, Ogun SA, Ogunkolo OF, Salu LO, Oritogun KS, Oyegunle VA. Demographic pattern and haematological profile in people living with HIV/AIDS in a university teaching hospital. Scientific Research and Essay. 2007;2(8):315–318. [Google Scholar]
- 13.De Cock KM, Barrere B, Diaby L, Lafontaine MF, Gnaore E, Porter A, Pantobe D, Lafontant GC, Dago-Akribi A, Ette M, et al. AIDS—the leading cause of adult death in the West African City of Abidjan, Ivory Coast. Science. 1990;249(4970):793–796. doi: 10.1126/science.2167515. [DOI] [PubMed] [Google Scholar]
- 14.Ryder RW, Rayfield M, Quinn T. Transplacental HIV transmission in African newborns. 1998 [Google Scholar]
- 15.Amornkul PN, Vandenhoudt H, Nasokho P, Odhiambo F, Mwaengo D, Hightower A, Buve A, Misore A, Vulule J, Vitek C, Glynn J, Greenberg A, Slutsker L, De Cock KM. HIV prevalence and associated risk factors among individuals aged 13–34 years in Rural Western Kenya. PLoS One. 2009;4(7):e6470. doi: 10.1371/journal.pone.0006470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghana AIDS Commission, author. Ghana HIV/AIDS strategic framework: 2001–2005. Accra, Ghana: Ghana AIDS Commission; 2001. [Google Scholar]
- 17.Dabis F, Ekpini ER. HIV-1/AIDS and maternal and child health in Africa. Lancet. 2002;359(9323):2097–2104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- 18.WHO, author. AIDS epidemic update: December 2004. Geneva: 2004. p. 87. [Google Scholar]
- 19.WHO, author. Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: towards universal access. Recommendations for a public health approach (2006 revision) 2006
- 20.Woods MN, Spiegelman D, Knox TA, Forrester JE, Connors JL, Skinner SC, Silva M, Kim JH, Gorbach SL. Nutrient intake and body weight in a large HIV cohort that includes women and minorities. J Am Diet Assoc. 2002;102(2):203–211. doi: 10.1016/s0002-8223(02)90049-0. [DOI] [PubMed] [Google Scholar]
- 21.Oguntibeju OO, Van Den Heever WM, Van Schalkwyk FE. Effect of a liquid nutritional supplement on viral load and haematological parameters in HIV-positive/AIDS patients. Br J Biomed Sci. 2006;63(3):134–139. doi: 10.1080/09674845.2006.11732733. [DOI] [PubMed] [Google Scholar]
- 22.Bouic PJ, Clark A, Brittle W, Lamprecht JH, Freestone M, Liebenberg RW. Plant sterol/sterolin supplement use in a cohort of South African HIV-infected patients—effects on immunological and virological surrogate markers. S Afr Med J. 2001;91(10):848–850. [PubMed] [Google Scholar]
- 23.Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(1):29–33. doi: 10.1097/00042560-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, Pradier C, dArminio Monforte A, Ledergerber B, Lundgren JD. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13(8):943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 25.Levine AM, Berhane K, Masri-Lavine L, Sanchez M, Young M, Augenbraun M, Cohen M, Anastos K, Newman M, Gange SJ, Watts H. Prevalence and correlates of anemia in a large cohort of HIV-infected women: Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2001;26(1):28–35. doi: 10.1097/00126334-200101010-00004. [DOI] [PubMed] [Google Scholar]
- 26.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):27S–43S. doi: 10.1016/j.amjmed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Abrams DI, Steinhart C, Frascino R. Epoetin alfa therapy for anaemia in HIV-infected patients: impact on quality of life. Int J STD AIDS. 2000;11(10):659–665. doi: 10.1258/0956462001915020. [DOI] [PubMed] [Google Scholar]
- 28.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38(10):1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 29.Omoregie R, Omokaro EU, Palmer O, Ogefere HO, Egbeobauwaye A, Adeghe JE, Osakue SI, Ihemeje V. Prevalence of anaemia among HIV-infected patients in Benin City, Nigeria. Tanzania Journal of Health Research. 2009;11(1) doi: 10.4314/thrb.v11i1.43242. [DOI] [PubMed] [Google Scholar]
- 30.Burkes RL, Cohen H, Krailo M, Sinow RM, Carmel R. Low serum cobalamin levels occur frequently in the acquired immune deficiency syndrome and related disorders. Eur J Haematol. 1987;38(2):141–147. doi: 10.1111/j.1600-0609.1987.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 31.Beach RS, Mantero-Atienza E, Eisdorfer C, Fordyce-Baum MK. Altered folate metabolism in early HIV infection. JAMA. 1988;259(4):519. [PubMed] [Google Scholar]
- 32.Oudes P, Zittoun J, Sobel A. Folate, vitamin B12, and HIV infection. Lancet. 1990;335(8702):1401–1402. doi: 10.1016/0140-6736(90)91279-j. [DOI] [PubMed] [Google Scholar]
- 33.Hepburn MJ, Dyal K, Runser LA, Barfield RL, Hepburn LM, Fraser SL. Low serum vitamin B12 levels in an outpatient HIV-infected population. Int J STD AIDS. 2004;15(2):127–133. doi: 10.1258/095646204322764334. [DOI] [PubMed] [Google Scholar]
- 34.Remacha AF, Cadafalch J. Cobalamin deficiency in patients infected with the humanimmunodeficiency virus. Semin Hematol. 1999;36(1):75–87. [PubMed] [Google Scholar]
- 35.Gougeon ML, Montagnier L. Apoptosis in AIDS. Science. 1993;260(5112):1269–1270. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 36.Martinez G, Gonzalez I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tapanes R, Perez J, Leon OS. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res. 2003;47(3):217–224. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 37.Obirikorang C, Yeboah FA. Blood haemoglobin measurement as a predictive indicator for the progression of HIV/AIDS in resource-limited setting. Journal of Biomedical Science. 2009;16:102. doi: 10.1186/1423-0127-16-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curkendall SM, Richardson JT, Emons MF, Fisher AE, Everhard F. Incidence of anaemia among HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 2007;8(8):483–490. doi: 10.1111/j.1468-1293.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 39.Attili SVS, Singh VP, Rai M, Varma DV, Gulati AK, Sundar S. Hematological profile of HIV patients in relation to immune status - a hospital-based cohort from Varanasi, North India. Turk Journal of Hematology. 2008;25(1) [PubMed] [Google Scholar]
- 40.Pechere M, Samii K, Hirschel B. HIV-related thrombocytopenia. N Engl J Med. 1993;328(24):1785–1786. doi: 10.1056/NEJM199306173282414. [DOI] [PubMed] [Google Scholar]
- 41.Mwamburi DM, Ghosh M, Fauntleroy J, Gorbach SL, Wanke CA. Predicting CD4 count using total lymphocyte count: a sustainable tool for clinical decisions during HAART use. Am J Trop Med Hyg. 2005;73(1):58–62. [PubMed] [Google Scholar]
- 42.Crowe S, Turnbull S, Oelrichs R, Dunne A. Monitoring of human immunodeficiency virus infection in resource-constrained countries. Clin Infect Dis. 2003;37(Suppl 1):S25–S35. doi: 10.1086/375369. [DOI] [PubMed] [Google Scholar]
- 43.Kumarasamy N, Mahajan AP, Flanigan TP, Hemalatha R, Mayer KH, Carpenter CC, Thyagarajan SP, Solomon S. Total lymphocyte count (TLC) is a useful tool for the timing of opportunistic infection prophylaxis in India and other resource-constrained countries. J Acquir Immune Defic Syndr. 2002;31(4):378–383. doi: 10.1097/00126334-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 44.van der Ryst E, Kotze M, Joubert G, Steyn M, Pieters H, van der Westhuizan M, van Staden M, Venter C. Correlation among total lymphocyte count, absolute CD4+ percentage in a group of HIV-1 infected South African Patients. JAIDS Human Retroviral. 1998;19:238–244. doi: 10.1097/00042560-199811010-00005. [DOI] [PubMed] [Google Scholar]
- 45.Schreibman T, Friedland G. Use of total lymphocyte count for monitoring response to antiretroviral therapy. Clin Infect Dis. 2004;38(2):257–262. doi: 10.1086/380792. [DOI] [PubMed] [Google Scholar]