Abstract
Pulmonary arterial hypertension (PAH) is up to threefold more prevalent in women than men. Female mice overexpressing the serotonin transporter (SERT; SERT+ mice) exhibit PAH and exaggerated hypoxia-induced PAH, whereas male SERT+ mice remain unaffected. To further investigate these sex differences, microarray analysis was performed in the pulmonary arteries of normoxic and chronically hypoxic female and male SERT+ mice. Quantitative RT-PCR analysis was employed for validation of the microarray data. In relevant groups, immunoblotting was performed for genes of interest (CEBPβ, CYP1B1, and FOS). To translate clinical relevance to our findings, CEBPβ, CYP1B1, and FOS mRNA and protein expression was assessed in pulmonary artery smooth muscle cells (PASMCs) derived from idiopathic PAH (IPAH) patients and controls. In female SERT+ mice, multiple pathways with relevance to PAH were altered. This was also observed in chronically hypoxic female SERT+ mice. We selected 10 genes of interest for qRT-PCR analysis (FOS, CEBPβ, CYP1B1, MYL3, HAMP2, LTF, PLN, NPPA, UCP1, and C1S), and 100% concordance was reported. Protein expression of three selected genes, CEBPβ, CYP1B1, FOS, was also upregulated in female SERT+ mice. Serotonin and 17β-estradiol increased CEBPβ, CYP1B1, and FOS protein expression in PASMCs. In addition, CEBPβ, CYP1B1, and FOS mRNA and protein expression was also increased in PASMCs derived from IPAH patients. Here, we have identified a number of genes that may predispose female SERT+ mice to PAH, and these findings may also be relevant to human PAH.
Keywords: pulmonary arterial hypertension, estrogen, CCAAT enhancer binding protein, cytochrome P450 1B1, c-FOS
pulmonary arterial hypertension (PAH) is characterized by both remodeling and vasoconstriction of the pulmonary vasculature. Mutations in the gene encoding for the bone morphogenetic protein receptor type-2 (BMPR-II) are the predominant genetic cause of heritable PAH (HPAH) (27). BMPR-II mutations have been described in up to 80% of HPAH and 20% of idiopathic PAH (IPAH) patients. Despite this, penetrance is relatively low in BMPR-II mutation carriers as <20% of those actually develop PAH (26). Therefore, it is recognized that “second hit” risk factors contribute to disease pathogenesis.
A sex bias exists for both HPAH and IPAH, with females up to threefold more likely to present with disease (16, 28, 43). Despite this, the underlying reasons for these sex differences remain obscure. Estrogens are one possible risk factor in PAH. The use of oral contraceptives has been associated with the development of PAH (23). Genotyping studies have also revealed alterations in estrogen signaling in PAH. For example, female PAH patients exhibit increased expression levels of estrogen receptor-1, which is the gene encoding for estrogen receptor alpha, compared with unaffected females (30). Decreased expression of the estrogen-metabolizing enzyme cytochrome P450 1B1 (CYP1B1), leading to impaired estrogen metabolism, has also been described in female BMPR-II mutated PAH patients compared with unaffected female BMPR-II carriers (2).
Multiple studies have implicated serotonin in the pathobiology of PAH. In mice, peripheral serotonin synthesis is required for the development of both hypoxia-induced PAH (25) and dexfenfluramine-induced PAH (5), whereas the exogenous administration of serotonin uncovers a PAH phenotype in BMPR-II mutant mice (20). Mice overexpressing the SERT (SERT+ mice) also develop PAH and exaggerated hypoxia-induced PAH (21). Consistent with this, mice with targeted SERT overexpression in the PASMCs under the guidance of its own SM22 promoter also develop PAH and severe hypoxia-induced PAH (8). SERT expression is increased in human pulmonary artery smooth muscle cells (PASMCs) derived from IPAH patients, and this increased expression mediates enhanced serotonin-induced proliferation in these cells (6). Taken together, this evidence highlights the critical role of smooth muscle-SERT in mediating serotonin effects in experimental and human PAH.
In this study, we investigated genotypic differences in the development of PAH in SERT+ mice. Female SERT+ mice develop PAH and exaggerated hypoxia-induced PAH, whereas male SERT+ mice remain unaffected compared with their respective wild-type (WT) controls. This was only apparent at 5 mo of age. This experimental model of PAH is the first to exhibit female susceptibility and may provide insight into the female bias observed in human PAH. To investigate genotypic changes associated with the development and progression of PAH, microarray analysis was performed in the pulmonary arteries of 2 mo old SERT+ mice. Genes of interest were further assessed via quantitative RT-PCR and immunoblotting. With relevance to human PAH, this was also investigated in human PASMCs.
METHODS
Ethical information.
All animal procedures conform with the United Kingdom Animal Procedures Act (1986) and with the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996). Animal approval was granted by the University Committee Board. Experimental procedures using human PASMCs conform to the principles outlined in the Declaration of Helsinki.
SERT+ mice.
Female and male SERT+ mice (2 and 5 mo of age) were generated as previously described (21). Where appropriate, mice were exposed to 14 days of hypobaric hypoxia (equivalent to 10% O2). Age-matched C57B/6J×CBA littermate mice were studied as controls.
Hemodynamic measurements.
Heart rate, right ventricular pressure, and systemic arterial pressure were measured and analyzed as previously described (17). Briefly, right ventricular pressure was measured via transdiaphragmatic right heart catheterization and systemic arterial pressure was measured via cannulation of the left common carotid artery. Hemodynamic measurements from six to eight mice for each group were assessed.
Lung histology.
Sagittal sections of lung were elastica-Van Gieson stained and microscopically assessed for the muscularization of pulmonary arteries (<80 μm external diameter) in a blinded fashion as previously described (17). Remodeled arteries were confirmed by the presence of a double elastic laminae. Lung sections from five mice for each group were studied. Approximately 150 arteries from each lung section (∼750 arteries in total for each group) were assessed.
Right ventricular hypertrophy.
Right ventricular hypertrophy (RVH) was assessed by weight measurement of the right ventricular free wall (RV) and left ventricle plus septum (LV+S). The ratio expressed is RV/LV+S. RVH measurements from six to eight mice for each group were assessed.
Human PASMCs.
Human PASMCs were provided by Prof N. W. Morrell (University of Cambridge, Cambridge, UK). Briefly, PASMCs were derived from the pulmonary arteries (1–3 mm internal diameter) of three nonfamilial IPAH patients. PASMCs derived from macroscopically normal lung biopsies (pulmonary arteries, 1–3 mm internal diameter) excised from non-PAH donors were studied as control. The homogeneity of PASMCs was confirmed via cell morphology and positive staining for α-smooth muscle actin. PASMCs (passage 3–5) were seeded in six-well plates at a density of 20,000 per/well and grown to 80% confluence in Dulbecco's modified eagle medium (GIBCO) supplemented with 10% fetal bovine serum (Sera Laboratories International) prior to quiescence for 24 h. Where appropriate, PASMCs were stimulated with 1 μmol/l serotonin or 1 nmol/l 17β-estradiol for 24 h. Cell lysates were prepared for immunoblotting as previously described (5). All experiments were performed in triplicate.
Microarray analysis.
Microarray analysis was performed in the pulmonary arteries of female and male WT and SERT+ mice at 2 mo of age. To investigate the development and progression of PAH in SERT+ mice we assessed genotypic differences at 2 mo of age, where no PAH phenotype was reported. One advantage to this is the exclusion of possible compensatory gene expression changes associated with end-stage PAH, which may be apparent in SERT+ mice at 5 mo of age where a PAH phenotype is reported. This was also repeated in mice following exposure to chronic hypoxia. For the purpose of biological replicates, pulmonary arteries from individual mice were subject to microarray analysis (n = 4 each group, total 32 microarrays, Accession number E-MTAB-455). Total RNA was extracted from the main, left, and right pulmonary arteries using the TissueLyser and RNeasy Fibrous Mini Kit (Qiagen). RNA was subjected to an additional DNase purification step to eliminate genomic DNA contamination (RNase-free DNase, Qiagen). RNA integrity and quantification were assessed using the NanoDrop ND-1000 Spectrophotometer (Nano-Drop Technologies, Wilmington, DE) and Agilent 2100 Bioanalyzer system (Agilent Technologies). Absorbance of the RNA samples was quantified at 260 and 280 nm, and the 260/280 ratio was calculated. All samples showed a 260/280 ratio ≥ 1.9 and RNA integrity number ≥ 8.0, which was indicative of RNA purity. Complementary RNA (cRNA) was synthesized from RNA using the Illumina TotalPrep RNA Amplification Kit (Applied Biosystems). Briefly, cDNA was synthesized from 200 ng RNA with a T7 oligo(dT) RNA polymerase promoter. After second-strand synthesis, in vitro transcription was performed resulting in the synthesis of biotin-labeled antisense cRNA. For microarray analysis, 750 ng cRNA was hybridized to the Illumina MouseRef-8 v1.1 Expression BeadChip using the Whole-Genome Expression Direct Hybridization Kit and scanning performed with a BeadStation 500GX (Illumina). Of the total probe-sets (∼25,600) in each microarray, at least 24,613 were detected in each of the 32 arrays performed. Data were analyzed with BeadStudio software (Illumina). Hybridization signal strength was normalized to the median array and expression levels determined using the Average Normalization Beadstudio algorithm. For identifying differentially expressed genes, the following parameters recommended by Illumina were used: P value <0.05, diff. score >15, average signal >100.
Quantitative real-time PCR.
Quantitative real-time PCR (qRT-PCR) was employed for validation of the microarray data. Gene-specific primers corresponding to the PCR targets were designed based on published sequences in GenBank using the primer 3 program and synthesized by IDT (Integrated DNA Technologies; supplementary table).1 We confirmed the absence of nonspecific amplification by examining PCR products by agarose gel electrophoresis, ensuring amplification of single discrete bands with no primer-dimers. Real-time PCR was carried out in a DNA Engine OPTICON2 (MJ Research). Each reaction was performed according to the Brilliant II SYBRGreen PCR Master Mix (Agilent Technologies) protocol using 10 ng of RNA. Three replicates were performed for each sample plus template-free samples as negative controls. Cycling parameters consisted of an initial reverse transcription step for 30 min at 50°C, followed by a 10 min incubation at 95°C to fully activate the DNA polymerase and 40 amplification cycles at 95°C for 30 s, 56°C/58°C for 30 or 40 s, and 72°C for 30 s. Fluorescence measurements were assessed at the end of the annealing phase at 78, 82, and 86°C. The CT values were determined using the Opticon2 software, and the total amount of RNA was normalized against β-actin. Data are expressed as fold-change.
Western blotting.
Immediately following death, the pulmonary arteries were snap-frozen in liquid N2 and stored at −80°C until use. To obtain a sufficient concentration of protein for analysis, arteries from four mice were suspended in 250 μl lysis buffer (50 mmol/l Tris pH 7.4, 1 mmol/l DTT, 1× complete-protease inhibitor tablet; Roche Diagnostics, West Sussex, UK) and homogenized using a microrotary blade.
Human PASMCs (passage 3–5) were derived from both IPAH patients and control subjects and solubilized in RIPA buffer. Immunoblotting was performed as previously described (5). Experiments were repeated in triplicate, and α-tubulin was used for protein loading control. Densitometrical analysis was performed using TotalLab TL100 software. Data are expressed as the ratio of protein density to α-tubulin density.
Statistical analysis.
Data were analyzed by a two-way ANOVA followed by Bonferroni's post hoc test, one-way ANOVA followed by Dunnett's post hoc test or unpaired t-test as appropriate. Data are expressed as means ± SE.
RESULTS
Assessment of PAH.
In normoxia, right ventricular systolic pressure (RVSP), pulmonary vascular remodeling (PVR, % remodeled vessels), and right ventricular hypertrophy (RVH) were similar in 2 mo old male and female WT and SERT+ mice. However, at 5 mo of age female SERT+ mice exhibited PAH, as assessed by increased RVSP and RVP (Fig. 1), whereas male SERT+ mice remained unaffected (Fig. 2). Following exposure to chronic hypoxia, all groups developed hypoxia-induced PAH as assessed by significant increases in RVSP, PVR, and RVH. However, at 2 mo of age, chronically hypoxic female SERT+ mice exhibited increased RVH compared with WT mice. Similarly, at 5 mo of age these mice exhibited increased RVSP, PVR and RVH compared with 5 mo WT mice. Exaggerated hypoxia-induced PAH was not apparent in male SERT+ mice at 2 or 5 mo of age. There were no systemic effects reported in both female and male normoxic and chronically hypoxic SERT+ mice compared with WT mice, as assessed by no changes in systemic arterial pressure or heart rate (data not shown).
Fig. 1.
Right ventricular systolic pressure (RVSP, n = 6–8; A), pulmonary vascular remodeling (PVR, n = 5; B), and right ventricular hypertrophy (RVH, n = 6–8; C) measurements in female wild-type (WT) and SERT+ mice at 2 (2M) and 5 mo (5M) of age, in both normoxic and chronic hypoxia. In normoxia, no pulmonary arterial hypertension (PAH) phenotype was observed in 2 mo female SERT+ mice, however, was apparent at 5 mo of age, as assessed by increased RVSP and PVR. Hypoxia-induced elevations of RVSP, PVR and RVH were observed in all groups; 5 mo old SERT+ mice exhibit increased RVSP, PVR, and RVH. Data are expressed as means ± SE and analyzed by 2-way ANOVA followed by Bonferroni's post hoc test. *P < 0.05, **P < 0.01, cf. normoxic mice; §P < 0.05, §§P < 0.01, cf. WT mice.
Fig. 2.
RVSP (n = 6–8, A), PVR(n = 5, B), and RVH (n = 6–8, C) measurements in male WT and SERT+ mice at 2 (2M) and 5 mo (5M) of age, in both normoxic and chronic hypoxia. In both normoxia and chronic hypoxia, 2 and 5 mo old male SERT+ mice exhibit similar RVSP, PVR, and RVH compared with their respective WT controls. Data are expressed as means ± SE and analyzed by 2-way ANOVA followed by Bonferroni's post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001 cf. normoxic mice.
Genotypic differences in SERT+ mice.
We were interested in exploring the genotypic differences associated with the development and progression of PAH in female SERT+ mice. In total, we identified a total of 155 genes that were significantly (P < 0.05) differentially expressed in female SERT+ mice compared with their WT controls; 71 genes show increased expression (Table 1), while the remaining 84 genes show reduced expression (Table 2). To determine their biological relevance, we functionally categorized these genes by biological processes. A considerable number of these genes (>40%) were assigned to one or more biological processes, of which 15 categories were present in total (Fig. 3). Specifically, a large number of these genes were assigned to biological functions with relevance to PAH. These included oxidation-reduction, cell differentiation, regulation of transcription, apoptosis, muscle contraction, cellular calcium ion homeostasis, and glycolysis.
Table 1.
List of genes upregulated in the pulmonary arteries of 2 mo old female SERT+ mice compared with 2 mo old female wild-type mice, arranged by biological process
| Gene Symbol | Gene Name | Accession No. | Fold Change | False Discovery Rate |
|---|---|---|---|---|
| Oxidation Reduction | ||||
| CYP2S1 | cytochrome P450, family 2, subfamily s, polypeptide 1 | NM_028775.2 | 1.52 | 0.046 |
| SCD1 | stearoyl-Coenzyme A desaturase 1 | scl52445.7_23 | 2.51 | 0.009 |
| FASN | fatty acid synthase | scl014104.1_1 | 1.70 | 0.044 |
| ALDH1A7 | aldehyde dehydrogenase family 1, subfamily A7 | scl52665.13.1_14 | 2.20 | 0.018 |
| GPD1 | glycerol-3-phosphate dehydrogenase 1 | NM_010271.2 | 2.74 | 0.010 |
| CYP1B1 | cytochrome P450, family 1, subfamily b, polypeptide 1 | scl49594.5.189_22 | 1.54 | 0.037 |
| Cell Differentiation | ||||
| CEBPB | CCAAT/enhancer binding protein | NM_009883.1 | 1.50 | 0.049 |
| LGALS3 | lectin, galactose binding, soluble 3 | NM_010705.1 | 1.76 | 0.022 |
| DMKN | dermokine | scl32804.19.1_0 | 1.92 | 0.017 |
| Regulation of Transcription | ||||
| CEBPB | CCAAT/enhancer binding protein | NM_009883.1 | 1.50 | 0.049 |
| FOS | FBJ osteosarcoma oncogene | NM_010234.2 | 2.79 | 0.017 |
| Xbp1 | X-box binding protein 1 | NM_013842.2 | 1.56 | 0.029 |
| HOXA4 | homeo box A4 | NM_008265.2 | 2.02 | 0.018 |
| HOXB5 | homeo box B5 | NM_008268.1 | 1.90 | 0.015 |
| AXUD1 | AXIN1 upregulated 1 | scl35215.8_496 | 1.87 | 0.017 |
| Immune Response | ||||
| CFD | complement factor D | NM_013459.1 | 1.86 | 0.045 |
| SPON2 | spondin 2, extracellular matrix protein | NM_133903.2 | 1.76 | 0.017 |
| Apoptosis | ||||
| CIDEC | cell death-inducing DFFA-like effector c | NM_178373.2 | 2.91 | 0.014 |
| SRGN | Serglycin | scl019073.1_109 | 1.62 | 0.021 |
| AXUD1 | AXIN1 upregulated 1 | scl35215.8_496 | 1.87 | 0.017 |
| Metabolic Process | ||||
| UGT1A10 | UDP glycosyltransferase 1 family, polypeptide A10 | scl0394435.7_126 | 1.66 | 0.046 |
| ACLY | ATP citrate lyase | NM_134037.2 | 1.68 | 0.044 |
| FASN | fatty acid synthase | scl014104.1_1 | 1.70 | 0.044 |
| ALDH1A7 | aldehyde dehydrogenase family 1, subfamily A7 | scl52665.13.1_14 | 2.20 | 0.018 |
| AACS | acetoacetyl-CoA synthetase | NM_030210.1 | 1.94 | 0.026 |
| GPD1 | glycerol-3-phosphate dehydrogenase 1 | NM_010271.2 | 2.74 | 0.010 |
| UAP1 | UDP-N-acetylglucosamine pyrophosphorylase 1 | NM_133806.2 | 1.74 | 0.026 |
| Lipid Metabolic Process | ||||
| SCD1 | stearoyl-Coenzyme A desaturase 1 | NM_011182.2 | 2.51 | 0.009 |
| AACS | acetoacetyl-CoA synthetase | NM_030210.1 | 1.94 | 0.026 |
| Lipid Biosynthetic Process | ||||
| SCD1 | stearoyl-Coenzyme A desaturase 1 | scl52445.7_23 | 2.51 | 0.009 |
| ACLY | ATP citrate lyase | NM_134037.2 | 1.68 | 0.044 |
| FASN | fatty acid synthase | scl014104.1_1 | 1.70 | 0.044 |
| ELOVL6 | ELOVL family member 6 | scl00170439.1_29 | 2.16 | 0.014 |
| Brown Fat Cell Differentiation | ||||
| SCD1 | stearoyl-Coenzyme A desaturase 1 | scl52445.7_23 | 2.51 | 0.009 |
| ADIPOQ | adiponectin, C1Q and collagen domain containing | scl49310.3_131 | 2.66 | 0.017 |
| UCP1 | uncoupling protein 1 | NM_009463.2 | 15.14 | 0.000 |
| CEBPB | CCAAT/enhancer binding protein | NM_009883.1 | 1.50 | 0.049 |
| BC054059 | cDNA sequence BC054059 | scl19994.5.1_11 | 2.54 | 0.017 |
| Glycolysis | ||||
| ENO2 | enolase 2, gamma neuronal | NM_013509.2 | 1.61 | 0.045 |
Table 2.
List of genes downregulated in the pulmonary arteries of 2 mo old female SERT+ mice compared with 2 mo old female wild-type mice, arranged by biological process
| Gene Symbol | Gene Name | Accession No. | Fold Change | False Discovery Rate |
|---|---|---|---|---|
| Oxidation Reduction | ||||
| SC4MOL | sterol-C4-methyl oxidase-like | NM_025436.1 | 2.09 | 0.034 |
| PRDX2 | peroxiredoxin 2 | NM_011563.2 | 2.89 | 0.017 |
| Cell Differentiation | ||||
| TRIM54 | tripartite motif-containing 54 | NM_021447.1 | 2.58 | 0.026 |
| OBSCN | obscurin, cytoskeletal calmodulin and titin-interacting RhoGEF | scl40175.7.1_79 | 2.13 | 0.042 |
| CSRP3 | cysteine and glycine-rich protein 3 | NM_013808.3 | 5.14 | 0.003 |
| Regulation of Transcription | ||||
| TBX20 | T-box 20 | NM_194263.1 | 2.06 | 0.041 |
| Immune Response | ||||
| PRG4 | proteoglycan 4 | scl000882.1_25 | 2.57 | 0.019 |
| Apoptosis | ||||
| ACTC1 | actin, alpha, cardiac | NM_009608.1 | 2.17 | 0.037 |
| COMP | cartilage oligomeric matrix protein | scl33728.21.1_0 | 4.73 | 0.011 |
| Metabolic Process | ||||
| PGAM2 | phosphoglycerate mutase 2 | scl40555.3.1_120 | 8.59 | 0.003 |
| Lipid Metabolic Process | ||||
| CPT1B | carnitine palmitoyltransferase 1b, muscle | NM_009948.1 | 2.81 | 0.022 |
| LPL | lipoprotein lipase | scl0016956.1_234 | 1.92 | 0.047 |
| Heart Development | ||||
| MB | myoglobin | NM_013593.2 | 22.71 | 0.000 |
| TNNI3 | troponin I, cardiac | NM_009406.2 | 6.86 | 0.003 |
| MYL2 | myosin, light polypeptide 2, regulatory, cardiac, slow | scl27267.9.1_12 | 70.40 | 0.000 |
| TNNT2 | troponin T2, cardiac | NM_011619.1 | 3.18 | 0.015 |
| Muscle Contraction | ||||
| MYBPC3 | myosin binding protein C, cardiac | NM_008653.1 | 3.34 | 0.014 |
| ACTN2 | actinin alpha 2 | NM_016798.2 | 9.71 | 0.003 |
| TBX20 | T-box 20 | NM_194263.1 | 2.06 | 0.041 |
| MYOM2 | myomesin 2 | scl34033.37.1_91 | 2.40 | 0.028 |
| TTN | titin | scl19104.8.1_3 | 6.48 | 0.003 |
| TNNT2 | troponin T2, cardiac | NM_011619.1 | 3.18 | 0.015 |
| Lipid Biosynthetic Process | ||||
| SC4MOL | sterol-C4-methyl oxidase-like | NM_025436.1 | 2.09 | 0.034 |
| Cellular Calcium Ion Homeostasis | ||||
| PLN | phospholamban | scl38924.3_494 | 6.93 | 0.003 |
| TNNI3 | troponin I, cardiac | NM_009406.2 | 6.86 | 0.003 |
| CSRP3 | cysteine and glycine-rich protein 3 | NM_013808.3 | 5.14 | 0.003 |
| Brown Fat Cell Differentiation | ||||
| MB | myoglobin | NM_013593.2 | 22.71 | 0.000 |
| Glycolysis | ||||
| ENO3 | enolase 3, beta muscle | NM_007933.2 | 3.82 | 0.010 |
| PGAM2 | phosphoglycerate mutase 2 | scl40555.3.1_120 | 8.59 | 0.003 |
| Sarcomere Organization | ||||
| MYBPC3 | myosin binding protein C, cardiac | NM_008653.1 | 3.34 | 0.014 |
| MYH6 | myosin, heavy polypeptide 6, cardiac muscle, alpha | scl46291.1.1_325 | 2.60 | 0.018 |
| TTN | Titin | scl19104.8.1_3 | 6.48 | 0.003 |
| TNNT2 | troponin T2, cardiac | NM_011619.1 | 3.18 | 0.015 |
| Regulation of Heart Contraction | ||||
| MYBPC3 | myosin binding protein C, cardiac | NM_008653.1 | 3.34 | 0.014 |
| HRC | histidine rich calcium binding protein | NM_010473.1 | 3.71 | 0.011 |
| MYH6 | myosin, heavy polypeptide 6, cardiac muscle, alpha | scl46291.1.1_325 | 2.60 | 0.018 |
| TNNT2 | troponin T2, cardiac | NM_011619.1 | 3.18 | 0.015 |
Fig. 3.
Hierarchical cluster analysis of the differentially expressed genes in female and male WT and SERT+ mice (A). Representation of the differentially expressed genes in female SERT+ mice (B) and male SERT+ mice (C), arranged by biological processes.
To further investigate the genotypic changes underlying these sex differences in SERT+ mice, we also performed microarray analysis in the pulmonary arteries of male SERT+ mice. We observed that a total of 148 genes were significantly differentially expressed in male SERT+ mice compared with male WT mice. Of these, 110 genes were increased (Table 3), whereas the remaining 38 genes were decreased (Table 4). When categorized by biological processes, only 25% of these genes were assigned to biological function, and nine categories were represented in total.
Table 3.
List of genes upregulated in the pulmonary arteries of 2 mo old male SERT+ mice compared with 2 mo old male wild-type mice, arranged by biological process
| Gene Symbol | Gene Name | Accession No. | Fold Change | False Discovery Rate |
|---|---|---|---|---|
| Transport | ||||
| SCNN1G | sodium channel, nonvoltage-gated 1 gamma | scl32105.12.878_39 | 1.90 | 0.029 |
| CLIC6 | chloride intracellular channel 6 | NM_172469.1 | 2.28 | 0.009 |
| SLC39A4 | solute carrier family 39 | NM_028064.2 | 2.04 | 0.021 |
| RAB25 | RAB25, member RAS oncogene family | scl21969.5.1_66 | 2.04 | 0.021 |
| GABRP | gamma-aminobutyric acid | scl0001520.1_108 | 2.83 | 0.002 |
| Ltf | lactotransferrin | NM_008522.2 | 11.43 | 0.000 |
| Transport: Oxygen Transport | ||||
| MB | myoglobin | NM_013593.2 | 2.74 | 0.000 |
| HBB-B2 | hemoglobin, beta adult minor chain | NM_016956.2 | 2.35 | 0.000 |
| Signal Transduction | ||||
| FCER1G | Fc receptor, IgE, high affinity I, gamma polypeptide | scl15940.5.1_15 | 2.68 | 0.002 |
| GRB7 | growth factor receptor bound protein 7 | scl40936.14_9 | 1.86 | 0.021 |
| Cell Adhesion | ||||
| MUC4 | mucin 4 | scl0140474.33_123 | 1.82 | 0.031 |
| WISP2 | WNT1 inducible signaling pathway protein 2 | NM_016873.1 | 1.92 | 0.010 |
| SPON2 | spondin 2, extracellular matrix protein | NM_133903.2 | 1.54 | 0.045 |
| Oxidation Reduction | ||||
| CYP2E1 | cytochrome P450, family 2, subfamily e, polypeptide 1 | NM_021282.1 | 1.98 | 0.008 |
| CYP2F2 | cytochrome P450, family 2, subfamily f, polypeptide 2 | scl32906.13.1_13 | 12.08 | 0.000 |
| CYP2A5 | cytochrome P450, family 2, subfamily a, polypeptide 5 | NM_009997.1 | 3.32 | 0.004 |
| CYP4A12B | cytochrome P450, family 4, subfamily a, polypeptide 12B | scl013118.12_302 | 4.46 | 0.001 |
| ABP1 | amiloride binding protein 1 | NM_029638.1 | 1.62 | 0.044 |
| GPX2 | glutathione peroxidase 2 | NM_030677.1 | 2.20 | 0.004 |
| ALDH3A1 | aldehyde dehydrogenase family 3, subfamily A1 | NM_007436.1 | 2.10 | 0.013 |
| ALDH1A1 | aldehyde dehydrogenase family 1, subfamily A1 | scl011668.12_94 | 2.55 | 0.027 |
| PRDX2 | peroxiredoxin 2 | NM_011563.2 | 2.25 | 0.012 |
| Immune Response | ||||
| PGLYRP1 | peptidoglycan recognition protein 1 | scl33021.4.288_87 | 2.08 | 0.035 |
| CXCL15 | chemokine | scl27600.3.1_4 | 5.76 | 0.000 |
| SPON2 | spondin 2, extracellular matrix protein | NM_133903.2 | 1.54 | 0.045 |
| Metabolic Process | ||||
| ALDH3A1 | aldehyde dehydrogenase family 3, subfamily A1 | NM_007436.1 | 2.10 | 0.013 |
| ALDH1A1 | aldehyde dehydrogenase family 1, subfamily A1 | scl011668.12_94 | 2.55 | 0.027 |
| GSTA3 | glutathione S-transferase, alpha 3 | scl18127.10.1_92 | 2.14 | 0.014 |
| GSTO1 | glutathione S-transferase omega 1 | NM_010362.1 | 2.92 | 0.004 |
| Hemopoiesis | ||||
| CXCL15 | chemokine | scl27600.3.1_4 | 5.76 | 0.000 |
| Regulation of Transcription | ||||
| IRX5 | Iroquois related homeobox 5 | NM_018826.2 | 1.94 | 0.025 |
| OTX1 | orthodenticle homolog 1 | scl40460.6_595 | 1.72 | 0.038 |
| IRX3 | Iroquois related homeobox 3 | scl34499.5.1_0 | 1.69 | 0.024 |
| FOXA1 | forkhead box A1 | scl42430.2_236 | 1.84 | 0.015 |
Table 4.
List of genes downregulated in the pulmonary arteries of 2 mo old male SERT+ mice compared with 2 mo old male wild-type mice, arranged by biological process
| Gene Symbol | Gene Name | Accession No. | Fold Change | False Discovery Rate |
|---|---|---|---|---|
| Transport: Oxygen Transport | ||||
| HBB-B1 | hemoglobin, beta adult major chain | NM_008220.2 | 2.41 | 0.019 |
| Signal Transduction | ||||
| LGR6 | leucine-rich repeat-containing G protein-coupled receptor 6 | scl00329252.1_132 | 1.86 | 0.011 |
| Oxidation Reduction | ||||
| JARID1B | lysine (K)-specific demethylase 5B | scl17448.26_107 | 1.65 | 0.032 |
| SC4MOL | sterol-C4-methyl oxidase-like | NM_025436.1 | 2.25 | 0.003 |
| Hemopoiesis | ||||
| PICALM | phosphatidylinositol binding clathrin assembly protein | scl32408.23_56 | 3.80 | 0.001 |
| HBB-B1 | hemoglobin, beta adult major chain | NM_008220.2 | 2.41 | 0.019 |
| Regulation of Transcription | ||||
| TEF | thyrotroph embryonic factor | scl0002562.1_0 | 1.82 | 0.022 |
| DBP | D site albumin promoter binding protein | NM_016974.1 | 2.45 | 0.002 |
| PER2 | period homolog 2 | NM_011066.1 | 1.89 | 0.013 |
Hierarchal cluster analysis between the four normoxic groups (258 genes in total) revealed distinct gene expression patterns between female SERT+ and female WT that were not apparent in the identical male SERT+ and WT comparison.
Genotypic differences in hypoxic SERT+ mice.
We were also interested in investigating the genotypic differences associated with exaggerated hypoxia-induced PAH in female SERT+ mice. Following exposure to chronic hypoxia, female SERT+ mice exhibited a greater than twofold increase in the number of differentially expressed genes compared against the identical normoxic comparison. In total, 316 genes were differentially expressed. We observed that 254 genes were increased (Table 5), while the remaining 62 genes showed decreased expression (Table 6). When arranged by biological processes, 53% of genes were assigned to a total of 26 distinct pathways. Moreover, a significant number of these dysregulated pathways observed in chronically hypoxic female SERT+ mice have been previously associated with PAH including apoptosis, inflammation, transcription, and metabolism (Fig. 4).
Table 5.
List of genes upregulated in the pulmonary arteries of 2 mo old hypoxic female SERT+ mice compared with 2 mo old hypoxic female wild-type mice, arranged by biological process
| Gene Symbol | Gene Name | Accession No. | Fold Change | False Discovery Rate |
|---|---|---|---|---|
| Apoptosis | ||||
| PGLYRP1 | peptidoglycan recognition protein 1 | scl33021.4.288_87 | 3.30 | 0.000 |
| CIDEC | cell death-inducing DFFA-like effector c | NM_178373.2 | 1.84 | 0.000 |
| SRGN | serglycin | scl019073.1_109 | 1.32 | 0.048 |
| KRT8 | keratin 8 | scl0016691.1_37 | 3.13 | 0.000 |
| CIDEA | cell death-inducing DNA fragmentation factor, alpha subunit-like effector A | NM_007702.1 | 2.38 | 0.000 |
| Induction of Apoptosis | ||||
| CEBPB | CCAAT/enhancer binding protein | NM_009883.1 | 1.67 | 0.004 |
| CIDEC | cell death-inducing DFFA-like effector c | NM_178373.2 | 1.84 | 0.000 |
| ERN2 | endoplasmic reticulum | scl30713.22.1_242 | 2.08 | 0.003 |
| Brown Fat Cell Differentiation | ||||
| SCD1 | stearoyl-Coenzyme A desaturase 1 | scl52445.7_23 | 1.97 | 0.000 |
| ADIPOQ | adiponectin, C1Q and collagen domain containing | scl49310.3_131 | 1.87 | 0.025 |
| UCP1 | uncoupling protein 1 | NM_009463.2 | 2.60 | 0.000 |
| CEBPB | CCAAT/enhancer binding protein | NM_009883.1 | 1.67 | 0.004 |
| BC054059 | cDNA sequence BC054059 | scl19994.5.1_11 | 1.86 | 0.004 |
| NUDT7 | nudix NM_024446.2 1.57 | |||
| MRAP | melanocortin 2 receptor accessory protein | NM_029844.1 | 1.96 | 0.001 |
| ALDH6A1 | aldehyde dehydrogenase family 6, subfamily A1 | NM_134042.1 | 1.65 | 0.021 |
| PPARG | peroxisome proliferator activated receptor gamma | NM_011146.1 | 1.75 | 0.000 |
| Carbohydrate Metabolic Process | ||||
| AMY1 | amylase 1, salivary scl077379.3_13 1.66 | |||
| PDK4 | pyruvate dehydrogenase kinase, isoenzyme 4 | scl29310.11_209 | 2.95 | 0.005 |
| PYGL | liver glycogen phosphorylase | NM_133198.1 | 1.86 | 0.000 |
| GPD1 | glycerol-3-phosphate dehydrogenase 1 | NM_010271.2 | 2.00 | 0.001 |
| CHST1 | carbohydrate | NM_023850.1 | 1.42 | 0.031 |
| PPP1R3C | protein phosphatase 1, regulatory | NM_016854.1 | 1.54 | 0.025 |
| KLB | klotho beta | scl27771.5.1_89 | 1.87 | 0.005 |
| Cell Adhesion | ||||
| MYBPC2 | myosin binding protein C, fast-type | NM_146189.1 | 1.34 | 0.031 |
| LGALS3BP | lectin, galactoside-binding, soluble, 3 binding protein | scl39273.6_263 | 1.66 | 0.011 |
| 1110049B09RIK | RIKEN cDNA 1110049B09 gene | scl42544.15.6_29 | 1.39 | 0.017 |
| CDH5 | cadherin 5 | NM_009868.4 | 1.75 | 0.030 |
| CD93 | CD93 antigen | scl18542.4.1_65 | 1.99 | 0.034 |
| Cell-Cell Adhesion | ||||
| CDH5 | cadherin 5 | scl33446.12_65 | 1.75 | 0.030 |
| CD93 | CD93 antigen | scl18542.4.1_65 | 1.99 | 0.034 |
| Chemotaxis | ||||
| CYSLTR1 | cysteinyl leukotriene receptor 1 | NM_021476.2 | 1.33 | 0.041 |
| CMTM8 | CKLF-like MARVEL transmembrane domain containing 8 | NM_027294.1 | 1.34 | 0.047 |
| CXCL12 | chemokine | scl0001073.1_120 | 1.77 | 0.007 |
| Defense Response to Bacterium | ||||
| PGLYRP1 | peptidoglycan recognition protein 1 | scl33021.4.288_87 | 3.30 | 0.000 |
| HAMP2 | hepcidin antimicrobial peptide 2 | NM_183257.1 | 5.38 | 0.000 |
| FCER1G | Fc receptor, IgE, high affinity I, gamma polypeptide | scl15940.5.1_15 | 2.29 | 0.016 |
| H2-K1 | histocompatibility 2, K1, K region | scl0014972.1_210 | 1.61 | 0.016 |
| DNA Replication | ||||
| POLN | DNA polymerase N | scl0272158.1_149 | 1.69 | 0.031 |
| SUPT16H | suppressor of Ty 16 homolog | NM_033618.1 | 1.76 | 0.021 |
| POLK | polymerase | scl43651.15_178 | 1.29 | 0.047 |
| Immune Response | ||||
| PGLYRP1 | peptidoglycan recognition protein 1 | scl33021.4.288_87 | 3.30 | 0.000 |
| CXCL12 | chemokine | scl0001073.1_120 | 1.77 | 0.007 |
| CFD | complement factor D | NM_013459.1 | 1.68 | 0.007 |
| CD300LG | CD300 antigen like family member G | scl40868.8_408 | 1.72 | 0.049 |
| H2-T23 | histocompatibility 2, T region locus 23 | NM_010398.1 | 1.37 | 0.036 |
| H2-K1 | histocompatibility 2, K1, K region | scl0014972.1_210 | 1.61 | 0.016 |
| Inflammatory Response | ||||
| REG3G | regenerating islet-derived 3 gamma | NM_011260.1 | 2.97 | 0.049 |
| CHST1 | carbohydrate | NM_023850.1 | 1.42 | 0.031 |
| KNG1 | kininogen 1 | NM_023125.2 | 1.79 | 0.006 |
| PPARG | peroxisome proliferator activated receptor gamma | NM_011146.1 | 1.75 | 0.000 |
| Integrin-Mediated Signaling Pathway | ||||
| ADAM9 | a disintegrin and metallopeptidase domain 9 | NM_007404.1 | 1.39 | 0.018 |
| Lipid Biosynthetic Process | ||||
| SCD1 | stearoyl-Coenzyme A desaturase 1 | scl52445.7_23 | 1.97 | 0.000 |
| PCX | pyruvate carboxylase | scl000483.1_20 | 1.74 | 0.002 |
| ACLY | ATP citrate lyase | NM_134037.2 | 1.81 | 0.009 |
| FASN | fatty acid synthase | scl014104.1_1 | 1.97 | 0.004 |
| ELOVL6 | ELOVL family member 6, elongation of long chain fatty acids | scl00170439.1_29 | 2.86 | 0.000 |
| ELOVL5 | ELOVL family member 5, elongation of long chain fatty acids | NM_134255.2 | 1.39 | 0.031 |
| DGAT2 | diacylglycerol O-acyltransferase 2 | scl31009.10_67 | 2.20 | 0.000 |
| Lipid Metabolic Process | ||||
| HSD11B1 | hydroxysteroid 11-beta dehydrogenase 1 | scl000857.1_11 | 1.55 | 0.054 |
| SCD1 | stearoyl-Coenzyme A desaturase 1 | scl52445.7_23 | 1.97 | 0.000 |
| HADHB | hydroxyacyl-Coenzyme A dehydrogenase/3-ketoacyl-Coenzyme A thiolase/enoyl-Coenzyme A hydratase | NM_145558.1 | 1.52 | 0.001 |
| CPT1B | carnitine palmitoyltransferase 1b, muscle | NM_009948.1 | 1.94 | 0.013 |
| AACS | acetoacetyl-CoA synthetase | NM_030210.1 | 1.47 | 0.028 |
| CPT2 | carnitine palmitoyltrasferase 2 | scl000022.1_12 | 1.56 | 0.006 |
| ACADVL | acyl-Coenzyme A dehydrogenase, very long chain | scl40004.19.1_140 | 1.41 | 0.042 |
| ACAA2 | acetyl-Coenzyme A acyltransferase 2 | scl0002163.1_25 | 1.40 | 0.033 |
| ACADL | acyl-Coenzyme A dehydrogenase, long-chain | NM_007381.2 | 2.12 | 0.000 |
| ACSM3 | acyl-CoA synthetase medium-chain family member 3 | scl000249.1_5 | 1.67 | 0.010 |
| DGAT2 | diacylglycerol O-acyltransferase 2 | scl31009.10_67 | 2.20 | 0.000 |
| PNPLA2 | patatin-like phospholipase domain containing 2 | scl8719.1.1_106 | 1.79 | 0.006 |
| LPL | lipoprotein lipase | scl0016956.1_234 | 1.86 | 0.007 |
| ADIPOR2 | adiponectin receptor 2 | scl28480.7_231 | 1.50 | 0.041 |
| CIDEA | cell death-inducing DNA fragmentation factor, A alpha subunit-like effector | NM_007702.1 | 2.38 | 0.000 |
| Metabolic Process | ||||
| HSD11B1 | hydroxysteroid 11-beta dehydrogenase 1 | scl000857.1_11 | 1.55 | 0.005 |
| PCX | pyruvate carboxylase | scl000483.1_20 | 1.74 | 0.002 |
| ACLY | ATP citrate lyase | NM_134037.2 | 1.81 | 0.009 |
| FASN | fatty acid synthase | scl014104.1_1 | 1.97 | 0.004 |
| AMY1 | amylase 1, salivary | scl077379.3_13 | 1.66 | 0.005 |
| AGPAT2 | 1-acylglycerol-3-phosphate O-acyltransferase 2 | NM_026212.1 | 2.76 | 0.002 |
| ALAS2 | aminolevulinic acid synthase 2, erythroid | scl54562.12.1_64 | 1.29 | 0.034 |
| HADHB | hydroxyacyl-Coenzyme A dehydrogenase/3-ketoacyl-Coenzyme A thiolase/enoyl-Coenzyme A hydratase | NM_145558.1 | 1.52 | 0.001 |
| AACS | acetoacetyl-CoA synthetase | NM_030210.1 | 1.47 | 0.028 |
| GPD1 | glycerol-3-phosphate dehydrogenase 1 | NM_010271.2 | 2.00 | 0.000 |
| EPHX2 | epoxide hydrolase 2, cytoplasmic | scl45408.20.1_29 | 1.48 | 0.021 |
| ACO2 | aconitase 2, mitochondrial | NM_080633.1 | 1.89 | 0.000 |
| UAP1 | UDP-N-acetylglucosamine pyrophosphorylase 1 | NM_133806.2 | 3.48 | 0.000 |
| ACADVL | acyl-Coenzyme A dehydrogenase, very long chain | scl40004.19.1_140 | 1.41 | 0.042 |
| GSTA3 | glutathione S-transferase, alpha 3 | scl18127.10.1_92 | 2.43 | 0.000 |
| GSTO1 | glutathione S-transferase omega 1 | NM_010362.1 | 2.01 | 0.034 |
| ACAA2 | acetyl-Coenzyme A acyltransferase 2 | scl0002163.1_25 | 1.40 | 0.033 |
| EYA3 | eyes absent 3 homolog | NM_010166.2 | 2.10 | 0.002 |
| NAT8L | N-acetyltransferase 8-like | scl27919.3_374 | 1.47 | 0.040 |
| ALDH6A1 | aldehyde dehydrogenase family 6, subfamily A1 | NM_134042.1 | 1.65 | 0.021 |
| ACADL | acyl-Coenzyme A dehydrogenase, long-chain | NM_007381.2 | 2.12 | 0.000 |
| ACSM3 | acyl-CoA synthetase medium-chain family member 3 | scl000249.1_5 | 1.67 | 0.010 |
| ACSS1 | acyl-CoA synthetase short-chain family member 1 | NM_080575.1 | 1.47 | 0.012 |
| PNPLA2 | patatin-like phospholipase domain containing 2 | scl8719.1.1_106 | 1.79 | 0.006 |
| GSTA4 | glutathione S-transferase, alpha 4 | NM_010357.1 | 2.61 | 0.000 |
| Muscle Contraction | ||||
| MYBPC2 | myosin binding protein C, fast-type | NM_146189.1 | 1.34 | 0.031 |
| TBX20 | T-box 20 | NM_194263.1 | 1.57 | 0.034 |
| PPARG | peroxisome proliferator activated receptor gamma | NM_011146.1 | 1.75 | 0.000 |
| Oxidation Reduction | ||||
| CYP2S1 | cytochrome P450, family 2, subfamily s, polypeptide 1 | NM_028775.2 | 1.56 | 0.034 |
| HSD11B1 | hydroxysteroid 11-beta dehydrogenase 1 | scl000857.1_11 | 1.55 | 0.005 |
| SCD1 | stearoyl-Coenzyme A desaturase 1 | scl52445.7_23 | 1.97 | 0.000 |
| GPX2 | glutathione peroxidase 2 | NM_030677.1 | 1.53 | 0.045 |
| FASN | fatty acid synthase | scl014104.1_1 | 1.97 | 0.000 |
| GPX3 | glutathione peroxidase 3 | NM_008161.1 | 1.55 | 0.007 |
| CYP2E1 | cytochrome P450, family 2, subfamily e, polypeptide 1 | NM_021282.1 | 2.09 | 0.003 |
| GPD2 | glycerol phosphate dehydrogenase 2, mitochondrial | NM_010274.2 | 1.52 | 0.007 |
| ETFDH | electron transferring flavoprotein, dehydrogenase | NM_025794.1 | 1.64 | 0.047 |
| DLD | dihydrolipoamide dehydrogenase | NM_007861.2 | 1.41 | 0.046 |
| GPD1 | glycerol-3-phosphate dehydrogenase 1 | NM_010271.2 | 2.00 | 0.000 |
| ALDH3A1 | aldehyde dehydrogenase family 3, subfamily A1 | NM_007436.1 | 1.92 | 0.003 |
| ACADVL | acyl-Coenzyme A dehydrogenase, very long chain | scl40004.19.1_140 | 1.41 | 0.046 |
| CYP2F2 | cytochrome P450, family 2, subfamily f, polypeptide 2 | scl32906.13.1_13 | 8.61 | 0.000 |
| CYP2A5 | cytochrome P450, family 2, subfamily a, polypeptide 5 | NM_009997.1 | 1.85 | 0.022 |
| CYP4A12B | cytochrome P450, family 4, subfamily a, polypeptide 12B | scl013118.12_302 | 3.42 | 0.000 |
| ALDH6A1 | aldehyde dehydrogenase family 6, subfamily A1 | NM_134042.1 | 1.65 | 0.021 |
| ACADL | acyl-Coenzyme A dehydrogenase, long-chain | NM_007381.2 | 2.12 | 0.000 |
| PRDX2 | peroxiredoxin 2 | NM_011563.2 | 6.22 | 0.000 |
| Response to Toxin | ||||
| EPHX2 | epoxide hydrolase 2, cytoplasmic | scl45408.20.1_29 | 1.48 | 0.021 |
| CES3 | carboxylesterase 3 | scl34490.14.1_30 | 2.18 | 0.002 |
| CYP2F2 | cytochrome P450, family 2, subfamily f, polypeptide 2 | scl32906.13.1_13 | 8.61 | 0.000 |
| PON1 | paraoxonase 1 | NM_011134.1 | 2.09 | 0.001 |
| Signal Transduction | ||||
| RERG | RAS-like, estrogen-regulated, growth-inhibitor | NM_181988.1 | 2.02 | 0.001 |
| GPR109A | G protein-coupled receptor 109A | NM_030701.1 | 1.61 | 0.009 |
| CYSLTR1 | cysteinyl leukotriene receptor 1 | NM_021476.2 | 1.33 | 0.042 |
| ELTD1 | EGF, latrophilin seven transmembrane domain containing 1 | NM_133222.1 | 2.08 | 0.001 |
| FCER1G | Fc receptor, IgE, high affinity I, gamma polypeptide | scl15940.5.1_15 | 2.29 | 0.016 |
| Small GTPase-Mediated Signal Transduction | ||||
| KNDC1 | kinase noncatalytic C-lobe domain | scl31927.18.1_9 | 2.41 | 0.002 |
| RAB25 | RAB25, member RAS oncogene family | scl21969.5.1_66 | 1.31 | 0.047 |
| G3BP2 | GTPase activating protein | scl0023881.1_86 | 1.33 | 0.021 |
| Temperature Homeostasis | ||||
| GPX2 | glutathione peroxidase 2 | NM_030677.1 | 1.53 | 0.045 |
| ACADVL | acyl-Coenzyme A dehydrogenase, very long chain | scl40004.19.1_140 | 1.41 | 0.042 |
| ACADL | acyl-Coenzyme A dehydrogenase, long-chain | NM_007381.2 | 2.12 | 0.001 |
| CIDEA | cell death-inducing DNA fragmentation factor, alpha subunit-like effector A | NM_007702.1 | 2.38 | 0.000 |
| Transcription | ||||
| FOS | FBJ osteosarcoma oncogene | NM_010234.2 | 2.34 | 0.000 |
| SUPT16H | suppressor of Ty 16 homolog | NM_033618.1 | 1.76 | 0.021 |
| KLF5 | Kruppel-like factor 5 | scl45215.1.1_294 | 1.99 | 0.001 |
| CEBPB | CCAAT/enhancer binding protein | NM_009883.1 | 1.67 | 0.002 |
| TBX20 | T-box 20 | NM_194263.1 | 1.57 | 0.034 |
| PPARG | peroxisome proliferator activated receptor gamma | NM_011146.1 | 1.75 | 0.000 |
| ZFP367 | zinc finger protein 367 | NM_175494.2 | 1.22 | 0.031 |
| EYA3 | eyes absent 3 homolog | NM_010166.2 | 2.10 | 0.002 |
| Transport | ||||
| UCP1 | uncoupling protein 1 | NM_009463.2 | 2.60 | 0.000 |
| SLC5A6 | solute carrier family 5 | scl26744.20.688_4 | 1.38 | 0.024 |
| APOC1 | apolipoprotein C-I | NM_007469.2 | 1.95 | 0.001 |
| NDUFB4 NADH | dehydrogenase (ubiquinone) 1 beta subcomplex 4 | scl48493.1_0 | 1.34 | 0.028 |
| ETFB | electron transferring flavoprotein, beta polypeptide | NM_026695.2 | 1.29 | 0.044 |
| ETFA | electron transferring flavoprotein, alpha polypeptide | NM_145615.2 | 1.42 | 0.043 |
| ETFDH | electron transferring flavoprotein, dehydrogenase | NM_025794.1 | 1.64 | 0.047 |
| CPT1B | carnitine palmitoyltransferase 1b, muscle | NM_009948.1 | 1.94 | 0.013 |
| CPT2 | carnitine palmitoyltrasferase 2 | scl000022.1_12 | 1.56 | 0.007 |
| RAB25 | RAB25, member RAS oncogene family | scl21969.5.1_66 | 1.31 | 0.047 |
| FXYD3 | FXYD domain-containing ion transport regulator 3 | NM_008557.1 | 1.84 | 0.003 |
| GABRP | gamma-aminobutyric acid | scl0001520.1_108 | 2.35 | 0.022 |
| ATP5K | ATP synthase, H+ transporting, mitochondrial F1F0 complex, subunit e | scl011958.2_29 | 1.49 | 0.033 |
| G3BP2 | GTPase activating protein | scl0023881.1_86 | 1.33 | 0.021 |
| MFI2 | antigen p97 | scl0001844.1_62 | 1.45 | 0.014 |
| SLC25A1 | solute carrier family 25 | NM_153150.1 | 1.71 | 0.002 |
| MTCH2 | mitochondrial carrier homolog 2 | NM_019758.2 | 1.41 | 0.033 |
| Triglyceride Metabolic Process | ||||
| APOC1 | apolipoprotein C-I | NM_007469.2 | 1.95 | 0.001 |
| Other | ||||
| CISH | cytokine inducible SH2-containing protein | scl012700.3_170 | 1.60 | 0.004 |
| STMN2 | stathmin-like 2 | scl23400.7_310 | 1.65 | 0.000 |
| SOCS3 | suppressor of cytokine signaling 3 | NM_007707.2 | 1.41 | 0.022 |
| TUBA8 | tubulin, alpha 8 | scl29555.5_307 | 1.71 | 0.020 |
| DNAHC2 | dynein, axonemal, heavy chain 2 | scl40034.27.1_30 | 1.57 | 0.004 |
| SCGB1A1 | secretoglobin, family 1A, member 1 | NM_011681.1 | 9.55 | 0.000 |
| HSPA5 | heat shock 70 kDa protein 5 | NM_022310.2 | 1.75 | 0.005 |
| DUSP1 | dual specificity phosphatase 1 | NM_013642.1 | 1.64 | 0.021 |
| DUSP23 | dual specificity phosphatase 23 | NM_026725.2 | 1.45 | 0.044 |
| BMPER | BMP-binding endothelial regulator | NM_028472.1 | 1.95 | 0.004 |
Table 6.
List of genes downregulated in the pulmonary arteries of 2 mo old hypoxic female SERT+ mice compared with 2 mo old hypoxic female wild-type mice, arranged by biological process
| Gene Symbol | Gene Name | Accession No. | Fold Change | False Discovery Rate |
|---|---|---|---|---|
| Brown Fat Cell Differentiation | ||||
| MB | myoglobin | NM_013593.2 | 1.89 | 0.003 |
| Carbohydrate Metabolic Process | ||||
| IGF2 | insulin-like growth factor 2 | scl30469.7_1 | 1.82 | 0.015 |
| RPE | ribulose-5-phosphate-3-epimerase | scl0227227.1_0 | 2.13 | 0.019 |
| Cell Adhesion | ||||
| VCAN | versican | scl013003.1_89 | 1.11 | 0.025 |
| STAB1 | stabilin 1 | NM_138672.1 | 1.39 | 0.030 |
| SELP | selectin, platelet | NM_011347.1 | 1.38 | 0.021 |
| ITGA3 | integrin alpha 3 | NM_013565.2 | 1.25 | 0.017 |
| ITGB1 | integrin beta 1 | NM_010578.1 | 1.72 | 0.008 |
| Cell-Cell Adhesion | ||||
| TNXB | tenascin XB | NM_031176.1 | 1.71 | 0.021 |
| Cellular Iron Ion Homeostasis | ||||
| LTF | lactotransferrin | NM_008522.2 | 11.84 | 0.000 |
| ALAS2 | aminolevulinic acid synthase 2, erythroid | scl54562.12.1_64 | 1.29 | 0.033 |
| HAMP2 | hepcidin antimicrobial peptide 2 | NM_183257.1 | 5.38 | 0.000 |
| MFI2 | antigen p97 | scl0001844.1_62 | 1.45 | 0.014 |
| Chemotaxis | ||||
| CCL21B | chemokine | scl0018829.1_65 | 2.04 | 0.026 |
| DNA Replication | ||||
| NFIC | nuclear factor I/C | scl068530.1_6 | 2.03 | 0.005 |
| RBBP4 | retinoblastoma binding protein 4 | scl24919.4.1_260 | 2.09 | 0.021 |
| Heart Development | ||||
| MB | myoglobin | NM_013593.2 | 1.89 | 0.004 |
| MYL2 | myosin, light polypeptide 2, regulatory, cardiac, slow | scl27267.9.1_12 | 6.36 | 0.001 |
| VCAN | versican | scl013003.1_89 | 1.11 | 0.025 |
| OSR1 | oxidative-stress responsive 1 | scl35223.18_513 | 1.53 | 0.007 |
| Immune Response | ||||
| H2-EA | histocompatibility 2, class II antigen E alpha | NM_010381.2 | 1.17 | 0.030 |
| CCL21B | chemokine | scl0018829.1_65 | 2.04 | 0.026 |
| Inflammatory Response | ||||
| STAB1 | stabilin 1 | NM_138672.1 | 1.39 | 0.030 |
| CCL21B | chemokine | scl0018829.1_65 | 2.04 | 0.026 |
| SELP | selectin, platelet | NM_011347.1 | 1.38 | 0.021 |
| Integrin-Mediated Signaling Pathway | ||||
| ITGA3 | integrin alpha 3 | NM_013565.2 | 1.25 | 0.018 |
| ITGB1 | integrin beta 1 | NM_010578.1 | 1.72 | 0.009 |
| Lipid Biosynthetic Process | ||||
| PRKAG2 | protein kinase, AMP-activated, gamma 2 noncatalytic subunit | NM_145401.1 | 2.38 | 0.021 |
| Lipid Metabolic Process | ||||
| PTPN11 | protein tyrosine phosphatase, nonreceptor type 11 | NM_011202.2 | 1.88 | 0.040 |
| TNXB | tenascin XB | NM_031176.1 | 1.71 | 0.022 |
| Metabolic Process | ||||
| RPE | ribulose-5-phosphate-3-epimerase | scl0227227.1_0 | 2.13 | 0.019 |
| Muscle Contraction | ||||
| TTN | titin | scl19104.8.1_3 | 1.18 | 0.037 |
| ZBTB7A | zinc finger and BTB domain containing 7a | scl0016969.1_242 | 3.43 | 0.002 |
| Oxidation Reduction | ||||
| 4933406E20RIK | RIKEN cDNA 4933406E20 gene | NM_028944.2 | 1.24 | 0.009 |
| Signal Transduction | ||||
| RAP2C | RAP2C, member of RAS oncogene family | scl54266.5_48 | 2.00 | 0.025 |
| Transcription | ||||
| CREBBP | CREB binding protein | scl48815.9.1_11 | 2.07 | 0.033 |
| SKI | superkiller viralicidic activity 2-like (S.cerevisiae) | scl23441.8_64 | 1.98 | 0.003 |
| RBBP4 | retinoblastoma binding protein 4 | scl24919.4.1_260 | 2.09 | 0.021 |
| NFIC | nuclear factor I/C | scl068530.1_6 | 2.03 | 0.005 |
| Transport | ||||
| MB | myoglobin | NM_013593.2 | 1.89 | 0.004 |
| LTF | lactotransferrin | NM_008522.2 | 11.84 | 0.000 |
| TRAM1 | translocating chain-associating membrane protein 1 | NM_028173.1 | 1.72 | 0.036 |
| RAMP1 | receptor | scl17654.5.1_10 | 1.54 | 0.034 |
| RAB17 | RAB17, member RAS oncogene family | NM_008998.2 | 2.33 | 0.006 |
| Triglyceride Metabolic Process | ||||
| PTPN11 | protein tyrosine phosphatase, nonreceptor type 11 | NM_011202.2 | 1.88 | 0.040 |
| TNXB | tenascin XB | NM_031176.1 | 1.71 | 0.022 |
| Other | ||||
| GUCY1A3 | guanylate cyclase 1, soluble, alpha 3 | scl0060596.1_205 | 1.03 | 0.011 |
| BMX | BMX nonreceptor tyrosine kinase | NM_009759.2 | 1.62 | 0.030 |
| KIF1B | kinesin family member 1B | scl0002773.1_49 | 2.29 | 0.021 |
| ZBTB7A | zinc finger and BTB domain containing 7a | scl0016969.1_242 | 3.43 | 0.002 |
| PRRX1 | paired related homeobox 1 | scl018933.1_11 | 1.78 | 0.021 |
Fig. 4.
Hierarchical cluster analysis of the differentially expressed genes in female and male WT and SERT+ mice following exposure to chronic hypoxia (A). Representation of the differentially expressed genes in female SERT+ mice (B) and male SERT+ mice (C), arranged by biological processes.
In contrast, a large number of these changes were not apparent in hypoxic male SERT+ mice. A total of 145 genes were differentially expressed in male SERT+ mice, with 87 showing increased expression (Table 7) and 58 showing decreased expression (Table 8). When categorized by biological processes, 42% of these genes were assigned a biological function; 12 categories were represented in total.
Table 7.
List of genes upregulated in the pulmonary arteries of 2 mo old hypoxic male SERT+ mice compared with 2 mo old hypoxic male wild-type mice, arranged by biological process
| Gene Symbol | Gene Name | Accession No. | Fold Change | False Discovery Rate |
|---|---|---|---|---|
| Transport | ||||
| LBP | lipopolysaccharide binding protein | scl20002.16.7_10 | 1.92 | 0.019 |
| LTF | lactotransferrin | NM_008522.2 | 6.63 | 0.000 |
| APOC1 | apolipoprotein C-I | NM_007469.2 | 1.95 | 0.020 |
| SLC4A1 | solute carrier family 4 | NM_011403.1 | 2.28 | 0.004 |
| HBB-B1 | hemoglobin, beta adult major chain | NM_008220.2 | 3.05 | 0.032 |
| ABCC9 | ATP-binding cassette, sub-family C | scl0001165.1_35 | 2.25 | 0.015 |
| SLC1A3 | solute carrier family 1 | NM_148938.2 | 1.85 | 0.011 |
| LCN2 | lipocalin 2 | NM_008491.1 | 1.65 | 0.021 |
| Signal Transduction, Protein Binding | ||||
| FGL1 | fibrogen-like protein 1 | scl34887.7.1_10 | 2.48 | 0.007 |
| ANGPTL4 | angiopoietin-like 4 | scl50042.7_106 | 1.89 | 0.019 |
| TNC | tenascin C | scl0002731.1_70 | 1.77 | 0.018 |
| Immune Response | ||||
| CCL7 | chemokine | scl41159.3.1_10 | 2.02 | 0.014 |
| CFD | complement factor D | NM_013459.1 | 1.78 | 0.042 |
| CCL2 | chemokine | scl020296.2_11 | 2.03 | 0.014 |
| CLEC4D | C-type lectin domain family 4, member d | scl017474.5_5 | 1.54 | 0.034 |
| C3 | complement component 3 | scl49743.39.1_15 | 1.74 | 0.042 |
| C1S | complement component 1, s subcomponent | NM_144938.1 | 3.34 | 0.003 |
| PRG4 | PREDICTED: proteoglycan 4 | scl000882.1_25 | 2.34 | 0.003 |
| SPON2 | spondin 2, extracellular matrix protein | NM_133903.2 | 1.98 | 0.019 |
| CXCL14 | chemokine | scl43911.4.1_38 | 1.52 | 0.031 |
| Proteolysis | ||||
| DPEP2 | dipeptidase 2 | scl34381.6_178 | 1.40 | 0.047 |
| CTSK | cathepsin K | NM_007802.2 | 1.56 | 0.014 |
| CFD | complement factor D | NM_013459.1 | 1.78 | 0.042 |
| CPXM1 | carboxypeptidase X 1 | NM_019696.1 | 1.25 | 0.020 |
| CTSC | cathepsin C | NM_009982.2 | 1.75 | 0.020 |
| C1S | complement component 1, s subcomponent | NM_144938.1 | 3.34 | 0.003 |
| HP | haptoglobin | NM_017370.1 | 3.13 | 0.002 |
| Cell Adhesion | ||||
| CNTN2 | contactin 2 | scl0021367.1_277 | 2.19 | 0.017 |
| CPXM1 | carboxypeptidase X 1 | NM_019696.1 | 1.25 | 0.020 |
| COL8A2 | collagen, type VIII, alpha 2 | scl24964.1.1958_52 | 1.98 | 0.018 |
| CYR61 | cysteine rich protein 61 | NM_010516.1 | 1.66 | 0.039 |
| TNC | tenascin C | scl0002731.1_70 | 1.77 | 0.018 |
| SPP1 | secreted phosphoprotein 1 | NM_009263.1 | 2.51 | 0.006 |
| SPON2 | spondin 2, extracellular matrix protein | NM_133903.2 | 1.98 | 0.019 |
| COMP | cartilage oligomeric matrix protein | scl33728.21.1_0 | 1.85 | 0.025 |
| FN1 | fibronectin 1 | scl16639.44.189_5 | 1.57 | 0.013 |
| Apoptosis | ||||
| CIDEC | cell death-inducing DFFA-like effector c | NM_178373.2 | 1.90 | 0.046 |
| COMP | cartilage oligomeric matrix protein | scl33728.21.1_0 | 1.85 | 0.025 |
| Lipid Metabolic Process | ||||
| SLC27A3 | solute carrier family 27 | scl21918.10.1_222 | 1.74 | 0.031 |
| LPL | lipoprotein lipase | scl0016956.1_234 | 1.71 | 0.018 |
| Innate Immune Response | ||||
| LBP | lipopolysaccharide binding protein | scl20002.16.7_10 | 1.92 | 0.019 |
| CFD | complement factor D | NM_013459.1 | 1.78 | 0.042 |
| C3 | complement component 3 | scl49743.39.1_15 | 1.74 | 0.042 |
| C1S | complement component 1, s subcomponent | NM_144938.1 | 3.34 | 0.003 |
| Brown Fat Cell Differentiation | ||||
| ADIPOQ | adiponectin, C1Q and collagen domain containing | scl49310.3_131 | 2.30 | 0.003 |
| LRG1 | leucine-rich alpha-2-glycoprotein 1 | NM_029796.2 | 2.31 | 0.004 |
| Skeletal System Development | ||||
| RUNX1 | runt related transcription factor 1 | scl48188.1.1_190 | 2.07 | 0.011 |
| COL1A1 | procollagen, type I, alpha 1 | scl012842.26_28 | 2.15 | 0.007 |
| Blood Vessel Development | ||||
| COL3A1 | procollagen, type III, alpha 1 | NM_009930.1 | 1.57 | 0.039 |
| COL1A1 | procollagen, type I, alpha 1 | scl012842.26_28 | 2.15 | 0.007 |
Table 8.
List of genes downregulated in the pulmonary arteries of 2 mo old hypoxic male SERT+ mice compared with 2 mo old hypoxic male wild-type mice, arranged by biological process
| Gene Symbol | Gene Name | Accession No. | Fold Change | False Discovery Rate |
|---|---|---|---|---|
| Transport | ||||
| UCP1 | uncoupling protein 1 | NM_009463.2 | 3.84 | 0.000 |
| KCNAB1 | potassium voltage-gated channel, shaker-related 1 subfamily, beta member | NM_010597.2 | 1.68 | 0.025 |
| KCNH2 | potassium voltage-gated channel, subfamily H | NM_013569.1 | 1.74 | 0.014 |
| TOMM22 | translocase of outer mitochondrial membrane 22 homolog | scl47742.5_273 | 3.76 | 0.000 |
| RAMP1 | receptor (calcitonin) activity modifying protein 1 | scl17654.5.1_10 | 1.59 | 0.046 |
| RAB17 | RAB17, member RAS oncogene family | NM_008998.2 | 1.92 | 0.006 |
| Proteolysis | ||||
| MIPEP | mitochondrial intermediate peptidase | NM_027436.1 | 1.50 | 0.046 |
| DPEP1 | dipeptidase 1 | NM_007876.1 | 1.70 | 0.031 |
| CORIN | corin | NM_016869.1 | 1.84 | 0.014 |
| Cell Adhesion | ||||
| MCAM | melanoma cell adhesion molecule | NM_023061.1 | 1.51 | 0.046 |
| PKP4 | plakophilin 4 | scl0003206.1_31 | 1.70 | 0.025 |
| Apoptosis | ||||
| ACTC1 | actin, alpha, cardiac | NM_009608.1 | 1.14 | 0.018 |
| CIDEA | cell death-inducing DNA fragmentation factor, alpha subunit-like effector A | NM_007702.1 | 1.88 | 0.018 |
| PAWR | PRKC, apoptosis, WT1, regulator | scl38461.7.1_1 | 1.67 | 0.031 |
| Lipid Metabolic Process | ||||
| TNXB | tenascin XB | NM_031176.1 | 1.91 | 0.013 |
| CIDEA | cell death-inducing DNA fragmentation factor, alpha subunit-like effector A | NM_007702.1 | 1.88 | 0.018 |
| Heart Development | ||||
| PDLIM3 | PDZ and LIM domain 3 | NM_016798.2 | 1.59 | 0.028 |
| EDN1 | endothelin 1 | NM_010104.2 | 1.55 | 0.046 |
| TNNT2 | troponin T2, cardiac | NM_011619.1 | 1.62 | 0.004 |
| Brown Fat Cell Differentiation | ||||
| UCP1 | uncoupling protein 1 | NM_009463.2 | 3.84 | 0.000 |
| Skeletal System Development | ||||
| GJA5 | gap junction membrane channel protein alpha 5 | NM_008121.2 | 1.55 | 0.042 |
| Blood Vessel Development | ||||
| GJA5 | gap junction membrane channel protein alpha 5 | NM_008121.2 | 1.55 | 0.042 |
| GJA4 | gap junction membrane channel protein alpha 4 | NM_008120.2 | 1.80 | 0.014 |
Hierarchal cluster analysis of the differentially expressed genes between the four hypoxic groups revealed distinct gene expression patterns which were unique to female SERT+ mice. This may be critical to the exaggerated hypoxia-induced PAH phenotype observed in these mice.
Quantitative RT-PCR analysis.
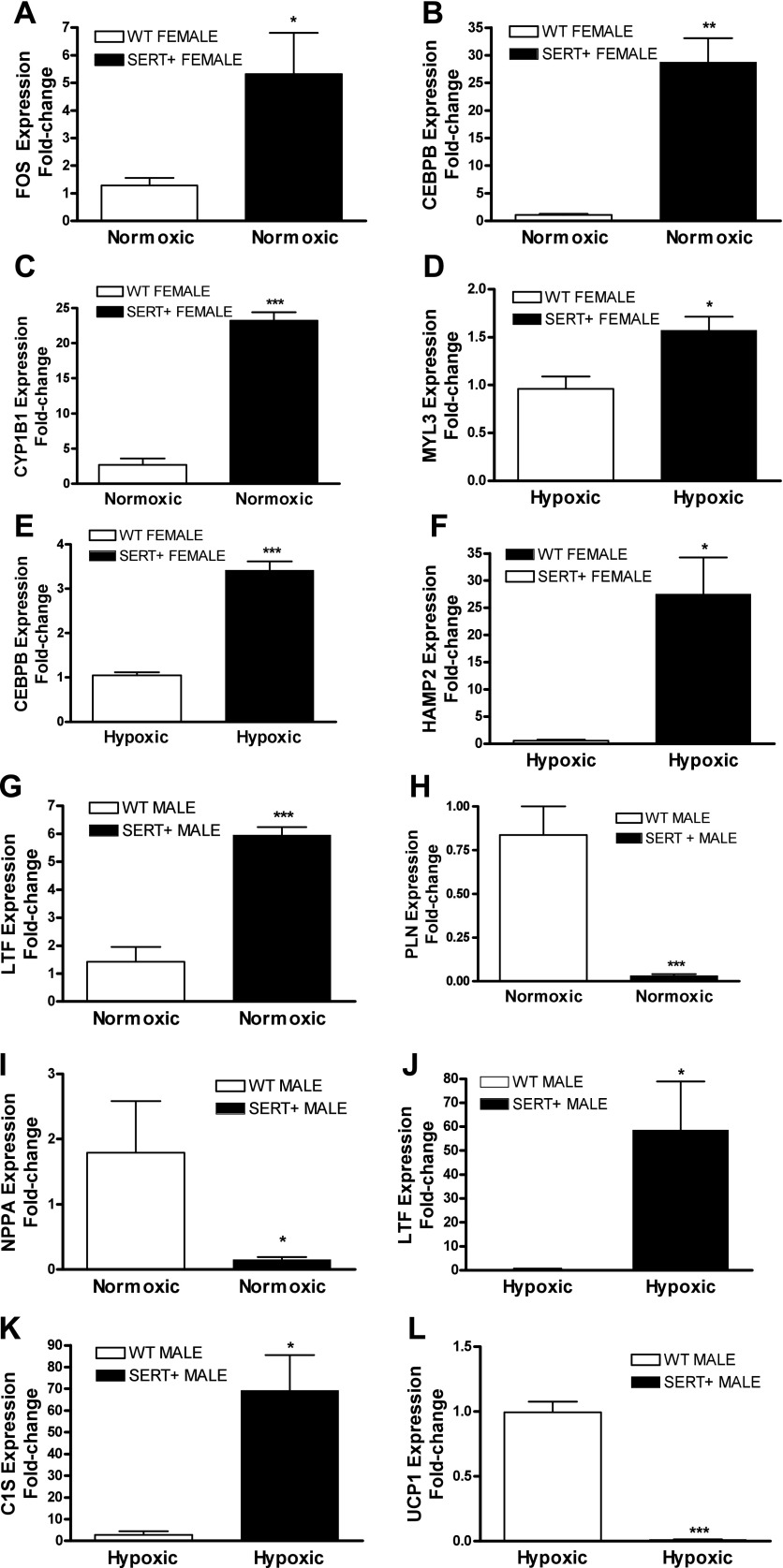
For validation of the microarray study, we employed qRT-PCR. To perform this, we selected three differentially expressed genes for each of the four group comparisons (Supplementary Table). Our genes of interest were FOS, CEBPβ, CYP1B1, MYL3, HAMP2, LTF, PLN, NPPA, UCP1, and C1S. In concordance with our microarray data, expression of these genes was significantly altered in relevant groups (Fig. 5). Of particular interest, qRT-PCR analysis confirmed that FOS, CEBPβ and CYP1B1 were considerably up-regulated (4-, 20-, and 8-fold, respectively) in female SERT+ mice.
Fig. 5.
Validation of microarray data using qRT-PCR analysis. qRT-PCR analysis performed on 3 differentially expressed genes (according to microarray) for each comparison. A–C: normoxic female WT mice cf. normoxic female SERT+ mice (FOS, CEBPβ, CYP1B1); D–F: hypoxic female WT mice cf. hypoxic female SERT+ mice (MYL3, CEBPβ, HAMP2); G–I: normoxic male WT mice cf. normoxic male SERT+ mice (LTF, PLN, NPPA); J–L: hypoxic WT male cf. hypoxic SERT+ male (LTF, C1S, UCP1). n = 4 and performed in triplicate. Data are expressed as means ± SE and analyzed by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001 cf. relevant comparison group.
CCAAT/enhancer-binding protein beta, CYP1B1, and c-FOS protein expression in female SERT+ mice.
To build on interesting gene expression differences observed in female SERT+ mice, we investigated expression of CCAAT/enhancer-binding protein beta (C/EBPβ), CYP1B1, and FOS at protein level. In agreement with our qRT-PCR findings, protein expression of C/EBPβ, CYP1B1, and c-FOS were also upregulated in the pulmonary arteries of female SERT+ mice (Fig. 6).
Fig. 6.
Representative immunoblotting and densitometric analysis confirming increased protein expression of C/EBPβ (A, B), CYP1B1 (C, D), and c-FOS (E, F) in the pulmonary arteries of female SERT+ mice compared with female WT mice. n = 4 and performed in triplicate. Data are expressed as means ± SE and analyzed by unpaired t-test. *P < 0.05 cf. female WT mice.
Serotonin and 17β-estradiol stimulate C/EBPβ, CYP1B1, and c-FOS expression in human PASMCs.
To determine if serotonin and 17β-estradiol stimulate expression of C/EBPβ, CYP1B1, and c-FOS, we investigated expression of these in PASMCs following 24 h stimulation with serotonin and 17β-estradiol. Stimulation with serotonin or 17β-estradiol was sufficient to increase C/EBPβ, CYP1B1, and c-FOS expression in PASMCs (Fig. 7).
Fig. 7.
Representative immunoblotting and densitometric analysis confirming increased protein expression of C/EBPβ (A, B), CYP1B1 (C, D), and c-FOS (E, F) in human PASMCs following stimulation with 1 μmol/l serotonin (5-HT) or 1 nmol/l 17β-estradiol (E2). n = 3 and performed in triplicate. Data are expressed as means ± SE and analyzed by 1-way ANOVA followed by Dunnett's post hoc test. *P < 0.05 cf. control PASMCs.
C/EBPβ, CYP1B1, and c-FOS expression in IPAH PASMCs.
To identify if these findings translate with relevance to human PAH, we investigated the expression of CEBPβ, CYP1B1, and FOS in PASMCs derived from IPAH patients. PASMCs from non-PAH donors were studied as controls. Interestingly, CEBPβ, CYP1B1, and FOS expression appeared significantly increased in mRNA extracted from IPAH PASMCs (Fig. 8). Similarly, Western blot analysis confirmed that protein expression of C/EBPβ, CYP1B1, and c-FOS is also increased in IPAH PASMCs compared with control PASMCs.
Fig. 8.
Quantitative RT-PCR analysis confirming the upregulation of CEBPβ (A), CYP1B1 (B), and FOS (C), and representative immunoblotting and densitometric analysis confirming increased protein expression of C/EBPβ (D, E), CYP1B1 (F, G), and c-FOS (H, I) in pulmonary artery smooth muscle cells (PASMCs) derived from IPAH patients compared with control PASMCs. n = 3 and performed in triplicate. Data are expressed as means ± SE and analyzed by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001 cf. control.
DISCUSSION
Despite increased mortality reported in men (15), the incidence of both IPAH and HPAH remains up to threefold more common in women. This is highlighted in recent epidemiological studies carried out in Scotland, France, and USA, where 60, 65, and 77% of the patients studied respectively were female (16, 28, 43). Established experimental models of PAH have failed to provide insight into this increased occurrence. Paradoxically, several experimental models of PAH exhibit male susceptibility compared with their female counterparts (12, 24, 29, 34). Here, we describe an experimental model of PAH that exhibits female susceptibility. Female SERT+ mice develop PAH and exaggerated hypoxia-induced PAH, whereas male SERT+ mice remain unaffected, when compared against their respective WT controls. We were interested in determining the genotypic differences associated with the development and progression of PAH in SERT+ mice. To investigate this, microarray analysis was performed in the pulmonary arteries of SERT+ mice at 2 mo of age, where no PAH phenotype is reported.
Through microarray analysis we have identified a large number of differentially expressed genes in the pulmonary arteries of SERT+ mice. In total, we identified 155 genes changed in female SERT+ mice, while 148 genes were changed in male SERT+ mice. Heat map analysis identified gene expression changes in females that were not apparent in males. When assigned to biological processes, we also identified that >40% of the differentially expressed genes in female SERT+ mice were directly involved in biological pathways. In total, 15 known biological pathways were dysregulated in female SERT+ mice and included oxidation-reduction, cell differentiation, regulation of transcription, apoptosis, muscle contraction, cellular calcium ion homeostasis, and glycolysis. This may be relevant to the development of PAH in SERT+ mice, as dysregulation of these pathways has been previously implicated in the pathogenesis of PAH (33, 35). Indeed, similar pathway changes have also been described in the lungs of VIP−/− mice (9) and BMPR-II mutant mice (40). In contrast to female SERT+ mice, only 25% of altered genes in male SERT+ mice were associated with biological function and as a consequence resulted in the dysregulation of pathways to a much lesser extent.
In chronic hypoxia, there were also a large number of differentially expressed genes in SERT+ mice compared with their respective WT controls. We observed a total of 316 genes altered in females, whereas less than half (154) of these were altered in males. In hypoxic female SERT+ mice, 53% of genes were associated with biological function. Similar to the normoxic female comparison, altered genes were related to apoptotic, inflammatory, transcription, and metabolic processes, all of which are well-described in PAH (13). In total, 26 biological pathways were identified as dysregulated. As expected, fewer genes were reported as changed in male SERT+ mice. These differences may help explain the exaggerated hypoxia-induced PAH phenotype in female SERT+ mice.
The female hormone 17β-estradiol is one risk factor in PAH. Decreased expression of the 17β-estradiol-metabolizing enzyme CYP1B1, resulting in altered estrogen metabolism, has been identified in female PAH patients harboring a BMPR-II mutation compared with unaffected female carriers (2). Multiple factors modulate the levels of estrogen-metabolizing enzymes in the liver and target tissues, and the biological effects of an estrogen will depend on the profile of metabolites formed and the biological activities of each of these metabolites (50). 17β-Estradiol is metabolized to both pro- and antiproliferative metabolites, and its effects will depend on its metabolism. 17β-Estradiol can be converted to estrone and subsequently metabolized to 16α-hydroxyestrone (16-OHE1) via CYP3A4. Or alternatively, 17β-estradiol is metabolized to 2-hydroxyestradiol (2-OHE) via the estrogen-metabolizing enzymes CYP1A1/2 and to a lesser extent via CYP1B1 (10, 46). 2-OHE can itself be metabolized to 2-methoxyestradiol (2-ME) via catechol O-methyltransferase (COMT). Both 2-OHE and 2-ME have antiproliferative effects on cells (44), whereas 16α-OHE1 stimulates proliferation by constitutively activating the estrogen receptor (39). Metabolism of 17β-estradiol will therefore be species, sex, and strain-dependent, and differential disruption in the balance of metabolites may therefore account for the differential effects of female hormones in different models of PAH. Consistent with this, our microarray findings show that CYP1B1 mRNA expression is increased in female SERT+ mice. In further support of this, immunoblotting confirmed that CYP1B1 protein expression is also increased in the pulmonary arteries of female SERT+ mice. Of further interest, both serotonin and 17β-estradiol stimulation increased CYP1B1 expression in PASMCs. Indeed, similar 17β-estradiol effects have been previously described in cancer cells (45). On this evidence, serotonin and 17β-estradiol may be accountable for increased CYP1B1 expression in female SERT+ mice.
C/EBPβ is a transcription factor encoded by the CEBPβ gene. C/EBPβ has been previously shown to regulate inflammation, cell differentiation, and cell proliferation (31). For example, C/EBPβ is essential in the pathogenesis of multiple proliferative disorders including skin, breast, and ovarian cancer (32, 38, 51). In line with this, C/EBPβ-deficient mice appear resistant to tumorigenesis (37). The role of C/EBPβ in the development of PAH is poorly defined. Increased C/EBPβ expression has been reported in the lungs of chronically hypoxic rats (42), where it appears to stimulate inducible nitric oxide synthase expression. Reduced CEBPβ expression has also been previously reported in the lungs of SERT knockout mice (3). Conversely, our microarray data show the upregulation CEBPβ in female SERT+ mice. Increased CEBPβ mRNA expression was confirmed by qRT-PCR analysis. We also identified that C/EBPβ protein expression was increased in the pulmonary arteries of female SERT+ mice. In support of this, we observed that serotonin and 17β-estradiol increased C/EBPβ expression in human PASMCs. These findings suggest that serotonin and 17β-estradiol may stimulate C/EBPβ expression in vivo, and this contributes to the pathogenesis of PAH in female SERT+ mice.
We observed increased FOS expression in the pulmonary arteries of female SERT+ mice. FOS is a proto-oncogene that exists as an immediate early gene transcription factor and is transactivated in response to various stimuli (14). For example, FOS expression is increased in the heart following exposure to hypoxia (4). In bovine PASMCs, serotonin is also a potent inducer of FOS expression via a MAPK-dependent pathway (36). In agreement with this, we observed that serotonin stimulation also increased c-FOS expression in human PASMCs. Of interest, expression was also increased in 17β-estradiol-stimulated cells. Similar effects have also been described in rat hepatoctyes (19). In vivo, c-FOS expression is increased in the pulmonary arteries of female SERT+ mice. Here, our evidence suggests serotonin and 17β-estradiol stimulate c-FOS expression, and this may be relevant to the pathogenesis of PAH in female SERT+ mice.
With relevance to human PAH we further examined CEBPβ, CYP1B1, and FOS expression in PASMCs derived from IPAH patients. We observed that expression of these three genes (CEBPβ, CYP1B1 and FOS) was increased in IPAH PASMCs. We observed at least fivefold increases in CEBPβ and FOS mRNA expression compared with control PASMCs. Immunoblotting confirmed that the upregulation of C/EBPβ and c-FOS was also apparent at protein level. Since these genes are involved in inflammation and proliferation, both of which are essential components in disease pathogenesis (47), our findings suggest their importance in human PAH. We also observed increased CYP1B1 mRNA and protein expression in IPAH PASMCs, suggesting the importance of CYP1B1-mediated estrogen metabolism in PAH. However, these findings are inconsistent with previous studies in Epstein-Barr virus immortalized B cells derived from female BMPR-II PAH patients (48), where decreased CYP1B1 mRNA expression was described. Most likely, this is attributable to the differences in cell type investigated. This study focuses on changes in PASMCs, which represent a more physiologically relevant cell type in PAH.
We have previously reported that SERT+ mice develop elevated RVSP in the absence of RVH (21). This phenomenon is particular to normoxic mice as we, like others, have shown that mice develop RVH following exposure to hypoxia (18, 22). We are not alone in observing this phenomenon as other studies have similarly demonstrated elevated RVSP in transgenic mice in the absence of RVH. For example, mice that express BMPR-IIR899X in smooth muscle or molecular loss of BMPR-II signaling in smooth muscle demonstrate elevated RVSP with no change in RVH (41, 49). The observation that this only occurs in normoxic mice suggests that hypoxia induces an effect on RVH that may indeed be independent of RVSP.
The distal arteries are typically those most susceptible to pulmonary vascular remodeling in PAH; however, microarray analysis was performed in the proximal pulmonary arteries of mice as these were the smallest that could be practically dissected out from whole lung. Therefore, these gene changes may not be entirely representative of gene expression changes in smaller resistance arteries. For example, our microarray results show that hypoxic female SERT+ mice exhibit increased PPAR-γ expression relative to hypoxic female WT mice. However, previous observations confirm that PPAR-γ expression is reduced in the distal pulmonary arteries of PAH patients (1), and its targeted deletion in pulmonary artery smooth muscle or endothelial cells is sufficient to cause PAH in mice (7, 11). This contrast in findings may well result from the effect of hypoxia per se or an indirect compensatory change in response to PAH in SERT+ mice.
In conclusion, through microarray analysis we have identified a large number of differentially expressed genes in the pulmonary arteries of SERT+ mice. These findings offer further insight into the gender differences observed in this serotonin-dependent model of PAH. At least three of these genes (CEBPβ, CYP1B1, and FOS) are also upregulated at protein level in these mice. With relevance to human PAH, we identified that mRNA and protein expression of CEBPβ, CYP1B1, and FOS was also increased in PASMCs derived from IPAH patients. This study has described genotypic differences in a serotonin-dependent model of PAH and these findings at least in part, may be relevant to the pathogenesis observed in human PAH.
GRANTS
K. White is supported by a Capacity Building Award in Integrative Mammalian Biology funded by the Biotechnology and Biological Sciences Research Council (BBSRC), British Pharmacological Society, Knowledge Transfer Network, Medical Research Council, and Scottish Funding Council. Z. Maqbool is supported by the BBSRC. Y. Dempsie is supported by the Medical Research Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Professor Nicholas W. Morrell (University of Cambridge, UK) for the supply of human PASMCs.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 92: 1162–1169, 2003. [DOI] [PubMed] [Google Scholar]
- 2. Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, Wheeler LA, Parl FF, Loyd JE, Phillips JA., III Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J 34: 1093–1099, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crona D, Harral J, Adnot S, Eddahibi S, West J. Gene expression in lungs of mice lacking the 5-hydroxytryptamine transporter gene. BMC Pulm Med 9: 19, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deindl E, Kolar F, Neubauer E, Vogel S, Schaper W, Ostadal B. Effect of intermittent high altitude hypoxia on gene expression in rat heart and lung. Physiol Res 52: 147–157, 2003. [PubMed] [Google Scholar]
- 5. Dempsie Y, Morecroft I, Welsh DJ, Macritchie NA, Herold N, Loughlin L, Nilsen M, Peacock AJ, Harmar A, Bader M, MacLean MR. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation 117: 2928–2937, 2008. [DOI] [PubMed] [Google Scholar]
- 6. Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, Hamon M, Adnot S. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension - Critical role for serotonin-induced smooth muscle hyperplasia. Circulation 113: 1857–1864, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Guignabert C, Alvira CM, Alastalo TP, Sawada H, Hansmann G, Zhao M, Wang L, El-Bizri N, Rabinovitch M. Tie2-mediated loss of peroxisome proliferator-activated receptor-gamma in mice causes PDGF receptor-beta-dependent pulmonary arterial muscularization. Am J Physiol Lung Cell Mol Physiol 297: L1082–L1090, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guignabert C, Izikki M, Tu LI, Li ZL, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res 98: 1323–1330, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Hamidi SA, Prabhakar S, Said SI. Enhancement of pulmonary vascular remodelling and inflammatory genes with VIP gene deletion. Eur Respir J 31: 135–139, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Hanna IH, Dawling S, Roodi N, Guengerich FP, Parl FF. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res 60: 3440–3444, 2000. [PubMed] [Google Scholar]
- 11. Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118: 1846–1857, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ, Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation 115: 1275–1284, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54: S10–S19, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Herschman HR. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem 60: 281–319, 1991. [DOI] [PubMed] [Google Scholar]
- 15. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jais X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 173: 1023–1030, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT1B receptor to hypoxia-induced pulmonary hypertension - Converging evidence using 5-HT1B-receptor knockout mice and the 5-HT1B/1D-receptor antagonist GR127935. Circ Res 89: 1231–1239, 2001. [DOI] [PubMed] [Google Scholar]
- 18. Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT1B receptor to hypoxia-induced pulmonary hypertension - Converging evidence using 5-HT1B-receptor knockout mice and the 5-HT1B/1D-receptor antagonist GR127935. Circ Res 89: 1231–1239, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Lee CH, Edwards AM. Stimulation of DNA synthesis and c-fos mRNA expression in primary rat hepatocytes by estrogens. Carcinogenesis 22: 1473–1481, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Long L, MacLean MR, Jeffery TK, Morecroft I, Yang XD, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res 98: 818–827, 2006. [DOI] [PubMed] [Google Scholar]
- 21. MacLean MR, Deuchar GA, Hicks MN, Morecroft I, Shen SB, Sheward J, Colston J, Loughlin L, Nilsen M, Dempsie Y, Harmar A. Overexpression of the 5-hydroxytryptamine transporter gene - Effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation 109: 2150–2155, 2004. [DOI] [PubMed] [Google Scholar]
- 22. MacLean MR, Deuchar GA, Hicks MN, Morecroft I, Shen SB, Sheward J, Colston J, Loughlin L, Nilsen M, Dempsie Y, Harmar A. Overexpression of the 5-hydroxytryptamine transporter gene - Effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation 109: 2150–2155, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Masi AT. Editorial: Pulmonary hypertension and oral contraceptive usage. Chest 69: 451–453, 1976. [DOI] [PubMed] [Google Scholar]
- 24. Miller AA, Hislop AA, Vallance PJ, Haworth SG. Deletion of the eNOS gene has a greater impact on the pulmonary circulation of male than female mice. Am J Physiol Lung Cell Mol Physiol 289: L299–L306, 2005. [DOI] [PubMed] [Google Scholar]
- 25. Morecroft I, Dempsie Y, Bader M, Walther DJ, Kotnik K, Loughlin L, Nilsen M, MacLean MR. Effect of Tryptophan Hydroxylase 1 Deficiency on the Development of Hypoxia-Induced Pulmonary Hypertension. Hypertension 49: 232–236, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Newman JH, Trembath RC, Morse JA, Grunig E, Loyd JE, Adnot S, Coccolo F, Ventura C, Phillips JA, III, Knowles JA, Janssen B, Eickelberg O, Eddahibi S, Herve P, Nichols WC, Elliott G. Genetic basis of pulmonary arterial hypertension: current understanding and future directions. J Am Coll Cardiol 43: 33S––39S., 2004. [DOI] [PubMed] [Google Scholar]
- 27. Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA, Loyd JE. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med 345: 319–324, 2001. [DOI] [PubMed] [Google Scholar]
- 28. Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 30: 104–109, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary-hypertension of chronic hypoxia and on recovery. Am J Physiol Heart Circ Physiol 240: H62–H72, 1981. [DOI] [PubMed] [Google Scholar]
- 30. Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol 298: H1235–H1248, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365: 561–575, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raught B, Gingras AC, James A, Medina D, Sonenberg N, Rosen JM. Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein beta isoform and up-regulation of the eukaryotic translation initiation factor 2alpha are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res 56: 4382–4386, 1996. [PubMed] [Google Scholar]
- 33. Rehman J, Archer SL. A proposed mitochondrial-metabolic mechanism for initiation and maintenance of pulmonary arterial hypertension in fawn-hooded rats: the Warburg model of pulmonary arterial hypertension. Adv Exp Med Biol 661: 171–185, 2010. [DOI] [PubMed] [Google Scholar]
- 34. Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 115: 1260–1268, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res 10: 95, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simon AR, Severgnini M, Takahashi S, Rozo L, Andrahbi B, Agyeman A, Cochran BH, Day RM, Fanburg BL. 5-HT induction of c-fos gene expression requires reactive oxygen species and Rac1 and Ras GTPases. Cell Biochem Biophys 42: 263–276, 2005. [DOI] [PubMed] [Google Scholar]
- 37. Sterneck E, Zhu S, Ramirez A, Jorcano JL, Smart RC. Conditional ablation of C/EBP beta demonstrates its keratinocyte-specific requirement for cell survival and mouse skin tumorigenesis. Oncogene 25: 1272–1276, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sundfeldt K, Ivarsson K, Carlsson M, Enerback S, Janson PO, Brannstrom M, Hedin L. The expression of CCAAT/enhancer binding protein (C/EBP) in the human ovary in vivo: specific increase in C/EBPbeta during epithelial tumour progression. Br J Cancer 79: 1240–1248, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Swaneck GE, Fishman J. Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc Natl Acad Sci USA 85: 7831–7835, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tada Y, Majka S, Carr M, Harral J, Crona D, Kuriyama T, West J. Molecular effects of loss of BMPR2 signaling in smooth muscle in a transgenic mouse model of PAH. Am J Physiol Lung Cell Mol Physiol 292: L1556–L1563, 2007. [DOI] [PubMed] [Google Scholar]
- 41. Tada Y, Majka S, Carr M, Harral J, Crona D, Kuriyama T, West J. Molecular effects of loss of BMPR2 signaling in smooth muscle in a transgenic mouse model of PAH. Am J Physiol Lung Cell Mol Physiol 292: L1556–L1563, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Teng X, Li D, Catravas JD, Johns RA. C/EBP-beta mediates iNOS induction by hypoxia in rat pulmonary microvascular smooth muscle cells. Circ Res 90: 125–127, 2002. [DOI] [PubMed] [Google Scholar]
- 43. Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J 30: 1103–1110, 2007. [DOI] [PubMed] [Google Scholar]
- 44. Tofovic SP, Zhang XC, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vasc Pharmacol 45: 358–367, 2006. [DOI] [PubMed] [Google Scholar]
- 45. Tsuchiya Y, Nakajima M, Kyo S, Kanaya T, Inoue M, Yokoi T. Human CYP1B1 is regulated by estradiol via estrogen receptor. Cancer Res 64: 3119–3125, 2004. [DOI] [PubMed] [Google Scholar]
- 46. Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett 227: 115–124, 2005. [DOI] [PubMed] [Google Scholar]
- 47. Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, Haworth SG. Development and pathology of pulmonary hypertension. J Am Coll Cardiol 54: S3–S9, 2009. [DOI] [PubMed] [Google Scholar]
- 48. West J, Cogan J, Geraci M, Robinson L, Newman J, Phillips JA, Lane K, Meyrick B, Loyd J. Gene expression in BMPR2 mutation carriers with and without evidence of Pulmonary Arterial Hypertension suggests pathways relevant to disease penetrance. BMC Med Genomics 1: 45, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 94: 1109–1114, 2004. [DOI] [PubMed] [Google Scholar]
- 50. Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 19: 1–27, 1998. [DOI] [PubMed] [Google Scholar]
- 51. Zhu S, Yoon K, Sterneck E, Johnson PF, Smart RC. CCAAT/enhancer binding protein-beta is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc Natl Acad Sci USA 99: 207–212, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.