Abstract
Phospholamban (PLN), the reversible inhibitor of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), is a key regulator of myocyte Ca2+ cycling with a significant role in heart failure. We previously showed that the single amino acid difference between human and mouse PLN results in increased inhibition of Ca2+ cycling and cardiac remodeling and attenuated stress responses in transgenic mice expressing the human PLN (hPLN) in the null background. Here we dissect the molecular and electrophysiological processes triggered by the superinhibitory hPLN in the mouse. Using a multidisciplinary approach, we performed global gene expression analysis, electrophysiology, and mathematical simulations on hPLN mice. We identified significant changes in a series of Na+ and K+ homeostasis genes/proteins (including Kcnd2, Scn9a, Slc8a1) and ionic conductance (including L-type Ca2+ current, Na+/Ca2+ exchanger, transient outward K+ current). Simulation analysis suggests that this electrical remodeling has a critical role in rescuing cardiac function by improving sarcoplasmic reticulum Ca2+ load and overall Ca2+ dynamics. Furthermore, multiple structural and transcription factor gene expression changes indicate an ongoing structural remodeling process, favoring hypertrophy and myogenesis while suppressing apoptosis and progression to heart failure. Our findings expand current understanding of the hPLN function and provide additional insights into the downstream implications of SERCA2a superinhibition in the mammalian heart.
Keywords: hypertrophy, Ca2+ cycling, electrophysiology, genomics, microarrays
phospholamban (PLN) is a key regulator of sarcoplasmic reticulum (SR) Ca2+ cycling in the heart. Through its interaction with sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a, it reversibly inhibits the affinity of SERCA2a for Ca2+, thus inhibiting Ca2+ entry to the SR (23). The interaction of PLN with SERCA2a is Ca2+- and phosphorylation dependent. Upon β-adrenergic stimulation, phosphorylation of PLN relieves this inhibitory effect on SERCA2a, leading to enhanced SR Ca2+ transport (18, 39).
The role of PLN in SR Ca2+ cycling and cardiomyocyte contractility is well defined, and increased PLN inhibition of SR Ca2+ cycling is a major characteristic of human and experimental heart failure (5). Furthermore, the identification of human PLN mutations linked to dilated or hypertrophic cardiomyopathy (11, 33) has provided support for the functional significance of PLN activity in the heart. As a consequence, the regulation of SR Ca2+ uptake represents a promising therapeutic target. Characterization of the downstream molecular and electrophysiological cascades associated with PLN modifications is therefore a critical step.
The coding sequence of PLN is highly conserved across species, with the exception of amino acid position 27, where human PLN (hPLN) contains a lysine and mouse (m)PLN contains an asparagine. Notably, expression of the hPLN in the mouse heart was associated with superinhibition of SERCA2a leading to reduced cardiac contractility and hypertrophy yet, ultimately, cardiac remodeling and survival (46). Therefore, this mouse model represents a valuable means for deciphering the spectrum of molecular and cellular pathways in which hPLN is implicated, and for better understanding the role of SR Ca2+ cycling in cardiac remodeling.
To dissect these processes, the present study utilized global gene expression analysis combined with electrophysiology and mathematical simulations. Our findings reveal significant electrical, structural, and transcriptional remodeling in hPLN mouse hearts and point to the specific molecular players orchestrating these processes. Importantly, the hPLN-induced superinhibition of SERCA2a results in molecular changes that resemble previously described markers of hypertrophy and heart failure only to some extent, while multiple others are new and thus attribute novel roles to hPLN. Deciphering these molecular pathways and associating them with specific electrophysiological adaptations involved in the hPLN mouse phenotype provide insights into the downstream effects of PLN in cardiac function and could ultimately unveil promising new therapeutic targets.
MATERIALS AND METHODS
Tissue specimens.
Hearts from male FVB/N mice at 11 wk of age with cardiac-specific expression of the human PLN gene (N27K-PLN) in the PLN-deficient mouse background (mouse lines TGL1 and TGL2) and age-matched wild-type (WT) mice were collected at the University of Cincinnati (46). WT mice were chosen over PLN-deficient mice with α-MHCp-driven mPLN expression since the highest level of mouse PLN expression driven by α-MHCp in the null background is only 70% of WT levels.
The ventricles were dissected and snap frozen. Alternatively, ventricular myocytes were dissociated according to a standard protocol. The research protocol was approved by the institutional ethical committee and conformed with the principles outlined in the Declaration of Helsinki (1) and the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health (NIH Pub. No. 85-23, revised 1996).
Global gene expression analysis by microarrays.
The CodeLink Mouse Whole Genome Bioarrays were used according to recommended protocols (GE Healthcare). These arrays contain 35,587 30-mer oligonucleotide probes, representative of 36,142 transcripts, including 300 positive and 320 negative controls, on a three-dimensional surface (7, 36). The correlation between the biological replicates used in this study was very high, with intragroup (among TGL2 or among WT) correlation coefficient values for probes marked “good” with <1.5 fold change ranging between 98.4% and 99.5%. Each array successfully passed all the quality thresholds set by the Codelink Expression Analysis software (Bioarray QC metrics), and all the positive and negative controls were within the recommended range.
RNA isolation.
Each TGL2 and WT sample was homogenized in TRIzol (Invitrogen) followed by phase separation in chloroform as previously described (31). Only high-quality total RNA samples were included in the microarray analysis, with 260 nm-to-280 nm absorbance ratios between 1.9 and 2.1 and 28S-to-18S rRNA ratios close to 2, on 1.5% agarose gels. In total 12 samples were used, of which 6 were derived from transgenic and 6 from WT animals.
cRNA target preparation, microarray hybridization, and detection.
Initially, 2 μg of total RNA from each sample and bacterial control mRNAs were used for single-strand cDNA synthesis starting with a T7 oligo(dT) primer as per GE Healthcare recommendations. Double-stranded cDNA was then synthesized and purified with the QIAquick purification kit (Qiagen) followed by biotin-labeled cRNA synthesis, for 14 h in an air incubator at 37°C, with the CodeLink Expression Assay Reagent Kit (GE Healthcare). Only cRNA samples with sufficient quantity and high quality (260 nm-to-280 nm absorbance ratios between 1.8 and 2.1) were used for the preparation of the hybridization reaction mixtures. The samples were hybridized to Codelink Mouse Whole Genome Bioarrays at 37°C and 300 rpm for 18 h. After hybridization, the bioarrays were washed and stained with Cy5-streptavidin (Amersham). Each bioarray was scanned with a GenePix 4200AL Array Scanner (Axon Instruments) with the laser set at 635 nm, 100% laser power, the photomultiplier tube set at 600 V, and pixel size of 5 μm.
Bioinformatic data analysis.
The 12 scanned images were initially analyzed with Codelink Expression Analysis v4.2 software for bioarray quality evaluation and data extraction. The resulting median-normalized data were further processed with GeneSifter (VizX Labs). Specifically, only probes with “good” or “low” signal flags were included in the analysis. These flags are assigned by the Codelink Expression Analysis software to each probe of each hybridized bioarray, based on proprietary algorithms, to indicate quality of signal. Significant gene expression changes between hPLN and WT samples were determined with Student's t-test (2-tailed) on log2-transformed data, with thresholds of P < 0.01 and fold change > 1.5. All the raw and normalized data are available at the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under the series number GSE20172.
Protein extraction.
Cardiac homogenates from WT and transgenic (TGL1 and TGL2) mice were prepared in ice-cold lysis buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich Chemie, Schnelldorf, Germany) and 1 mM PMSF. The Quant-iT protein assay kit (Invitrogen, Eugene, OR) was used to determine protein concentration on a Qubit fluorometer (Invitrogen).
Immunoblotting.
SDS-PAGE and immunoblotting analysis were performed, as previously described (12), with one of the following primary antibodies: rabbit polyclonal anti-Scn8a/Nav1.6 (1:200; Sigma-Aldrich Chemie), rabbit polyclonal anti-Kcnd2/Kv4.2 (1:200; Sigma-Aldrich Chemie), rabbit polyclonal anti-Kcnq1/Kv7.1 (1:200; Sigma-Aldrich Chemie), and mouse monoclonal anti-GAPDH (1:10,000; Abcam, Cambridge, UK) and a peroxidase-conjugated goat anti-rabbit secondary antibody (3:10,000; Bio-Rad Laboratories, Hercules, CA) or goat anti-mouse (1:14,000; Sigma-Aldrich Chemie).
Electrophysiological recordings.
Ventricular myocytes from WT and TGL2 mice were dissociated with standard methods. The action potential, the transient outward current (Ito), the L-type Ca2+ current (ICaL), and the Na+/Ca2+ exchanger (NCX) were recorded with whole cell patch clamp (6, 44).
Mathematical modeling of mouse ventricular myocytes.
Modeling of mouse ventricular myocytes was done with the Physiome CellML Environment (PCEnv v0.4). The model was obtained from the CellML model repository (3). SERCA2a half-saturating constant (i.e., SERCA2a Ca2+ affinity, Km,up) was 0.32 μM for WT and 0.5 μM for hPLN myocytes based on our previous studies (46). For the hPLN myocyte model, fast transient outward current (Ito-f) and NCX conductances were decreased by 50% and 40%, respectively, and ICaL conductance was increased by 40%. Simulations were performed at 0.2-ms time resolution and were run for at least 300 s. Longer runs were performed to verify that the results were at steady state.
RESULTS
Molecular pathways implicated in cardiac remodeling of hPLN mice.
TGL2 hPLN and WT mouse hearts were analyzed in this study unless otherwise noted. After normalization, filtering, and log2 transformation, the 12 data sets (6 hPLN and 6 WT) were analyzed with GeneSifter software. Significant expression changes (P < 0.01 and >1.5 fold change) were identified in 320 different genes and expressed sequence tags (ESTs) (Supplemental Table S1): 205 genes were significantly upregulated, with values as high as 34-fold, and 115 genes were significantly downregulated, with values as low as −6-fold.1 These observations suggest a considerable change in the variety and levels of expressed transcripts in the left ventricle, in response to the N27K single amino acid change of PLN.
To decipher the biological implications of these changes, Gene Ontology annotations were used to group the significantly changed genes based on the “cellular component,” “biological process,” and “molecular function” of their corresponding proteins.
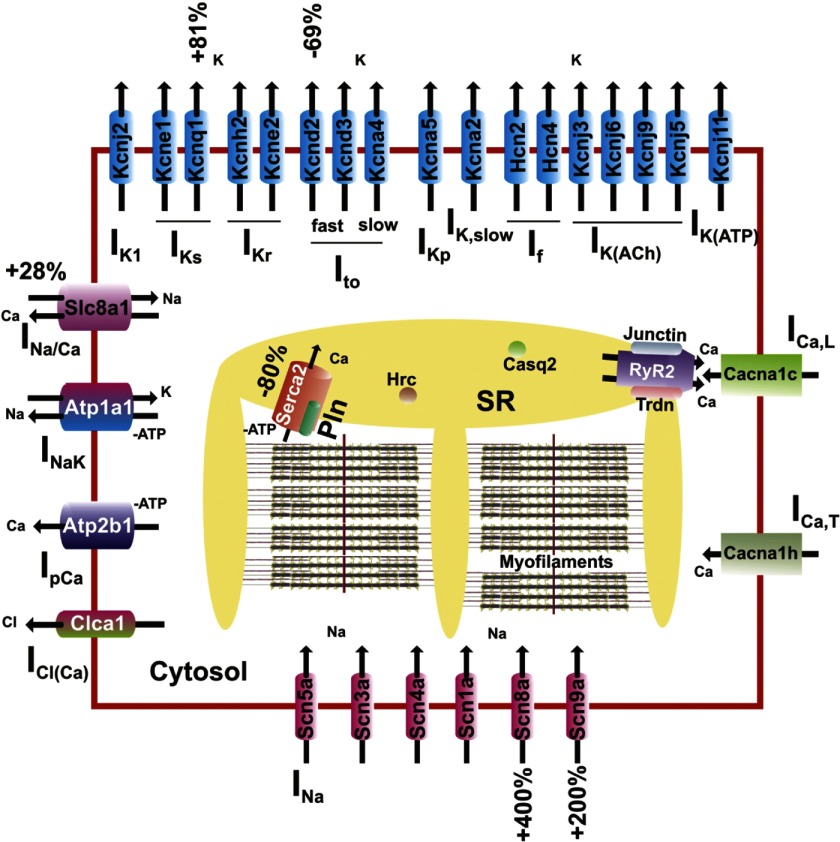
At the level of “biological process,” >100 significantly changed genes were involved in protein metabolism (e.g., Prkcb1, Usp53, Map3k6), nucleic acid metabolism (the vast majority of which were transcription related; see below), and signal transduction (e.g., Pde7b, Rgs11, Ctgf). Furthermore, numerous cell differentiation and cell cycle genes (e.g., Wwox, Pten, Tgfb2) were significantly changed. Importantly, >25 genes were associated with transport and ∼10 of them with ion transport. The ion transport group of genes included sodium (e.g., Slc5a12, Slc13a4, Scn8a), potassium (e.g., Kcnq1, Kcnd2), calcium (e.g., Prkcb1), and zinc (e.g., Slc39a3) ion transport genes (Fig. 1).
Fig. 1.
Replacement of mouse (m) phospholamban (PLN) with human (h)PLN, and the subsequent superinhibition of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a, results in significant expression changes of cardiomyocyte ion transport-related genes. Percentages indicate a statistically significant difference between hPLN (TGL2) and wild-type (WT) mice. Such changes may be part of the overall compensatory mechanisms activated in hPLN mouse hearts. SR, sarcoplasmic reticulum.
The Gene Ontology “molecular function” categories presenting with the highest numbers of significantly changed genes were those of protein binding (69 genes), ion binding (37 genes), and nucleic acid binding (28 genes). Consistent with the marked changes in ion transport genes, a large number of ion binding genes were significantly changed, relating to sodium, potassium, calcium, zinc, magnesium (e.g., Fah), copper (e.g., Snai3, Loxl2), and iron (e.g., Cyp1b1) ion binding. Genes associated with transcription included both transcription activators (e.g., Stat3, Nfatc2) and transcription repressors (e.g., Zfhx1b, Aebp2). Overall, 14 transcription factors (e.g., Stat3, Wt1, Ebf1, Fos) and 5 transcription cofactors (Nrip1, Ifi204, Actn2, Ppargc1b, Aebp2) were significantly changed.
Effect of hPLN on cardiac ion transporter protein expression.
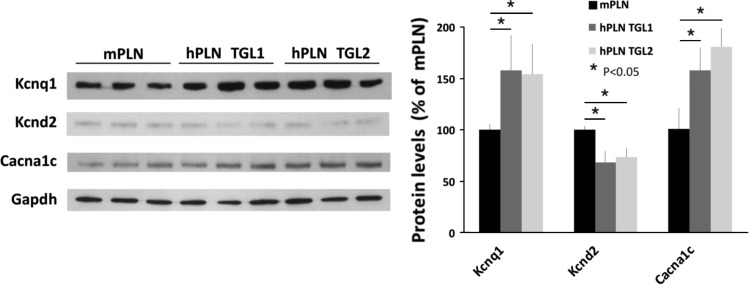
Western blotting analysis validated microarray findings and showed that the changes in ion channel and transporter protein expressions in the hPLN model agreed well with the changes at the gene expression level (Fig. 2). Notably, the Scn8a Na+ channel subunit was increased by 80% compared with WT, the Slc8a1 (or NCX1) Na+/Ca2+ exchanger was increased by 44%, the K+ channel subunit, Kcnd2, was decreased by 40% (while Kcnd3 was unchanged), and the slowly activating delayed rectifier K+ current (IKs) K+ channel subunit, Kcnq1, was increased by 65%. Interestingly, the L-type Ca2+ channel subunit, Cacna1c, was increased by 42% at the protein level, although there was no change at the gene expression level. Selected proteins were further evaluated in the TGL1 hPLN mouse line with similar results (Fig. 3), consistent with the superinhibitory effects exhibited by PLN mutations at amino acid 27 (N27K and N27A) (32, 45, 46). In addition, there were no alterations in SERCA, PLN, or calsequestrin protein expression levels in either TGL1 or TGL2 compared with WT controls (data not shown).
Fig. 2.
Expression changes in key ion transport proteins. Western blot analysis for key ion transport proteins confirmed the microarray gene expression findings and identified significant expression changes in the left ventricles of hPLN (TGL2) mice. Gapdh was used as an internal control. Data are averages of 3 or 4 hearts; error bars indicate SD. *P < 0.05 vs. WT.
Fig. 3.
Similarities in ion transport protein expression between hPLN mouse lines TGL1 and TGL2. Western blot analysis for selected ion transport proteins demonstrates high similarity between different transgenic mouse lines, TGL1 and TGL2. Gapdh was used as an internal control. Data are averages of 3 or 4 hearts; error bars indicate SD. *P < 0.05 vs. WT.
Effect of hPLN on cardiac cellular electrophysiological properties.
To understand the functional consequences of the observed alterations in ion transporter expression, cellular electrical properties of ventricular myocytes from hPLN hearts were examined with whole cell patch clamp. Compared with WT, hPLN myocytes had prolonged action potential duration (APD) (Fig. 4A), while the resting potential or action potential height was not changed. The average APD at 60% repolarization (APD60) was prolonged by 65%, from 2.67 ms in WT to 4.42 ms in hPLN myocytes (Fig. 4B).
Fig. 4.
Prolongation of action potential duration (APD) in hPLN ventricular myocytes. A: representative action potential traces recorded from WT (mPLN) and hPLN (TGL2) myocytes. Action potentials were triggered with 2-ms current steps at a rate of 1 Hz. B: average APDs at various percentages of repolarization for mPLN (n = 19) and hPLN (n = 23) myocytes; error bars indicate SE. *P < 0.01 vs. WT.
The prolongation of APD in hPLN myocytes correlated with a significant reduction of the K+ conductance (Fig. 5A). The peak K+ current density measured at +40 mV was reduced from 57 pA/pF in WT to 42 pA/pF in hPLN (P < 0.01, Fig. 5B). The K+ current in mouse ventricular myocytes has several components with distinct molecular identities (27). Consistent with the downregulation of Kcnd2, which underlies Ito-f, the reduction of total K+ conductance in hPLN myocytes was solely the result of decrease in Ito-f (Fig. 5C). Ito-f density was reduced by ∼50% in hPLN myocytes, while the densities of the slow component of Ito (Ito-s) and the steady-state current (Iss) were not changed.
Fig. 5.
Comparison of outward K+ currents and Na+/Ca2+ exchanger (NCX) in WT and hPLN ventricular myocytes. A: K+ currents activated by depolarizing steps from a holding potential of −60 mV to voltages ranging from −50 to +50 mV, in 10-mV increments. B: average peak current-voltage relationships of K+ currents in the 2 cell types [n = 34 for mPLN and 29 for hPLN (TGL2)]. C: comparison of densities of various K+ current components at +30 and +40 mV in the 2 myocyte types. Densities of fast (Ito-f) and slow (Ito-s) components of transient outward current were determined by fitting the current traces to a 2-exponential function, and the densities at the end of the 4.5-s voltage steps were regarded as the steady-state current (Iss). Inactivation time constants (τ) for Ito-f and Ito-s are indicated above the respective bars. D: comparison of NCX in WT and hPLN (TGL2) myocytes. Left: voltage clamp protocol for the recording of the NCX current and membrane current elicited by the ramp protocol and its blockade by 5 mM Ni2+. Right: average current density-voltage (Vm) relationships of NCX in mPLN (n = 25) and hPLN (n = 21) ventricular cells; error bars indicate SE.
Expression of human PLN also resulted in marked remodeling of the ICaL and the NCX current. Peak ICaL at 0 mV was increased by 39%, a result that agrees with the increase in Cacna1c protein expression (Fig. 2) and our previous findings (46). Interestingly, despite increases in Slc8a1 at the mRNA and protein levels, there was a 40% decrease in NCX activity in hPLN cells (Fig. 5D).
Mathematical simulation of hPLN ventricular myocytes:-role of ion transport remodeling.
We used a modified mouse ventricular myocyte model (3) to examine the contribution of ion transport changes to the cellular phenotype of the hPLN myocytes. The simulated hPLN action potential had a longer APD compared with WT (Fig. 6A), and this was largely accounted for by the reduction of Ito-f (Fig. 6D). The prolonged APD and increased ICaL conductance in the hPLN model resulted in increased Ca2+ influx during the action potential (Fig. 6A). We next compared the Ca2+ dynamics of WT, hPLN, and WT with an increase of SERCA2a Km,up alone without any ion transport remodeling (referred to below as the “Km,up↑ model”). Compared with WT, the Km,up↑ model showed a dramatic suppression of the peak Ca2+ transient level from 0.51 to 0.29 μM and a small increase in the diastolic Ca2+ level (Fig. 6B). The Km,up↑ model also showed marked decrease in both diastolic SR content (from 1.7 to 0.6 mM) and amplitude of triggered SR Ca2+ release (Fig. 6C). These abnormalities in Ca2+ dynamics were consistent with a decreased SR Ca2+ reuptake as a result of the decreased SERCA2a Ca2+ affinity. Importantly, with the ion transport remodeling incorporated into the model, the hPLN model showed significantly improved Ca2+ dynamics compared with the Km,up↑ model. In the hPLN model the Ca2+ transient levels were improved (peak = 0.43 μM; Fig. 6B), SR release was increased, and diastolic SR content was slightly higher (Fig. 6C).
Fig. 6.
Mathematical simulation of hPLN myocytes. A: action potential waveforms (top) and flux of the L-type Ca2+ current (ICaL) during the action potentials (bottom) in WT and hPLN myocyte models. B: Ca2+ transients for the WT and hPLN models and for the WT model with SERCA2a half-saturating constant Km,up increased from 0.32 to 0.5 μM (i.e., the Km,up↑ model; see results). [Ca]ct, cytosolic free Ca2+ concentration. Inset, comparison of Ca2+ transient decline kinetics of the WT and hPLN models. C: release of junctional sarcoplasmic reticulum (JSR) Ca2+ contents during Ca2+ transient in the 3 models. D: effect of reduction of the Ito-f conductance by 50% (blue line) on the action potential of the WT model. E: amplitude of the Ca2+ transients for the WT, hPLN, and Km,up↑ models and effects of individual ionic transport remodelings on the Ca2+ transient amplitude in the Km,up↑ model. F: diastolic JSR Ca contents for the WT, hPLN, and Km,up↑ models and effect of NCX reduction alone on the diastolic JSR Ca contents in the Km,up↑ model.
We next investigated the contribution of individual ion transport changes to the improved Ca2+ dynamics in the hPLN model by incorporating individual transport changes into the Km,up↑ model. Upregulation of ICaL and downregulation of Ito-f and NCX all individually improved Ca2+ transient amplitude, with downregulation of the NCX conductance making the smallest improvement (Fig. 6E). On the other hand, the increased SR Ca2+ content shown in Fig. 6C was solely a result of the decrease in NCX conductance density (Fig. 6F).
DISCUSSION
We have determined the specific molecular and electrical changes triggered through superinhibition of mouse SERCA2a by hPLN, thus uncovering new molecular players implicated in the cardiac response to Ca2+ cycling aberrations. The specific pathways contributing to compensation for the mild pathology of hPLN mice are unveiled and discussed.
Cellular electrical remodeling.
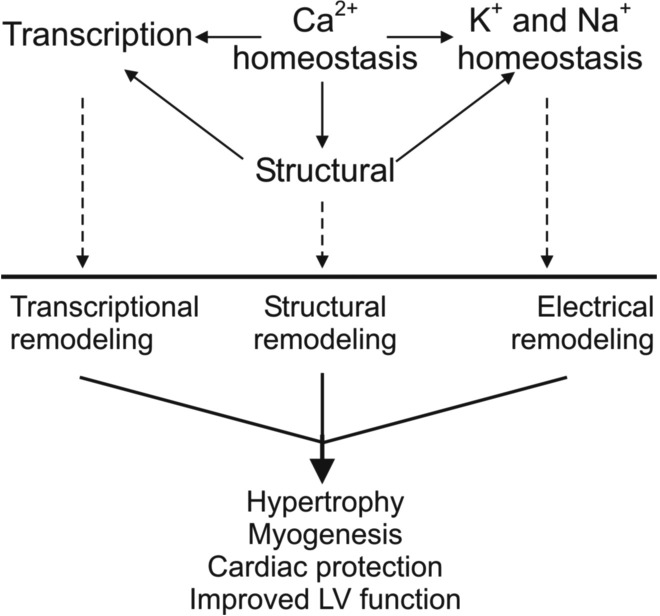
Alterations in hPLN cardiac gene expression are accompanied by significant changes in ventricular action potential morphology and K+ and Ca2+ conductance. Simulation studies show that incorporation of ion transport remodeling in the hPLN model improved SR Ca2+ cycling beyond that under suppressed SERCA2a function alone. The resulting Ca2+ transient amplitude relative to that in WT (Fig. 6B) agreed well with that observed experimentally in our previous findings (46). These simulation results suggest that the electrical remodeling may serve as a compensatory mechanism that enhances Ca2+ dynamics and attenuates what would otherwise be a more severe pathological phenotype (Fig. 7).
Fig. 7.
Hypothetical model of the molecular mechanisms triggered by hPLN, leading to electrical/structural remodeling and enabling long-term survival. LV, left ventricle.
Among the immediate downstream effects of aberrant Ca2+ homeostasis in the hPLN hearts appear to be alterations in K+ and Na+ ion transport. Kcnd2 (Kv4.2) expression and Ito-f density were reduced. This is in agreement with many, but not all, previous studies demonstrating downregulation of Kcnd and Ito-f in cardiac hypertrophy (9, 42). Decrease of Ito-f largely accounts for the moderate prolongation of APD in hPLN cells and results in increase of Ca2+ influx during the action potential. Interestingly, the two major changes in Ca2+ transport, i.e., upregulation of ICaL and downregulation of the NCX, appear to occur at the posttranscriptional/posttranslational levels. The mRNA and protein levels for NCX were increased in hPLN hearts, while a 40% decrease was found at the NCX functional level. Similar upregulation of NCX gene and protein expression and decrease in NCX function have been reported in a compensated pressure-overload cardiac hypertrophy model (41). Such remodeling of NCX in hypertrophy is in contrast with the situation in heart failure, where upregulation of both NCX protein and function has been consistently observed (37), and is believed to contribute to impaired excitation-contraction coupling and arrhythmias (2, 28). Simulation studies suggest that changes in both ICaL and NCX contribute to improved Ca2+ dynamics of the hPLN heart, although the individual effects of NCX downregulation on SR load and Ca2+ transient were fairly minor in the hPLN hearts.
Although the primary defect in the hPLN hearts is the altered structure and function of PLN, with direct implications in Ca2+ homeostasis, there were no major expression changes in Ca2+ transport-related genes. Significant gene expression changes were, however, observed in 16 Ca2+ binding proteins with roles in a variety of molecular pathways including ion homeostasis, cytoarchitecture, and regulation of transcription (Supplemental Table S1). Among them, S100a8 and S100a9, the most downregulated genes in this study (−4.91-fold and −6-fold, respectively), have been shown to interact with SERCA2a and affect Ca2+ flux, cardiac contractility, and ejection fraction (4).
Significant increases were also observed in the expression of the voltage-gated Na+ channel subunits (Scn8a and Scn9a), while the Prkcb1 underexpression could further promote their function (29, 40). These channels contribute, at least to some extent, to Na+ current (INa) in cardiomyocytes (13).
Structural remodeling.
Successful function of ion channels and membrane transporters requires tight control of their highly specialized subcellular localization. The significant expression changes in numerous structural genes in the hPLN mice could directly and/or indirectly affect these processes. For example, the actin cytoskeleton (Acta1, +1.81-fold) has been shown to participate in the organization of voltage-gated K+ channels through Actn2 (−1.94-fold) (21). Furthermore, emerging evidence shows that Actn2 can mediate the direct coupling of Ca2+ and Ca2+-activated K+ channels (21), which could translate into yet another mechanism bridging Ca2+ concentration aberrations with K+ changes in hPLN mice.
Another example involves syntrophins, actin-binding proteins and components of the dystrophin-associated protein complex, that can bind Na+ channels and to a lesser extent K+ channels, while the inward rectifier K+ channels associate with both syntrophins (Sntg1, +1.55-fold) and Cask (+1.76-fold) (10, 20). Through these interactions syntrophins and Cask may modulate the anchoring, clustering, and function of ion channels with direct implications in the propagation of depolarization and cardiac function.
In addition to their role in ion channel and membrane transporter localization and function, the multiple structural gene expression changes suggest extensive cytoarchitectural modifications in hPLN hearts. Significant gene expression changes were observed in sarcomeric/contractile (e.g., Myh6, Myom2, Myl1), cytoskeletal (e.g., Gas7, Shroom3, Sntg1), and intercalated disk proteins (Supplemental Table S1), suggesting a multilevel structural reorganization (14, 35). Since Ca2+ directly affects contractility and therefore sarcomere function, changes in Ca2+ dynamics such as those observed in hPLN cardiomyocytes could cause sarcomeric rearrangements that may be related to hypertrophy and/or compensate for the aberrant cardiomyocyte function (Fig. 7).
Transcriptional remodeling.
Approximately 8.5% of the 320 significantly changed genes in the hPLN model were associated with transcription. Importantly, the cytoskeletal membrane scaffold protein Cask (+1.76-fold) demonstrates nuclear translocation and transcriptional regulation, which may be dependent on Ca2+ concentrations in close proximity of the protein (16, 34). Similarly, Actn2 (−1.94-fold) has been proposed to work as a primary coactivator for the nuclear receptor family of transcription factors (17).
Both Cask and Actn2 as well as Ppargc1b (+1.91-fold) have been shown to bind Grip (+3.19-fold), while the latter two also enhance Grip1 transcriptional activity, thus contributing to the cross talk between cytoskeletal organization and transcriptional regulation in cardiomyocytes (17, 19). Grip1, in turn, is an important coactivator of the Mef2 family of myogenesis-related transcription factors. Consistent with a pattern of increased myogenesis in the hPLN mouse hearts, the myoblast fusion regulator Nfatc2 (+1.51-fold), as well as its downstream mediator, Il4 (+2.91-fold), were significantly overexpressed, while the negative regulator of myogenesis, Wt1, was significantly underexpressed (−1.65-fold) (15, 25). The reduced Stat3 expression (−1.67-fold) could be contributing to these processes by enabling the switch from myoblast proliferation to differentiation (38). Ca2+ concentration changes are likely the primary activator of the myogenic machinery, via calcineurin activation of Nfatc2 (26). The increased myogenesis and muscle growth in hPLN hearts could serve as a compensatory mechanism to facilitate cardiac function (Fig. 7).
Nfatc2 upregulation could contribute to Tgfb2 upregulation (+1.8-fold), which has been implicated in the development of cardiac hypertrophy (30). Meantime, Tfgb is also known to negatively regulate the proapoptotic Irf1 (24). Consistently with this notion, Irf1 was significantly underexpressed, together with the proapoptotic Fos and Wwox (−1.55-, −2.73-, and −1.63-fold, respectively). Furthermore, Fos downregulation appears to contribute to improved left ventricular function in failing hearts after cardiac support device therapy (43). This evidence points to the inactivation of apoptotic pathways possibly contributing to the absence of a progressive pathological phenotype in hPLN mice (Fig. 7).
Other remodeling processes.
The overexpressed Pten has been shown to promote cardiac contractility (8), whereas Tgfa, the most upregulated gene (34-fold) in hPLN mice, is likely to play an important, yet to be elucidated, role. Only limited changes were observed in energy metabolism, and there were no major changes in inflammatory response-related genes, further supporting the notion that the combined action of hPLN and the downstream activated pathways gives rise to a relatively mild pathological phenotype.
Study limitations.
A limitation of the present study is the use of nontransgenic WT mice instead of α-MHC promoter-driven mPLN transgenic mice as controls. Unfortunately, the highest level of mPLN expression, obtained in the knockout hearts, is only 70% of that in WT mice (22). Thus it was not appropriate to use a model with 70% expression of mPLN to examine the effects elicited by 100% expression of hPLN in the null background. For this reason, WT mice were used as control in our study. In addition, we cannot exclude that some of the observed alterations may be associated with promoter artifacts in the hPLN hearts. However, this is not likely since a second transgenic line (TGL1) exhibited some protein alterations that were similar to those in TGL2.
Conclusions.
We demonstrate that hPLN, through superinhibition of SERCA2a, causes significant changes in Ca2+ homeostasis and in turn a series of electrical, structural, and transcriptional remodeling processes (Fig. 7). A network of interconnected molecular and electrophysiological adaptations appears to promote hypertrophy, myogenesis, cardiac protection, and survival of the transgenic mice in the presence of the superinhibitory hPLN. A clear understanding of this cellular network and the underlying control mechanisms that fine-tune gene expression may be of significant future therapeutic value.
GRANTS
This study was supported by research funds from the Hellenic Cardiological Society, the Leducq Foundation Trans-Atlantic alliance, the European Union 6th Framework Program VALAPODYN No. LSHG-CT-2006-037277, the European Union 7th Framework Program EUTrigTreat No. HEALTH-F2-2009-241526 (to D. Sanoudou and E. G. Kranias), American Heart Association Predoctoral Fellowships (to M. Dong and C. K. Lam), and National Institutes of Health Grants HL-084539 and ES-017263 (to H.-S. Wang) and HL-26057 and HL-64018 (to E. G. Kranias).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1.Anonymous. World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res 35: 2–3, 1997. [PubMed] [Google Scholar]
- 2.Bers DM, Pogwizd SM, Schlotthauer K. Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res Cardiol 97, Suppl 1: I36–I42, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bondarenko VE, Szigeti GP, Bett GC, Kim SJ, Rasmusson RL. Computer model of action potential of mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 287: H1378–H1403, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res 102: 1239–1246, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Dash R, Frank KF, Carr AN, Moravec CS, Kranias EG. Gender influences on sarcoplasmic reticulum Ca2+-handling in failing human myocardium. J Mol Cell Cardiol 33: 1345–1353, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Dong M, Sun X, Prinz AA, Wang HS. Effect of simulated Ito on guinea pig and canine ventricular action potential morphology. Am J Physiol Heart Circ Physiol 291: H631–H637, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Dorris DR, Nguyen A, Gieser L, Lockner R, Lublinsky A, Patterson M, Touma E, Sendera TJ, Elghanian R, Mazumder A. Oligodeoxyribonucleotide probe accessibility on a three-dimensional DNA microarray surface and the effect of hybridization time on the accuracy of expression ratios. BMC Biotechnol 3: 6, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank K, Kranias EG. Phospholamban and cardiac contractility. Ann Med 32: 572–578, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa T, Kurokawa J. Potassium channel remodeling in cardiac hypertrophy. J Mol Cell Cardiol 41: 753–761, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci 18: 128–137, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, Fan GC, Tsiapras D, Parekh RR, Dorn GW, 2nd, MacLennan DH, Kremastinos DT, Kranias EG. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci USA 103: 1388–1393, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrer JM, Kiss E, Kranias EG. Application of the immunoblot technique for quantitation of protein levels in cardiac homogenates. Biotechniques 18: 995–998, 1995. [PubMed] [Google Scholar]
- 13.Haufe V, Camacho JA, Dumaine R, Gunther B, Bollensdorff C, von Banchet GS, Benndorf K, Zimmer T. Expression pattern of neuronal and skeletal muscle voltage-gated Na+ channels in the developing mouse heart. J Physiol 564: 683–696, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell 99: 485–497, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell 113: 483–494, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Hsueh YP, Wang TF, Yang FC, Sheng M. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature 404: 298–302, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Huang SM, Huang CJ, Wang WM, Kang JC, Hsu WC. The enhancement of nuclear receptor transcriptional activation by a mouse actin-binding protein, alpha actinin 2. J Mol Endocrinol 32: 481–496, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Inui M, Chamberlain BK, Saito A, Fleischer S. The nature of the modulation of Ca2+ transport as studied by reconstitution of cardiac sarcoplasmic reticulum. J Biol Chem 261: 1794–1800, 1986. [PubMed] [Google Scholar]
- 19.Kressler D, Hock MB, Kralli A. Coactivators PGC-1beta and SRC-1 interact functionally to promote the agonist activity of the selective estrogen receptor modulator tamoxifen. J Biol Chem 282: 26897–26907, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR, 3rd, Vandenberg CA. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J Biol Chem 279: 22331–22346, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res 100: 112–120, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Luo W, Chu G, Sato Y, Zhou Z, Kadambi VJ, Kranias EG. Transgenic approaches to define the functional role of dual site phospholamban phosphorylation. J Biol Chem 273: 4734–4739, 1998. [DOI] [PubMed] [Google Scholar]
- 23.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577, 2003. [DOI] [PubMed] [Google Scholar]
- 24.McCartney-Francis NL, Wahl SM. Dysregulation of IFN-gamma signaling pathways in the absence of TGF-beta1. J Immunol 169: 5941–5947, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Miyagawa K, Kent J, Moore A, Charlieu JP, Little MH, Williamson KA, Kelsey A, Brown KW, Hassam S, Briner J, Hayashi Y, Hirai H, Yazaki Y, van Heyningen V, Hastie ND. Loss of WT1 function leads to ectopic myogenesis in Wilms' tumour. Nat Genet 18: 15–17, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Mochida S, Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449: 336–340, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Oudit GY, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Backx PH. The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J Mol Cell Cardiol 33: 851–872, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med 14: 61–66, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Qu Y, Rogers J, Tanada T, Scheuer T, Catterall WA. Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc Natl Acad Sci USA 91: 3289–3293, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 63: 423–432, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Sanoudou D, Haslett JN, Kho AT, Guo S, Gazda HT, Greenberg SA, Lidov HG, Kohane IS, Kunkel LM, Beggs AH. Expression profiling reveals altered satellite cell numbers and glycolytic enzyme transcription in nemaline myopathy muscle. Proc Natl Acad Sci USA 100: 4666–4671, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt AG, Zhai J, Carr AN, Gerst MJ, Lorenz JN, Pollesello P, Annila A, Hoit BD, Kranias EG. Structural and functional implications of the phospholamban hinge domain: impaired SR Ca2+ uptake as a primary cause of heart failure. Cardiovasc Res 56: 248–259, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299: 1410–1413, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Schuh K, Uldrijan S, Gambaryan S, Roethlein N, Neyses L. Interaction of the plasma membrane Ca2+ pump 4b/CI with the Ca2+/calmodulin-dependent membrane-associated kinase CASK. J Biol Chem 278: 9778–9783, 2003. [DOI] [PubMed] [Google Scholar]
- 35.She BR, Liou GG, Lin-Chao S. Association of the growth-arrest-specific protein Gas7 with F-actin induces reorganization of microfilaments and promotes membrane outgrowth. Exp Cell Res 273: 34–44, 2002. [DOI] [PubMed] [Google Scholar]
- 36.MACQ Consortium. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol 24: 1151–1161, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sipido KR, Volders PG, Vos MA, Verdonck F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy. Cardiovasc Res 53: 782–805, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Sun L, Ma K, Wang H, Xiao F, Gao Y, Zhang W, Wang K, Gao X, Ip N, Wu Z. JAK1-STAT1-STAT3, a key pathway promoting proliferation and preventing premature differentiation of myoblasts. J Cell Biol 179: 129–138, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tada M, Katz AM. Phosphorylation of the sarcoplasmic reticulum and sarcolemma. Annu Rev Physiol 44: 401–423, 1982. [DOI] [PubMed] [Google Scholar]
- 40.Vijayaragavan K, Boutjdir M, Chahine M. Modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by protein kinase A and protein kinase C. J Neurophysiol 91: 1556–1569, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Nolan B, Kutschke W, Hill JA. Na+-Ca2+ exchanger remodeling in pressure overload cardiac hypertrophy. J Biol Chem 276: 17706–17711, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Wasson S, Reddy HK, Dohrmann ML. Current perspectives of electrical remodeling and its therapeutic implications. J Cardiovasc Pharmacol Ther 9: 129–144, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Wellner M, Dechend R, Park JK, Shagdarsuren E, Al-Saadi N, Kirsch T, Gratze P, Schneider W, Meiners S, Fiebeler A, Haller H, Luft FC, Muller DN. Cardiac gene expression profile in rats with terminal heart failure and cachexia. Physiol Genomics 20: 256–267, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Q, Fan GC, Dong M, Altschafl B, Diwan A, Ren X, Hahn HH, Zhao W, Waggoner JR, Jones LR, Jones WK, Bers DM, Dorn GW, 2nd, Wang HS, Valdivia HH, Chu G, Kranias EG. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation 115: 300–309, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Zhai J, Schmidt AG, Hoit BD, Kimura Y, MacLennan DH, Kranias EG. Cardiac-specific overexpression of a superinhibitory pentameric phospholamban mutant enhances inhibition of cardiac function in vivo. J Biol Chem 275: 10538–10544, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Zhao W, Yuan Q, Qian J, Waggoner JR, Pathak A, Chu G, Mitton B, Sun X, Jin J, Braz JC, Hahn HS, Marreez Y, Syed F, Pollesello P, Annila A, Wang HS, Schultz Jel J, Molkentin JD, Liggett SB, Dorn GW, 2nd, Kranias EG. The presence of Lys27 instead of Asn27 in human phospholamban promotes sarcoplasmic reticulum Ca2+-ATPase superinhibition and cardiac remodeling. Circulation 113: 995–1004, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.