Abstract
Increased circulating levels of resistin have been proposed as a possible link between obesity and insulin resistance; however, many of the potential metabolic effects of resistin remain to be investigated, including systemic versus local resistin action. We investigated potential autocrine effects of resistin on lipid and glucose metabolism in 2- and 16-mo-old transgenic spontaneously hypertensive rats (SHR) expressing a nonsecreted form of mouse resistin under control of the aP2 promoter. To search for possible molecular mechanisms, we compared gene expression profiles in adipose tissue in 6-wk-old transgenic SHR versus control rats, before development of insulin resistance, by digital transcriptional profiling using high-throughput sequencing. Both young and old transgenic rats showed moderate expression of the resistin transgene in adipose tissue but had serum resistin levels similar to control SHR and undetectable levels of transgenic resistin in the circulation. Young transgenic rats exhibited mild glucose intolerance. In contrast, older transgenic rats displayed marked glucose intolerance in association with near total resistance of adipose tissue to insulin-stimulated glucose incorporation into lipids (6 ± 2 vs. 77 ± 19 nmol glucose·g−1·2 h−1, P < 0.00001). Ingenuity Pathway Analysis of differentially expressed genes revealed calcium signaling, Nuclear factor-erythroid 2-related factor-2 (NRF2)-mediated oxidative stress response, and actin cytoskeletal signaling canonical pathways as those most significantly affected. Analysis using DAVID software revealed oxidative phosphorylation, glutathione metabolism, pyruvate metabolism, and peroxisome proliferator-activated receptor (PPAR) signaling as top Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. These results suggest that with increasing age autocrine effects of resistin in fat tissue may predispose to diabetes in part by impairing insulin action in adipose tissue.
Keywords: transgenic rat, adipose tissue, insulin resistance, autocrine effects
serum concentrations of resistin are known to be increased in rodent models of obesity and type 2 diabetes, and it has been suggested that resistin might represent a molecular link between obesity, insulin resistance, and type 2 diabetes (31). This hypothesis is supported by the observation that acute in vivo administration of large amounts of recombinant resistin is associated with hepatic insulin resistance and impaired glucose tolerance (26, 31). Hyperresistinemia induced by intravenous administration of an adenovirus encoding mouse resistin (29) or by ectopic overexpression of mouse resistin in liver of transgenic mice (27) has also been associated with disturbances in hepatic glucose production. Interestingly, despite the achievement of supraphysiological concentrations of circulating resistin in these studies, the observed metabolic effects were rather modest. For example, in transgenic mice a near 200-fold increase in the circulating concentration of resistin was associated with only very mild impairment of glucose tolerance and no changes in nonfasting serum lipids, glucose, or insulin (27). Although resistin transgenic mice have also been shown to have increased glucose production during hyperinsulinemic-euglycemic clamp studies (27), these results suggest that increased circulating resistin levels per se might not have a major impact on glucose tolerance or circulating lipid levels. The metabolic effects of resistin in humans are controversial, and most published studies also suggest little impact of circulating resistin levels on metabolic features of type 2 diabetes (reviewed in Refs. 3, 12, 18, 28). Although hyperresistinemia may exert only modest effects on glucose or lipid metabolism, it is possible that autocrine/paracrine effects of resistin in adipose tissue might further contribute to metabolic disturbances associated with obesity and increased risk for diabetes. Recently, we found (22, 23) that moderate fat-specific expression of the mouse resistin transgene in young (2 mo old) spontaneously hypertensive rats (SHR) was associated with decreased fatty acid reesterification in adipose tissue, increased serum fatty acid and muscle triglyceride levels, impaired skeletal muscle glucose metabolism, and mild glucose intolerance in the absence of any changes in serum resistin concentrations. Since transgenic resistin cannot be detected in the circulation of these animals, this model offers unique opportunities for studying the autocrine effects of resistin in adipose tissue. Although insulin resistance has long been associated with aging in both humans and rats (4, 5, 20), mechanisms playing a role in the deterioration of tissue insulin sensitivity and other metabolic changes associated with aging are not fully understood. In the present study, we investigated whether transgenic expression of resistin in adipose tissue, independent of changes in serum resistin levels, is associated with age-related changes in glucose tolerance and sensitivity of adipose tissue to insulin action. To search for possible molecular pathways involved in the autocrine effects of resistin, we also analyzed adipose tissue gene expression profiles in young resistin transgenic rats before development of overt insulin resistance in adipose tissue.
METHODS
Animals.
Transgenic SHR (referred to as SHR-Retn) with the resistin transgene under control of the aP2 promoter were derived as previously described (22). Briefly, transgenic SHR were derived by microinjections of zygotes with a mouse resistin cDNA construct that was prepared by reverse transcriptase PCR of RNA from fat tissue of a BALB/c mouse. There is a 75% homology between the rat and murine amino acid sequences, and thus mouse and rat resistin are likely to have similar targets (15). The construct contained in addition to cDNA of the mouse resistin gene rabbit β-globin intron 2, a growth hormone poly(A) signal, and the aP2 promoter. The rats were housed in an air-conditioned animal facility and allowed free access to food and water. Baseline metabolic phenotypes were assessed in nonfasted young and old male rats that were fed a diet with 60% fructose (K4102.0 diet, Hope Farms) for 15 days: 1) control SHR (n = 10) and resistin transgenic SHR (n = 10) at the age of 2 mo and 2) control SHR (n = 10) and resistin transgenic SHR (n = 10) at the age of 16 mo. Gene expression profiles were measured in epididymal fat isolated from 6-wk-old male transgenic rats (n = 5) and age-matched SHR control rats (n = 5) to search for gene expression changes preceding the development of metabolic disturbances in older animals. All experiments were performed in accordance with the Animal Protection Law of the Czech Republic (311/1997) and were approved by the Ethics Committee of the Institute of Physiology, Academy of Sciences of the Czech Republic.
Oral glucose tolerance testing.
Oral glucose tolerance tests (OGTT) were performed with a glucose load of 300 mg/100 g body wt after overnight fasting. Blood was drawn from the tail without anesthesia before the glucose load (0 min time point) and at 30, 60, and 120 min thereafter.
Basal and insulin-stimulated glucose oxidation and glycogen synthesis.
Insulin-stimulated glucose oxidation was determined in isolated soleus muscle by measuring the effects of insulin on incorporation of [U-14C]glucose into CO2 according to Vrana et al. (35). After decapitation of the rats, the soleus muscles were attached to a stainless steel frame in situ at in vivo length by special clips, separated from other muscles and tendons, and immediately incubated for 2 h in Krebs-Ringer bicarbonate buffer, pH 7.4, that contained 5.5 mM unlabeled glucose, 0.5 μCi/ml of [U-14C]glucose, and 3 mg/ml bovine serum albumin (BSA) (Armour, fraction V) with or without 250 μU/ml insulin. After 2-h incubation, 0.3 ml of 1 M hyamine hydroxide was injected into the central compartment of the incubation vessel and 0.5 ml of 1 M H2SO4 into the main compartment to liberate CO2. The vessels were incubated for another 30 min; the hyamine hydroxide was then quantitatively transferred into the scintillation vial containing 10 ml of toluene-based scintillation fluid for counting of radioactivity. For measurement of insulin-stimulated incorporation of glucose into glycogen, soleus muscles were incubated for 2 h in 95% O2 + 5% CO2 in Krebs-Ringer bicarbonate buffer, pH 7.4, containing 0.1 μCi/ml of [U-14C]glucose, 5 mM of unlabeled glucose, and 2.5 mg/ml of BSA (Armour, fraction V), with or without 250 μU/ml insulin. Glycogen was extracted, and insulin-stimulated incorporation of glucose into glycogen was determined as previously described (34).
Glucose utilization in isolated epididymal adipose tissue.
Distal parts of the epididymal adipose tissue were rapidly dissected and incubated for 2 h in Krebs-Ringer bicarbonate buffer with 5 mM glucose, 0.1 μCi/ml [U-14C]glucose (UVVR, Prague, Czech Republic), and 2% BSA and gaseous-phase 95% O2 and 5% CO2 in the presence (250 μU/ml) or absence of insulin in incubation medium. All incubations were performed at 37°C in sealed vials in a shaking water bath. Estimation of [14C]glucose incorporation into neutral lipids was performed as described previously (10, 25, 34). Briefly, adipose tissue was removed from incubation medium, rinsed in saline, and immediately put into chloroform. The pieces of tissue were dissolved with a Teflon pestle homogenizer, methanol was added [chloroform-methanol (2:1)], and lipids were extracted at 4°C overnight. The remaining tissue was removed, KH2PO4 was added and a clear extract was taken for further analysis. An aliquot was evaporated and reconstituted in scintillation liquid, and the radioactivity was measured by scintillation counting. Incremental glucose utilization was calculated as the difference between the insulin-stimulated and basal incorporation of glucose into neutral lipids.
Lipolysis in isolated epididymal adipose tissue.
Measurement of basal and epinephrine-stimulated lipolysis was performed as previously described (23). Distal parts of the epididymal adipose tissue were incubated in Krebs-Ringer phosphate buffer containing 3% BSA (Armour, fraction V) at 37°C, pH 7.4 with or without epinephrine (0.25 μg/ml). The tissue was incubated for 2 h, and the concentrations of free fatty acids (FFA) and glycerol in the medium were determined.
Tissue triglyceride measurements.
For determination of triglycerides in liver and soleus muscle, tissues were powdered under liquid N2 and extracted for 16 h in chloroform-methanol, after which 2% KH2PO4 was added and the solution was centrifuged. The organic phase was removed and evaporated under N2. The resulting pellet was dissolved in isopropyl alcohol, and triglyceride content was determined by enzymatic assay (Pliva-Lachema, Brno, Czech Republic).
Biochemical analyses.
Rat serum resistin and mouse serum resistin were measured with antibodies from Biovendor (Brno, Czech Republic). Blood glucose levels were measured by glucose oxidase assay (Pliva-Lachema) using tail vein blood drawn into 5% trichloroacetic acid and promptly centrifuged. Nonesterified fatty acid (NEFA) levels were determined with an acyl-CoA oxidase-based colorimetric kit (Roche Diagnostics, Mannheim, Germany). Serum triglyceride concentrations were measured by standard enzymatic methods (Pliva-Lachema). Glycerol was determined with a commercially available analytical kit (Sigma). Serum insulin concentrations were determined with a rat insulin ELISA kit (Mercodia, Uppsala, Sweden).
Gene expression profiles.
For multiplex sequencing determination of gene expression profiles, RNA was extracted from epididymal fat of five males each from 6-wk-old SHR and SHR-Retn transgenic rats. The epididymal fat was crushed to a fine powder by pestle and mortar in liquid nitrogen. Approximately 150 mg of powdered sample was homogenized in 1.5 ml of TRIzol (Invitrogen, Paisley, UK) and subsequently purified with the RNeasy Mini Plus Kit (Qiagen). Library preparation and sequencing were carried out essentially as described previously (16); however, oligonucleotide primers were modified to accommodate sequencing on the Illumina Genome Analyzer I (Wakimoto H, Seidman CE, and Seidman JG, unpublished). We pooled adipose tissue from animals per strain and used one lane per strain for analysis of the pooled tissue samples, which yielded >7 million reads for each pooled sample. Tag sequences were mapped back to the genome with customized software (Wakimoto H and Seidman JG, unpublished). To exclude tags containing single nucleotide polymorphisms (SNPs) between SHR and SHR-Retn and to ensure that sequenced tags are likely to represent a single locus, tags that were observed zero times in any strain and/or were found to align more than four times to the genome were removed from the data set. The number of tags was normalized across both strains. For the SHR-Retn transgenic strain, the tag count fold change compared with SHR was calculated. Raw P values were calculated by Fisher's exact test for those tags that had an absolute fold change of tag abundance ≥1.5. P values were corrected for multiple testing with the Benjamini and Hochberg false discovery rate method. Tags showing both tag abundance ratios ≥1.5 and P < 0.05 were considered to be robustly differentially expressed. Gene pathways were analyzed by Ingenuity Pathways Analysis. The significance of the association between the data set and the canonical pathway was determined based on two parameters: 1) a ratio of the number of differentially expressed genes from the data set that map to the pathway divided by the total number of genes that map to the canonical pathway and 2) a P value calculated with Fisher's exact test determining the probability that the association between the genes in the data set and the canonical pathway is due to chance alone (Ingenuity Systems; www.ingenuity.com). In addition, a functional analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was performed with DAVID online software (14).
Gene expression determined by real-time PCR.
Total RNA was extracted from epididymal fat and intraperitoneal macrophages with TRIzol reagent (Invitrogen), and cDNA was prepared and analyzed by real-time PCR testing using QuantiTect SYBR Green reagents (Qiagen) on an Opticon continuous fluorescence detector (MJ Research). Gene expression levels were normalized relative to the expression of peptidylprolyl isomerase A (Ppia) (cyclophilin) gene, which served as the internal control, with results being determined in triplicate. Primers used for validation of differentially expressed genes selected from significant pathways are given in Supplemental Table S1.1
Statistical analysis.
All data are expressed as means ± SE. Differences between control and experimental groups were evaluated by paired or nonpaired t-tests as appropriate. Statistical analysis of the gene expression data was performed with the REST XL program that tests for significance by a randomization procedure (21). For metabolic measurements, statistical significance was defined as P < 0.05. Statistical analysis of the transcription profiling studies was performed as described in Gene expression profiles above.
RESULTS
Expression of endogenous and transgenic resistin genes and serum levels of endogenous and transgenic resistin protein in young and old transgenic SHR and age-matched control rats.
As previously reported (22), we observed modest levels of Retn transgene expression in epididymal fat tissue of the transgenic rats. We did not detect any significant differences in Retn transgene expression between the young and old transgenic SHR tested in the present studies (Supplemental Table S2). As expected, no expression of the resistin transgene could be detected in the transgene negative control rats. There were no significant differences in expression levels of the endogenous rat Retn gene between young transgenic rats and their controls or between old transgenic rats and their controls (Supplemental Table S2). In contrast to strong expression of the Retn transgene in epididymal and subcutaneous fat, we found little or no expression of the Retn transgene in heart, liver, soleus muscle, and macrophages isolated from SHR-Retn (Supplemental Fig. S1).
In previous studies with an antibody that cross-reacts with both rat and mouse resistin, we found no significant difference in serum levels of resistin between young transgenic rats and control rats (22). In the present studies, we also found no significant differences in serum levels of resistin between the old transgenic rats and control rats (11.9 ± 1.5 vs. 10.7 ± 1.2 ng/ml, P = 0.73). In addition, using a monoclonal antibody that is specific for mouse resistin and that does not cross-react with rat resistin, we found no transgenic mouse resistin in the circulation in either the young or old transgenic rats, confirming that the transgenic resistin is not secreted into the circulation in detectable amounts.
Effects of resistin transgene on body weight, serum phenotypes, and tissue lipid levels.
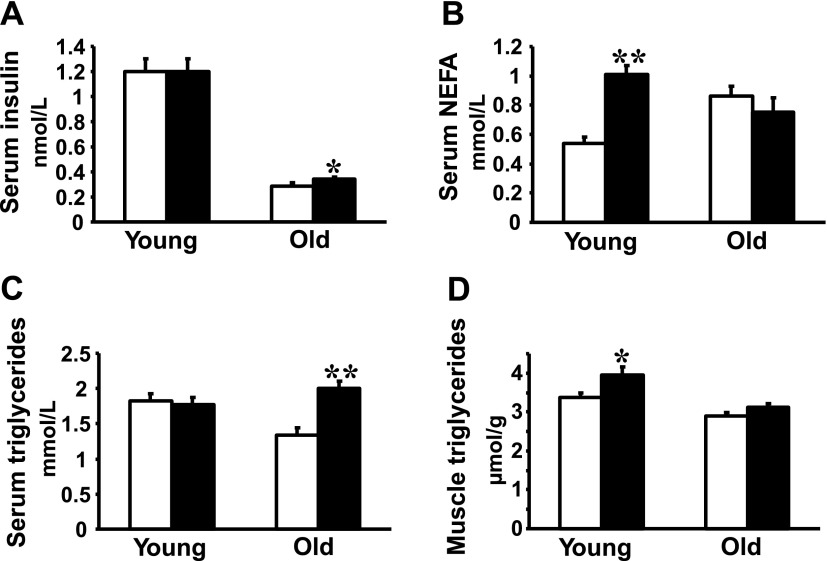
At the time of death, there were no statistically significant differences between young transgenic rats and their controls with respect to either body weight (249 ± 6 vs. 240 ± 3 g, P = 0.20) or epididymal fat weight (0.94 ± 0.05 vs. 0.89 ± 0.04 g/100 g body wt, P = 0.23). On the other hand, 16-mo-old transgenic rats exhibited a small but significant increase versus age-matched control rats in body weight (410 ± 10 g vs. 386 ± 10 g, P = 0.001) and in relative epididymal fat pad weights (0.92 ± 0.03 vs. 0.80 ± 0.03 g/100 g body wt, P = 0.01). Nonfasting serum insulin levels were similar in young transgenic rats and in age-matched control rats; however, old transgenic rats exhibited slightly but significantly increased concentrations of serum insulin compared with age-matched control rats (Fig. 1A). There were no significant differences in nonfasting serum glucose levels between young resistin transgenic rats compared with young control rats (5.7 ± 0.2 vs. 5.8 ± 0.2 mmol/l) and between old resistin transgenic and their controls (6.0 ± 0.3 vs. 5.8 ± 0.3 mmol/l). Serum NEFA levels were significantly increased in young transgenic rats compared with control rats but were similar between old transgenic rats and age-matched control rats (Fig. 1B). Serum triglycerides were similar in young transgenic and control rats but were significantly increased in old transgenic rats compared with their respective controls (Fig. 1C). In young transgenic rats but not in old transgenic rats, skeletal muscle triglycerides were modestly increased compared with those in age-matched controls (Fig. 1D). There were no significant differences in hepatic triglycerides between young transgenic and control rats (9.9 ± 0.5 vs. 9.8 ± 0.4 μmol/g) and between old transgenic and control rats (9.7 ± 1.4 vs. 9.5 ± 1.1 μmol/g).
Fig. 1.
Metabolic parameters in young (2 mo old) and old (16 mo old) spontaneously hypertensive rat (SHR)-Retn transgenic rats (filled bars) and in age-matched transgene negative control rats (open bars). A: nonfasting serum insulin concentrations were similar between young transgenic rats and control rats, while old transgenic rats exhibited a small but significant increase in insulin levels compared with their controls. Old transgenic rats had significantly lower insulin levels compared with young transgenic rats. B: serum nonesterified fatty acid (NEFA) levels were significantly increased in young transgenic rats compared with age-matched control rats, while no significant difference was observed between old transgenic rats and age-matched control rats. C: serum triglyceride levels were significantly increased in old transgenic rats but not in young transgenic rats compared with their respective control rats. D: young transgenic rats, but not old transgenic rats, showed significantly increased muscle triglyceride concentrations compared with respective control rats. *P < 0.05, **P < 0.0001.
Effects of resistin transgene on insulin resistance in muscle and adipose tissue.
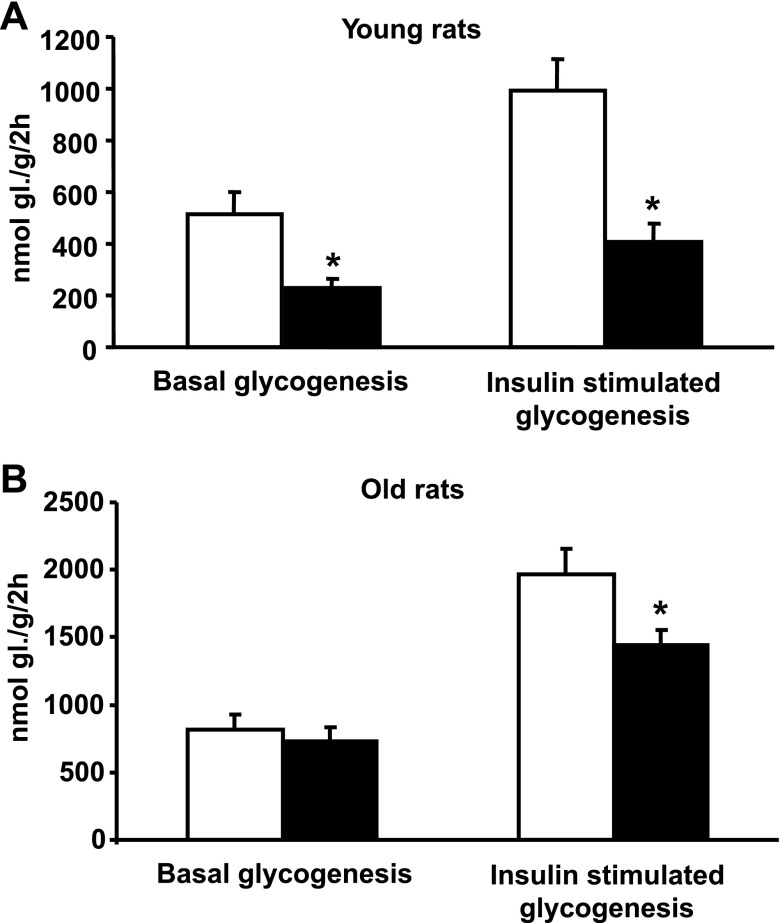
In soleus muscle isolated from young transgenic SHR, glucose incorporation into glycogen was significantly reduced in both the presence and absence of insulin compared with age-matched control rats (Fig. 2A). In contrast, the old transgenic SHR were characterized by normal levels of basal glycogenesis and only modest reductions in insulin-stimulated glycogenesis compared with age-matched control rats (Fig. 2B). Glucose oxidation in isolated soleus muscle was not significantly different between young transgenic versus age-matched control rats (221 ± 32 vs. 206 ± 29 nmol glucose·g tissue−1·2 h−1). In old transgenic rats, basal glucose oxidation was reduced compared with age-matched controls (117 ± 12 vs. 194 ± 19 nmol glucose·g tissue−1·2 h−1, P < 0.005). However, insulin-stimulated glucose oxidation was similar in both young and old transgenic rats compared with respective age-matched control rats (data not shown).
Fig. 2.
Basal and insulin-stimulated incorporation of glucose into soleus muscle glycogen in young (2 mo old) and old (16 mo old) SHR resistin transgenic rats (filled bars) and in transgene negative age-matched control rats (open bars). A: both basal and insulin-stimulated incorporation of radioactively labeled glucose into muscle glycogen were significantly reduced in young transgenic rats compared with control rats. B: in old transgenic rats, there was a modest but significant decrease in insulin stimulated incorporation of glucose into muscle glycogen, while basal glycogenesis was similar to that in age-matched control rats. *P < 0.05.
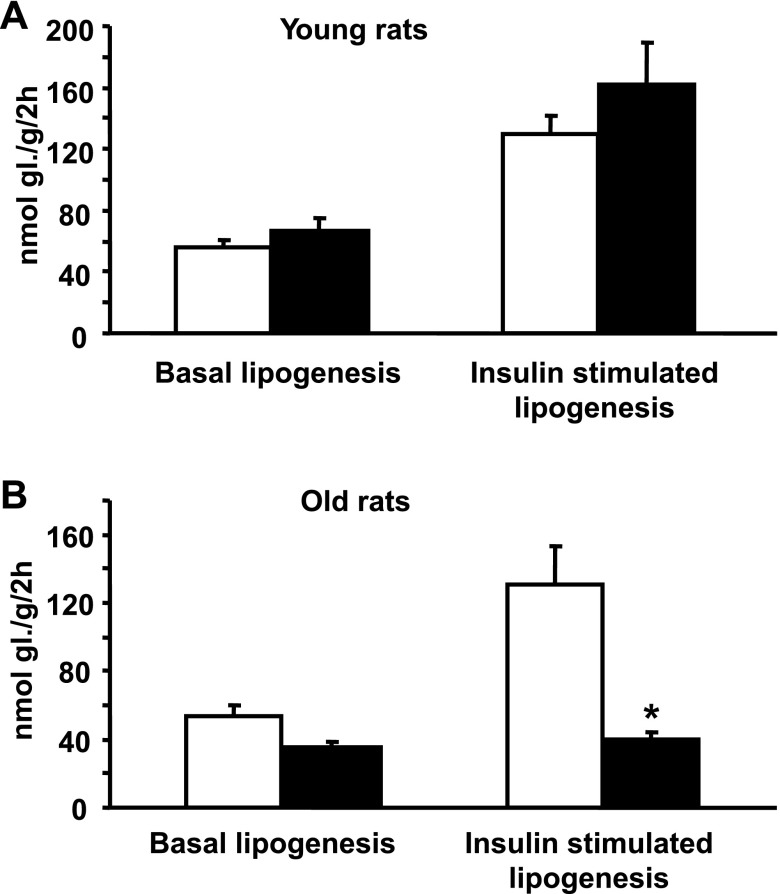
There were no significant differences between young transgenic rats and young control rats in basal or insulin-stimulated incorporation of glucose into adipose tissue lipids (Fig. 3A). In contrast to the results in young rats, insulin-stimulated glucose incorporation into lipids in the older transgenic rats was markedly decreased compared with that in age-matched control rats (Fig. 3B). In old transgenic rats, the insulin-induced increase in glucose incorporation into lipids, 6 ± 2 nmol glucose·g−1·2 h−1, was markedly lower than that in age-matched control rats, 77 ± 19 nmol glucose·g−1·2 h−1 (P < 0.00001), suggesting near total resistance of adipose tissue to insulin-stimulated glucose incorporation into lipids.
Fig. 3.
Basal and insulin-stimulated glucose utilization in epididymal fat in young (2 mo old) and old (16 mo old) SHR resistin transgenic rats (filled bars) and in age-matched transgene negative control rats (open bars). A: there were no significant differences in basal and insulin-stimulated glucose incorporation into adipose tissue lipids in young transgenic rats compared with age-matched control rats. B: old transgenic rats showed near total resistance to insulin-stimulated glucose incorporation into adipose tissue lipids. *P < 0.01.
Effects of resistin transgene on NEFA reesterification.
In young resistin transgenic rats, the NEFA-to-glycerol ratio during epinephrine-stimulated lipolysis was significantly greater than in transgene negative control rats (2.41 ± 0.20 vs. 1.58 ± 0.07, P = 0.001), while there was no significant difference in NEFA-to-glycerol ratio in old resistin transgenic rats compared with age-matched control rats (2.89 ± 0.27 vs. 2.59 ± 0.22, P = 0.39). These results suggest that only ∼20% of NEFA are reesterified during epinephrine-stimulated lipolysis in young transgenic rats (2.4/3 = 0.8 or 80% released NEFA and 20% reesterified) compared with 47% of NEFA reesterified in control rats (1.6/3 = 0.53 or 53% released NEFA and 47% reesterified). These results are consistent with our previous findings (23) and suggest that the prodiabetic effect of resistin in the young transgenic animals may be related to skeletal muscle insulin resistance secondary to increased release of NEFA from adipose tissue and attendant accumulation of lipids in skeletal muscle. In contrast, the prodiabetic effect of resistin in the old transgenic animals appears to largely involve resistance to insulin-stimulated glucose incorporation into adipose tissue lipids.
Effects of resistin transgene on oral glucose tolerance.
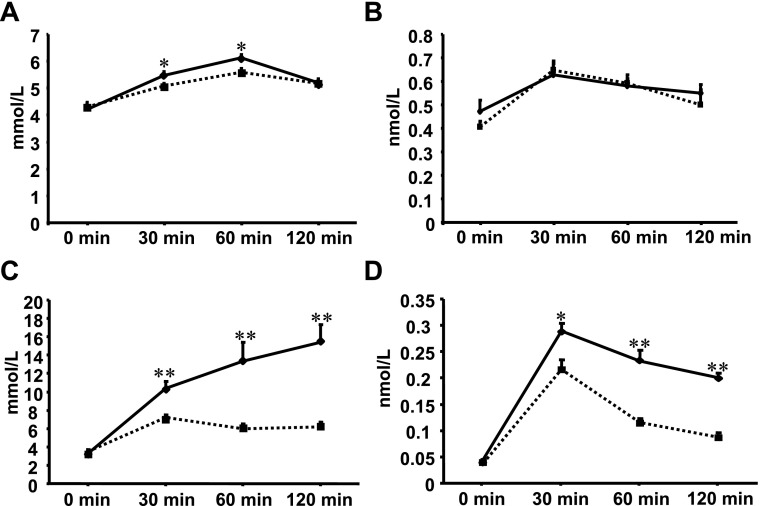
The young resistin transgenic rats displayed mild glucose intolerance during the OGTT (Fig. 4, A and B), with the area under the glucose curve being slightly but significantly greater in young transgenic rats compared with age-matched control rats (653 ± 8 vs. 623 ± 8 mmol·l−1·2 h−1, P = 0.01). In contrast, the old resistin transgenic rats exhibited very high glucose concentrations after glucose loading compared with age-matched control rats (Fig. 4C). The area under the glucose curve was markedly greater in the old transgenic rats compared with age-matched control rats (1,429 ± 169 vs. 725 ± 14 mmol·l−1·2 h−1, P = 0.005). Insulin levels during OGTT were also significantly higher in transgenic rats compared with control rats (Fig. 4D).
Fig. 4.
Effects of transgenic resistin on oral glucose tolerance test in young and old transgenic rats (♦, solid lines) and respective control rats (■, dotted lines). A: glucose concentrations after glucose loading were modestly increased in young transgenic rats compared with age-matched control rats at 30 and 60 min after glucose loading. B: insulin concentrations after glucose loading were similar in young transgenic rats and age-matched control rats. C: glucose concentrations after glucose loading were markedly increased in old transgenic rats compared with age-matched control rats. D: insulin concentrations after glucose loading were significantly increased in old transgenic rats compared with age-matched control rats. *P < 0.05, **P < 0.001.
Changes in gene expression profiles associated with resistin transgene.
To search for molecular mechanisms that link expression of the resistin transgene to adipose tissue insulin resistance, we compared gene expression profiles, using next-generation sequencing in epididymal fat isolated from transgenic SHR versus age-matched control rats at the age of 6 wk, i.e., before development of insulin resistance in adipose tissue. Altogether, we found 1,287 genes with significantly (adjusted P value <0.001) up- or downregulated expression (Supplemental Table S3). Directional differences in the expression of selected genes from Ingenuity Pathway Analysis and KEGG pathway analysis were confirmed by quantitative RT-PCR (Supplemental Figs. S2 and S3). Table 1 shows the top canonical pathways identified by Ingenuity Pathway Analysis of differentially expressed genes. Calcium signaling, Nuclear factor-erythroid 2-related factor-2 (NRF2) oxidative stress response, and actin cytoskeletal signaling canonical pathways were identified as the most significantly affected by transgene expression. Table 2 shows oxidative phosphorylation, glutathione metabolism, pyruvate metabolism, and peroxisome proliferator-activated receptor (PPAR) signaling as top KEGG pathways identified by the analysis of differentially expressed genes using DAVID online software. Genomewide expression profiling did not reveal any distinct effects on genes in the insulin receptor substrate 1 (IRS-1) signaling pathway. Real-time PCR testing of selected genes coding for proteins in the IRS-1-coupled pathway (Irs1, Irs2, Akt1, Akt2, Pik3r1, and Prkaa1) also did not reveal any significant differences in transcript levels between young and old transgenic rats compared with age-matched control rats (data not shown).
Table 1.
List of genes from top canonical pathways identified by Ingenuity Pathway Analysis
| Canonical Pathway | P Value | Ratio | Genes |
|---|---|---|---|
| Calcium signaling | 2.2E-10 | 22/206 (0.107) | Myl1↓, Calr↑, Tnni2↓, Camk2b↑, Ppp3r2↑, Myh2↓, Tnnt1↓, Acta2↑, Itpr3↑, Atp2a1↓, Trdn↓, Myl3↓, Ryr1↓, Myh6↓, Chrne↑, Tnnc2↓, Acta1↓, Trpv6↑, Calm2↑, Tpm1↑, Tnnt3↓, Rcan2↑ |
| NRF2-mediated oxidative stress response | 3.7E-06 | 16/185 (0.086) | Gstm5↓, Pik3r1↓, Herpud1↑, Fkbp5↑, Acta2↑, Dnajc3↑, Gstm3↓, Actg2↓, Gstm1↑, Erp29↑, Sod2↓, Dnajb9↑, Acta1↓, Ppib↑, Dnajc10↑, Dnajb11↑ |
| Actin cytoskeleton signaling | 1.1E-04 | 15/237 (0.063) | Mylpf↓, Myl1↓, Pik3r1↓, Pip5k1b↑, Rock1↓, Myh2↓, Acta2↑, Actn3↓, Myl3↓, Actg2↓, Myh6↓, Pfn2↑, Ttn↓, Acta1↓, Lbp↓ |
↑ and ↓, Up- and downregulated, respectively, in spontaneously hypertensive rats (SHR) vs.
SHR-Retn transgenic rats; Ratio, number of differentially expressed genes from data set that map to the pathway divided by total number of genes known to map to the canonical pathway; NRF2, Nuclear factor-erythroid 2-related factor-2.
See methods for column definitions and description of statistical analysis.
Table 2.
List of genes from top KEGG pathways
| Pathway | P Value | % | Genes |
|---|---|---|---|
| Oxidative phosphorylation | 6.8E-5 | 3.4 | Cox5b↓, Cox5a↓, Atp5i↓, Atp5j↓, Cox4i1↓, Uqcrh↓, Cox8h↓, Ndufab1↓, Ndufb7↓, Atp5d↓, Atp5o↓, Ndufb9↓, Atp5e↓, Atp5a1↓, Atp5b↓, Sdhc↓, Atp5c1↓, Uqcrc1↓, Ndufb4↓, Cyc1↓, Atp5g3↓, Cox7b↓, Cox6a2↓, Sdha↓, Uqcrb↓ |
| Pyruvate metabolism | 1.2E-2 | 1.4 | Mdh1↓, Ldhc↑, Me1↓, Pc↓, Acaca↓, Pdha1↓, Pdhb↓, Ldhb↓, Aldh1a7↑, Akr1b4↓ |
| Glutathione metabolism | 1.2E-2 | 1.4 | Ggtla1↑, Mgst1↓, Mgst2↑, Gpx3↓, Gstm5↓, Gstm3↓, Gpx4↓, Gpx1↓, Idh1↓, Ggt1↑ |
| PPAR signaling | 1.2E-2 | 2.0 | Fabp5↓, Lpl↓, Gyk↑, Plin↓, Pparg↓, Fabp4↓, Acsl1↓, Adipoq↓, Fabp7↑, Scd2↑, Scd1↓, Fabp3↓, Me1↓, Apoc3↓, Acadm↓ |
↑ and ↓, Up- and downregulated, respectively, in SHR vs. SHR-Retn transgenic rats;
%, percentage of differentially expressed genes divided by total number of genes in the given Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways; PPAR, peroxisome proliferator-activated receptor.
Modified Fisher's exact test P value, EASE score is Benjamini-Hochberg corrected (14).
DISCUSSION
In the present studies, we have found that autocrine effects of transgenic resistin predominantly expressed in adipose tissue are associated with age-related 1) increases in body weight and adiposity, 2) marked decreases in glucose tolerance, and 3) severely impaired insulin-stimulated glucose incorporation into adipose tissue lipids with only modestly impaired insulin sensitivity in skeletal muscle. The metabolic disturbances in the transgenic rats were observed in the absence of detectable amounts of transgenic resistin in the circulation and with serum levels of endogenous resistin comparable to those in transgene negative control rats. These findings suggest that in old transgenic rats autocrine effects of resistin may serve to promote disordered glucose metabolism in large part by impairing insulin action in adipose tissue. Peripheral glucose disposal is primarily mediated by skeletal muscle; however, it has also been shown that adipose tissue can play an important role in whole body glucose homeostasis (1, 8, 29). Although little or no transgenic resistin was expressed in skeletal muscle, increases in body weight and in circulating lipid levels may have contributed to the impaired insulin sensitivity that was also observed in skeletal muscle of the transgenic rats.
Although the mechanisms by which resistin impairs insulin action in fat and other tissues are not completely defined, some studies have suggested that resistin may adversely affect insulin signaling pathways. Satoh et al. (29) reported that supraphysiological plasma resistin concentrations, detected in Wistar rats 7 days after intravenous injection of adenovirus expressing a mouse resistin transgene, were associated with impaired insulin signaling due to significantly decreased content and activation of IRS-1 and IRS-2. These investigators concluded that chronic hyperresistinemia is associated with decreased signaling in adipose tissue via the IRS-1-coupled pathway due to downregulation of IRS-1 content and activity. However, other investigators have reported that resistin can inhibit cellular glucose uptake in the absence of distinct effects on insulin receptor signaling (19). In the present studies, genomewide expression profiling and real-time PCR analysis did not reveal any distinct effects of the resistin transgene on transcript levels for genes related to IRS-1 signaling. However, because mRNA abundance does not always correlate with protein abundance or activity, these results should be interpreted with caution and do not exclude a potential contribution of altered IRS-1 signaling to resistin-induced disturbances in adipose tissue glucose metabolism.
In contrast to rodents, humans produce resistin mainly in mononuclear cells rather than in adipocytes. Recently, Qatanani et al. (24) reported derivation of “humanized resistin mice” lacking a functional endogenous Retn gene (C57BL/6 Retn−/− mice) but expressing a human RETN transgene specifically in macrophages. These mice showed circulating levels of human resistin similar to or greater than those observed in humans and increased susceptibility to adipose tissue inflammation as reflected by increased expression of genes coding for a variety of inflammatory protein markers (24). These studies point to a potentially important role of the macrophage and inflammatory mechanisms in mediating the adverse metabolic effects of resistin in humans. In the present studies, the resistin transgene was predominantly expressed in visceral and subcutaneous fat, and we found relatively little or no expression of either the aP2 promoter-driven Retn transgene or the endogenous Retn gene in macrophages or other tissues isolated from transgenic SHR or control rats. Although not definitive, these findings suggest that expression of the resistin transgene in adipocytes may play a more important role in the impaired insulin action observed in adipose tissue of the old transgenic rats than its expression in monocytes or other tissues.
To search for mechanistic pathways that might contribute to the age-related disturbances in glucose metabolism observed in older resistin transgenic rats, we performed gene expression profile analyses of adipose tissue samples obtained before the development of adipose tissue insulin resistance. Analyses of adipose tissue gene expression profiles revealed calcium signaling, NRF2-mediated responses to oxidative stress, and actin cytoskeleton signaling as the top canonical pathways by Ingenuity Pathway Analysis. Oxidative phosphorylation, glutathione metabolism, pyruvate metabolism, and PPAR signaling were the top KEGG pathways identified by DAVID software analysis.
Resistin has been reported to increase cytosolic calcium concentrations, which can mediate proinflammatory processes (6). The results of the present study suggest differential expression of calcium signaling genes and genes involved in NRF2-mediated oxidative stress responses in adipose tissue as the most significant canonical pathways affected in the resistin transgenic rats. NRF2 is a key transcription factor for cellular defense against oxidative stress since it binds to antioxidant response elements (ARE) located in the promoter regions of antioxidant genes such as Gpx (glutathione peroxidase) or Gst (glutathione S-transferase). In the present studies, differential expression of genes involved in glutathione metabolism was noted in the KEGG pathway analysis. NRF2 signaling also plays an important role in attenuating inflammatory processes (17). Thus dysregulated expression of genes involved in NRF2 signaling could be contributing to the metabolic effects of transgenic resistin by predisposing to both increased oxidative stress and inflammation. Ingenuity Pathway Analysis further highlighted an effect on actin cytoskeletal signaling, which is of potential functional interest given the known role of actin signaling filaments in insulin-stimulated translocation of GLUT4 to adipocyte membranes and in controlling glucose uptake by adipocytes (7, 9).
In the KEGG pathway analysis, the gene expression changes related to oxidative phosphorylation and PPAR signaling are particularly noteworthy from a metabolic perspective. For example, the downregulated expression of genes coding for enzymes involved in oxidative phosphorylation suggests that impaired mitochondrial activity in adipose tissue might be contributing to the observed metabolic disturbances that ultimately develop in older transgenic rats. The identification of reduced Pparg gene expression in adipose tissue of the transgenic rats is interesting in light of the known relationship between the PPARγ and resistin protein function pathways. Resistin was originally identified by screening for genes that are induced during adipocyte differentiation and that are downregulated by exposure of mature adipocytes to thiazolidinedione ligands of PPARγ (31). Rosiglitazone has been shown to suppress transcription of resistin through PPARγ activation (32). The present findings are consistent with the possibility that overexpression of resistin might influence insulin sensitivity of adipose tissue through effects on PPARγ-related pathways. Taken together, the present results are analogous to a recently published proteomic analysis of human adipose tissue collected after rosiglitazone treatment in which major quantitative changes were also observed among proteins involved in cytoskeletal rearrangement, calcium signaling, and inflammatory and redox signaling (2).
As with most gene expression profiling analyses, it is difficult to determine which particular pathways are actually mediating the functional effects observed in vivo and which are simply reflecting secondary or coincidental phenomena. In the present studies, we attempted to minimize this problem by identifying changes in gene expression in adipose tissue that emerged before the development of adipose tissue insulin resistance. This raises the possibility that some of the identified pathways might be involved in driving development of the age-related metabolic disturbances associated with transgenic expression of resistin in adipose tissue. However, additional studies will be required to determine whether changes in any of these pathways actually initiate or mediate resistin-induced disturbances in adipose tissue glucose metabolism or simply reflect secondary or coincidental phenomena unrelated to disease pathogenesis. Thus the present results should serve to motivate future experiments including comprehensive proteomic analyses and targeted gene manipulation studies to help definitively sort out the cause-and-effect relationships suggested by these new gene expression findings.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-35018, HL-56028, and HL-63709 to T. W. Kurtz, Grants ME08006 and 1M6837805002 from the Ministry of Education of the Czech Republic to M. Pravenec, Grants MZ000023001 and NS9757-3 from the Ministry of Health of the Czech Republic to L. Kazdová and M. Pravenec, Grant IAA500110805 from the Grant Agency of the Academy of Sciences of the Czech Republic to V. Zídek, research project AV0Z 50110509, and Fondation Leducq Grant 06 CVD 03.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409: 729–733, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed M, Neville MJ, Edelmann MJ, Kessler BM, Karpe F. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity (Silver Spring) 18: 27–34, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Arner P. Resistin: yet another adipokine tells us that men are not mice. Diabetologia 48: 2203–2205, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Barzilai N, Rossetti L. Relationship between changes in body composition and insulin responsiveness in models of the aging rat. Am J Physiol Endocrinol Metab 269: E591–E597, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Bechtold M, Palmer J, Valtos J, Iasiello C, Sowers J. Metabolic syndrome in the elderly. Curr Diab Rep 6: 64–71, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Bertolani C, Sancho-Bru P, Failli P, Bataller R, Aleffi S, DeFranco R, Mazzinghi B, Romagnani P, Milani S, Ginés P, Colmenero J, Parola M, Gelmini S, Tarquini R, Laffi G, Pinzani M, Marra F. Resistin as an intrahepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol 169: 2042–2053, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brozinick JT, Jr, Berkemeier BA, Elmendorf JS. “Actin”g on GLUT4: membrane and cytoskeletal components of insulin action. Curr Diab Rev 3: 111–122, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducluzeau PH, Fletcher LM, Vidal H, Laville M, Tavare JM. Molecular mechanisms of insulin-stimulated glucose uptake in adipocytes. Diab Metab 28: 85–92, 2002. [PubMed] [Google Scholar]
- 9.Eyster CA, Olson AL. Compartmentalization and regulation of insulin signaling to GLUT4 by the cytoskeleton. Vitam Horm 80: 193–215, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 11.Fu Y, Luo L, Luo N, Garvey WT. Proinflammatory cytokine production and insulin sensitivity regulated by overexpression of resistin in 3T3-L1 adipocytes. Nutr Metab (Lond) 3: 28, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haluzik M, Haluzikova D. The role of resistin in obesity-induced insulin resistance. Curr Opin Investig Drugs 7: 306–311, 2006. [PubMed] [Google Scholar]
- 13.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 100: 15712–15717, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Kim KH, Lee K, Moon YS, Sul HS. A cysteine-rich adipose tissue-specific secretory factor inhibits adipocyte differentiation. J Biol Chem 276: 11252–11256, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Kim JB, Porreca GJ, Song L, Greenway SC, Gorham JM, Church GM, Seidman CE, Seidman JG. Polony multiplex analysis of gene expression (PMAGE) in mouse hypertrophic cardiomyopathy. Science 316: 1481–1484, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690: 12–23, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and type II diabetes. Clin Sci (Lond) 109: 243–256, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Moon B, Kwan JJ, Duddy N, Sweeney G, Begum N. Resistin inhibits glucose uptake in L6 cells independently of changes in insulin signaling and GLUT4 translocation. Am J Physiol Endocrinol Metab 285: E106–E115, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Mykkänen L, Laakso M, Pyörälä K. Association of obesity and distribution of obesity with glucose tolerance and cardiovascular risk factors in the elderly. Int J Obes Relat Metab Disord 16: 695–704, 1992. [PubMed] [Google Scholar]
- 21.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30: e36, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pravenec M, Kazdova L, Landa V, Zidek V, Mlejnek P, Jansa P, Wang J, Qi N, Kurtz TW. Transgenic and recombinant resistin impair skeletal muscle glucose metabolism in the spontaneously hypertensive rat. J Biol Chem 278: 45209–45215, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Pravenec M, Kazdova L, Cahova M, Landa V, Zidek V, Mlejnek P, Simakova M, Wang J, Qi N, Kurtz TW. Fat-specific transgenic expression of resistin in the spontaneously hypertensive rat impairs fatty acid re-esterification. Int J Obes (Lond) 30: 1157–1159, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest 119: 531–539, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi NR, Wang J, Zidek V, Landa V, Mlejnek P, Kazdova L, Pravenec M, Kurtz TW. A new transgenic rat model of hepatic steatosis and the metabolic syndrome. Hypertension 45: 1004–1011, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Rajala MW, Obici S, Scherer PE, Rossetti L. Adipose-derived resistin and gut-derived resistin-like molecule-beta selectively impair insulin action on glucose production. J Clin Invest 111: 225–230, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rangwala SM, Rich AS, Rhoades B, Shapiro JS, Obici S, Rossetti L, Lazar MA. Abnormal glucose homeostasis due to chronic hyperresistinemia. Diabetes 53: 1937–1941, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Rea R, Donnelly R. Resistin: an adipocyte-derived hormone. Has it a role in diabetes and obesity? Diab Obes Metab 6: 163–170, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Satoh H, Nguyen MT, Miles PD, Imamura T, Usui I, Olefsky JM. Adenovirus-mediated chronic “hyper-resistinemia” leads to in vivo insulin resistance in normal rats. J Clin Invest 114: 224–231, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafrir E, Raz I. Diabetes: mellitus or lipidus? Diabetologia 46: 433–440, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature 409: 307–312, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Tomaru T, Steger DJ, Lefterova MI, Schupp M, Lazar MA. Adipocyte-specific expression of murine resistin is mediated by synergism between peroxisome proliferator-activated receptor gamma and CCAAT/enhancer-binding proteins. J Biol Chem 284: 6116–6125, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigouroux C, Bourut C, Guerci B, Ziegler O, Magre J, Capeau J, Meyer L. A new missense mutation in the calcium-sensing receptor in familial benign hypercalcaemia associated with partial lipoatrophy and insulin resistant diabetes. Clin Endocrinol (Oxf) 53: 393–398, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Vrana A, Fabry P, Kazdova L. Effect of dietary fructose on fatty acid synthesis in adipose tissue and on triglyceride concentration in blood in the rat. Nutr Metab 15: 305–313, 1973. [DOI] [PubMed] [Google Scholar]
- 35.Vrána A, Poledne R, Fábry P, Kazdová L. Palmitate and glucose oxidation by diaphragm of rats with fructose-induced hypertriglyceridemia. Metabolism 27: 885–888, 1978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.