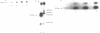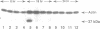Abstract
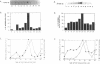
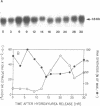
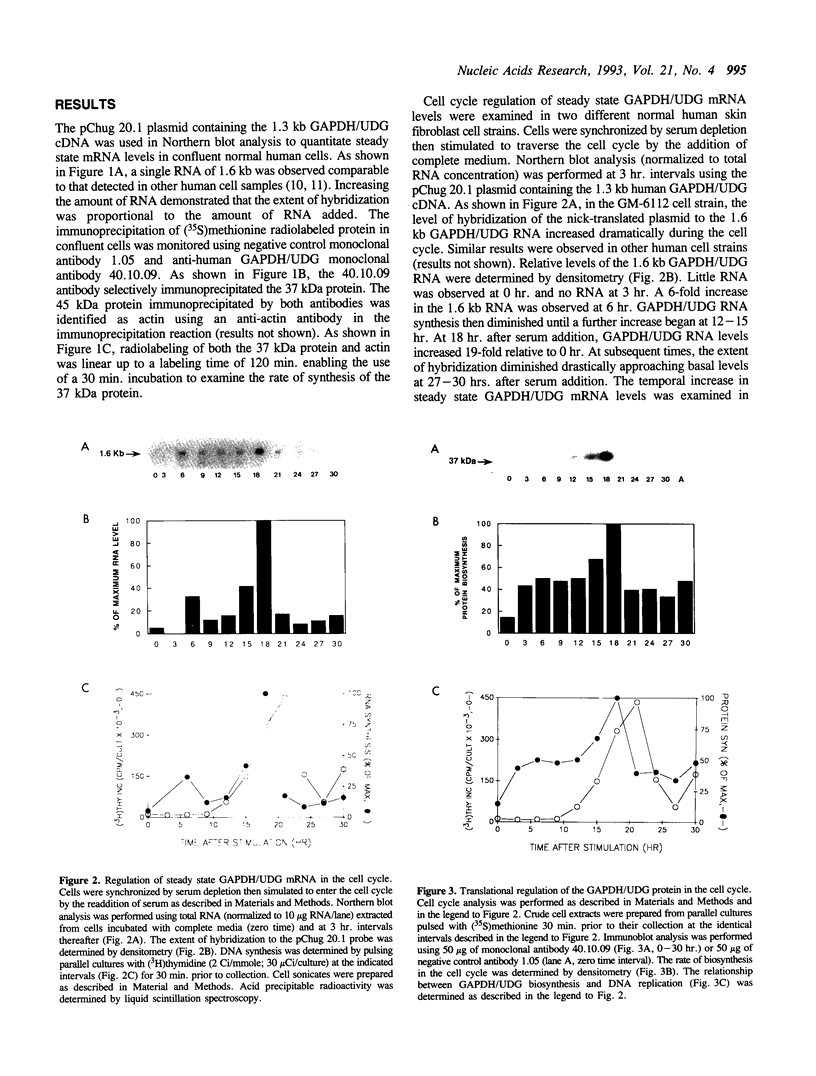
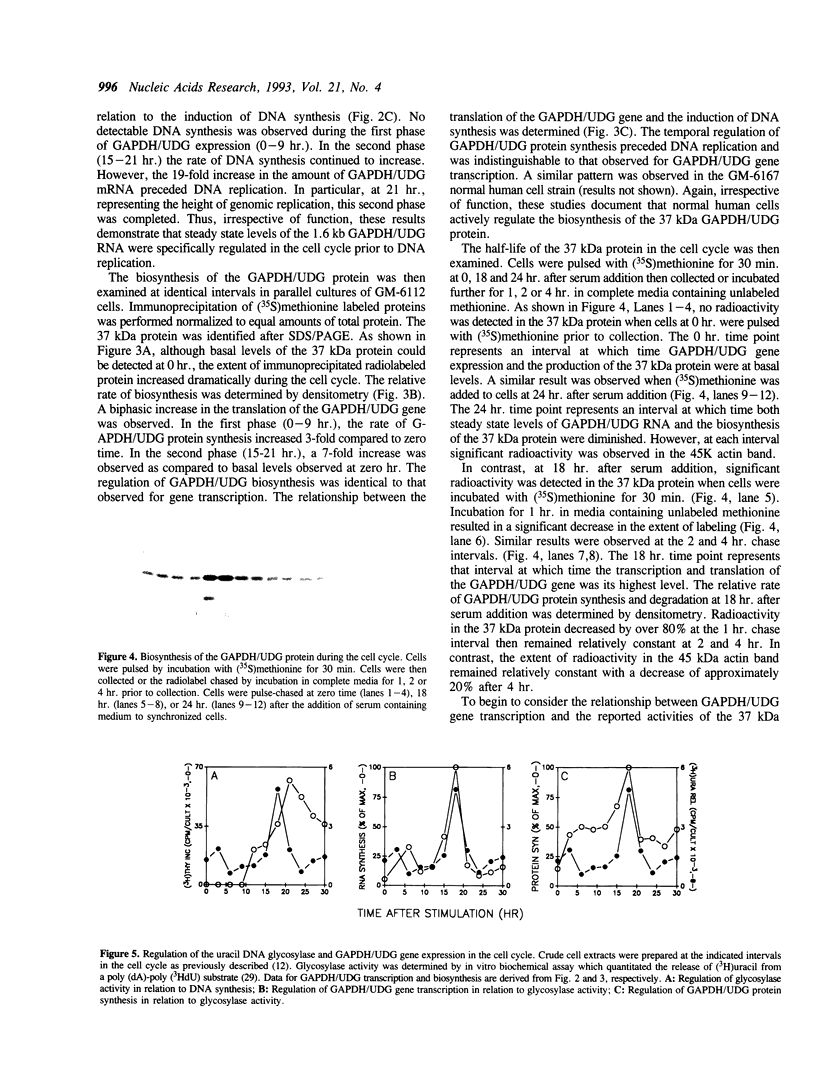
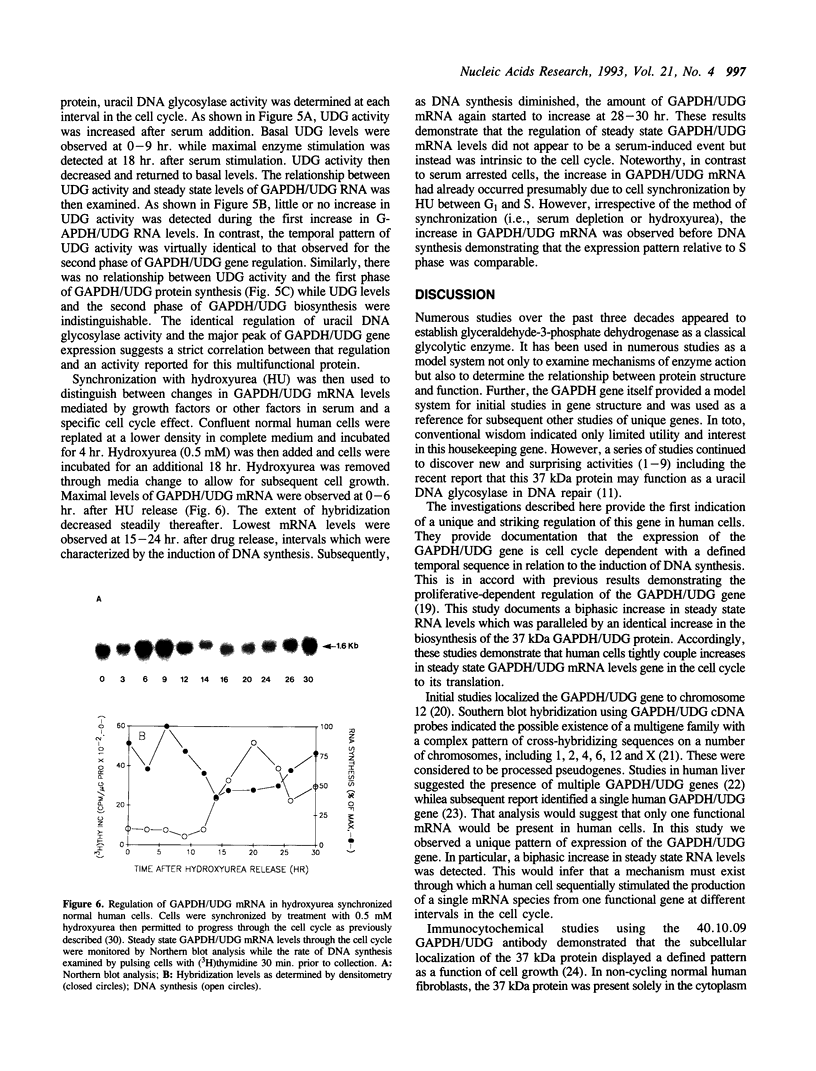
The cell cycle regulation of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH)/uracil DNA glycosylase (UDG) gene was examined in normal human cells. Steady state RNA levels were monitored by Northern blot analysis using a plasmid (pChug 20.1) which contained the 1.3 kb GAPDH/UDG cDNA. The biosynthesis of the 37 kDa GAPDH/UDG protein was determined using an anti-human placental GAPDH/UDG monoclonal antibody to immunoprecipitate the radiolabeled protein. Increases in steady state GAPDH/UDG mRNA levels were cell cycle specific. A biphasic pattern was observed resulting in a 19-fold increase in the amount of GAPDH/UDG mRNA. The biosynthesis of the 37 kDa GAPDH/UDG protein displayed a similar biphasic regulation with a 7-fold increase. Pulse-chase experiments revealed a remarkably short half life of less than 1 hr. for the newly synthesized 37 kDa protein, comparable to that previously documented for a number of oncogenes. GAPDH/UDG mRNA levels were markedly reduced at 24 hr. when DNA synthesis was maximal. These results define the GAPDH/UDG gene as cell cycle regulated with a characteristic temporal sequence of expression in relation to DNA synthesis. The cell cycle synthesis of a labile 37 kDa monomer suggests a possible regulatory function for this multidimensional protein. Further, modulation of the GAPDH/UDG gene in the cell cycle may preclude its use as a reporter gene when the proliferative state of the cell is not kept constant.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. W., Trach K. A., Hoch J. A. Identification of the 37-kDa protein displaying a variable interaction with the erythroid cell membrane as glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1987 Jan 15;262(2):649–653. [PubMed] [Google Scholar]
- Arenaz P., Sirover M. A. Isolation and characterization of monoclonal antibodies directed against the DNA repair enzyme uracil DNA glycosylase from human placenta. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5822–5826. doi: 10.1073/pnas.80.19.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell A. H., Corbett A. M. Interaction of glyceraldehyde-3-phosphate dehydrogenase with isolated microsomal subfractions of skeletal muscle. J Biol Chem. 1985 Jun 10;260(11):6892–6898. [PubMed] [Google Scholar]
- Gupta P. K., Sirover M. A. Sequential stimulation of DNA repair and DNA replication in normal human cells. Mutat Res. 1980 Sep;72(2):273–284. doi: 10.1016/0027-5107(80)90042-1. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Steck T. L. Association of glyceraldehyde-3-phosphate dehydrogenase with the human red cell membrane. A kinetic analysis. J Biol Chem. 1980 Jul 10;255(13):6314–6321. [PubMed] [Google Scholar]
- Morgenegg G., Winkler G. C., Hübscher U., Heizmann C. W., Mous J., Kuenzle C. C. Glyceraldehyde-3-phosphate dehydrogenase is a nonhistone protein and a possible activator of transcription in neurons. J Neurochem. 1986 Jul;47(1):54–62. doi: 10.1111/j.1471-4159.1986.tb02830.x. [DOI] [PubMed] [Google Scholar]
- Perucho M., Salas J., Salas M. L. Identification of the mammalian DNA-binding protein P8 as glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1977 Dec;81(3):557–562. doi: 10.1111/j.1432-1033.1977.tb11982.x. [DOI] [PubMed] [Google Scholar]
- Tsai I. H., Murthy S. N., Steck T. L. Effect of red cell membrane binding on the catalytic activity of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1982 Feb 10;257(3):1438–1442. [PubMed] [Google Scholar]
- Vollberg T. M., Siegler K. M., Cool B. L., Sirover M. A. Isolation and characterization of the human uracil DNA glycosylase gene. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8693–8697. doi: 10.1073/pnas.86.22.8693. [DOI] [PMC free article] [PubMed] [Google Scholar]