Blocking 5-HT2B receptor provides a therapeutic target for fibrotic diseases caused by activated platelet release of serotonin during vascular damage.
Abstract
Vascular damage and platelet activation are associated with tissue remodeling in diseases such as systemic sclerosis, but the molecular mechanisms underlying this association have not been identified. In this study, we show that serotonin (5-hydroxytryptamine [5-HT]) stored in platelets strongly induces extracellular matrix synthesis in interstitial fibroblasts via activation of 5-HT2B receptors (5-HT2B) in a transforming growth factor β (TGF-β)–dependent manner. Dermal fibrosis was reduced in 5-HT2B−/− mice using both inducible and genetic models of fibrosis. Pharmacologic inactivation of 5-HT2B also effectively prevented the onset of experimental fibrosis and ameliorated established fibrosis. Moreover, inhibition of platelet activation prevented fibrosis in different models of skin fibrosis. Consistently, mice deficient for TPH1, the rate-limiting enzyme for 5-HT production outside the central nervous system, showed reduced experimental skin fibrosis. These findings suggest that 5-HT/5-HT2B signaling links vascular damage and platelet activation to tissue remodeling and identify 5-HT2B as a novel therapeutic target to treat fibrotic diseases.
Systemic fibrotic diseases such as systemic sclerosis (SSc) and organ-specific fibrosis such as kidney fibrosis, idiopathic pulmonary fibrosis, and liver cirrhosis share common pathophysiological mechanisms (Bataller and Brenner, 2005; Varga and Abraham, 2007; Strieter and Mehrad, 2009). Tissue fibrosis results from an increased release of extracellular matrix from aberrantly activated fibroblasts (Varga and Abraham, 2007; Gabrielli et al., 2009). The accumulating extracellular matrix disrupts the physiological tissue structure, leading to organ dysfunction and contributing to the high morbidity and increased mortality of affected patients (Chung et al., 2007; Steen and Medsger, 2007). However, the mechanisms for pathological fibroblast activation are incompletely understood. Consequently, therapeutic approaches selectively targeting the molecular activation of fibroblasts are not yet available for clinical use.
Tissue fibrosis is preceded by microvascular injury in SSc, leading to a progressive loss of capillaries (Gabrielli et al., 2009). The vascular damage with exposure of subendothelial connective tissue results in ongoing activation of platelets. The levels of several platelet-derived molecules such as β-thrombomodulin, platelet factor 4, and platelet-derived growth factor are elevated in SSc patients (Kahaleh et al., 1982; Postlethwaite and Chiang, 2007). In addition, elevated levels of circulating platelet aggregates have also been described (Kahaleh et al., 1982). Moreover, platelets from SSc patients express markers of activation such as increased expression of the 65-kD receptor for type I collagen and enhanced activity of phosphatidylinositol-3 kinase (Postlethwaite and Chiang, 2007). However, the role of platelet activation in the pathogenesis of SSc and other fibrotic diseases has not yet been established.
5-hydroxytryptamine (5-HT; serotonin) is another potent mediator, which is released upon activation of platelets and is elevated in the blood of SSc patients (Stachów et al., 1979; Biondi et al., 1988; Hervé et al., 1995). The rate-limiting step of 5-HT synthesis is catalyzed by TPHs (tryptophan hydroxylases), whereof two isoforms have been described: TPH1 is mainly expressed in the periphery, whereas the expression of TPH2 is restricted to neuronal cells and the central nervous system (Walther et al., 2003a).
Based on (a) the activation of platelets upon microvascular injury, (b) the release of 5-HT during platelet activation, and (c) the potent biological effects of 5-HT, we hypothesized that 5-HT signaling might be involved in the pathogenesis of fibrosis. The cellular effects of 5-HT are mediated by seven families of 5-HT receptors, 5-HT1 to 5-HT7, some of which comprise several different members (Humphrey et al., 1993). Nonselective antagonists of 5-HT2 signaling such as terguride and cyproheptadine are already in current therapeutic use. A low rate of side effects has been observed in long-term follow-ups, suggesting that inhibition of 5-HT2 receptor signaling is well tolerated in humans (von Werner et al., 1989; Moertel et al., 1991). In addition, selective inhibitors have been developed targeting individual members of the 5-HT2 subfamily, including SB 204741, which inhibits 5-HT2B in pharmacologically relevant concentrations (Bonhaus et al., 1995; Glusa and Pertz, 2000). Using pharmacologic as well as genetic approaches and different experimental models of fibrosis, we demonstrate that 5-HT/5-HT2B signaling plays a central role in skin fibrosis. Together with the clinical availability of selective small molecular weight inhibitors, targeting 5-HT2B signaling might be a promising strategy for the treatment of fibrosis.
RESULTS
5-HT stimulates the production of extracellular matrix in SSc and healthy dermal fibroblasts
We first analyzed whether 5-HT stimulates the production of extracellular matrix. Therefore, fibroblasts from SSc patients and healthy subjects were stimulated with 5-HT at biologically relevant concentrations (Kasho et al., 1998; Wouters et al., 2007). 5-HT dose-dependently induced the expression of extracellular matrix proteins with maximal induction at a concentration of 1 µM (Fig. 1, A–D). Higher concentrations of 5-HT did not further increase the expression of extracellular matrix proteins but resulted in toxic effects (unpublished data). 5-HT increased the expression of col 1a1 by 211 ± 14% (P < 0.05; Fig. 1 A). In addition to col 1a1, 5-HT also induced the expression of col 1a2 and fibronectin-1 in a dose-dependent manner (Fig. 1, B and C). Similarly, the release of collagen protein from SSc fibroblasts was increased by up to 198 ± 53% (P < 0.05; Fig. 1 D). 5-HT also induced the expression of collagen in dermal fibroblasts from healthy individuals without significant differences to fibroblasts from SSc patients (Fig. 1, A–D). 5-HT did not enhance the stability of type I collagen or fibronectin-1 messenger RNA (mRNA; Fig. S1, A–C) but stimulated the release of collagen by increasing transcription in reporter assays (P = 0.05; Fig. S1 D).
Figure 1.
5-HT stimulates the production of extracellular matrix in dermal fibroblasts. (A–C) mRNA levels of col 1a1 (A), col 1a2 (B), and fibronectin-1 (C) after stimulation of SSc and healthy dermal fibroblasts with 5-HT. (D) The release of collagen protein from dermal fibroblasts upon 5-HT stimulation (n = 5 each). *, P < 0.05 compared with controls. The experiment was performed in two independent series. Error bars indicate SE.
The profibrotic effects of 5-HT are mediated by 5-HT2B
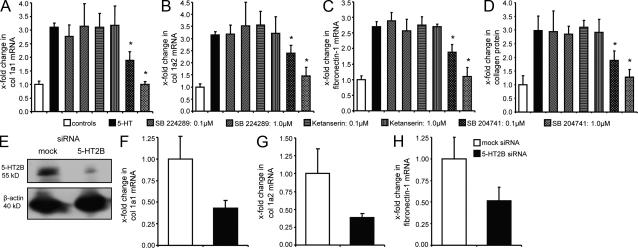
Consistent with a previous study, we observed that three different receptors for 5-HT are expressed in dermal fibroblasts: 5-HT1B, 5-HT2A, and 5-HT2B (Slominski et al., 2003). In contrast to 5-HT1B and 5-HT2A, the mRNA levels of 5-HT2B were up-regulated by 143 ± 17% in SSc fibroblasts compared with healthy controls (P < 0.05; unpublished data). To investigate which of these receptors mediates the profibrotic effects of 5-HT, dermal fibroblasts were incubated with selective serotonin receptor inhibitors. Inhibition of 5-HT1B by SB 224289 did not reduce the stimulatory effects of 5-HT on extracellular matrix production in SSc fibroblasts (Fig. 2, A–D). In contrast, the 5-HT2 inhibitor terguride decreased mRNA levels of col 1a1, col 1a2, and fibronectin-1 and also the release of collagen protein in a dose-dependent manner (Fig. S2, A–D). Most pronounced effects were observed at a concentration of 1.0 µM, with an almost complete blockade of 5-HT–induced extracellular matrix expression. To discriminate between 5-HT2A and 5-HT2B, the 5-HT2A inhibitor ketanserin and the 5-HT2B antagonist SB 204741 were used. No inhibitory effects were observed with ketanserin up to 1 µM, whereas SB 204741 at 1 µM completely prevented induction of extracellular matrix synthesis by 5-HT in SSc fibroblasts (Fig. 2, A–D). No differences in metabolic activity, as measured by conversion of microtiter tetrazolium (MTT), or in the number of annexin V– or propidium iodide–positive fibroblasts were observed upon incubation with terguride or SB 204741, demonstrating that the reduced synthesis of extracellular matrix proteins upon inhibition of 5-HT2B is not caused by toxicity of the inhibitors (unpublished data).
Figure 2.
Inhibition of 5-HT2B but not of 5-HT1B or 5-HT2A reduces the 5-HT–induced expression of extracellular matrix proteins. (A–D) Inhibition of 5-HT receptors by selective inhibitors at concentrations of 0.1 and 1.0 µM. mRNA levels of col 1a1 (A), col 1a2 (B), and fibronectin-1 (C) and release of collagen protein (D) after incubation with the 5-HT1B antagonist SB 224289, ketanserin (5-HT2A), and the 5-HT2B inhibitor SB 204741 (n = 7 each, two independent series). *, P < 0.05 compared with 5-HT–stimulated cells. (E–H) Knockdown of 5-HT2B by siRNA. (E) Expression levels of 5-HT2B protein after nucleofection with 5-HT2B and mock siRNA. (F–H) mRNA levels of col 1a1 (F), col 1a2 (G), and fibronectin-1 (H) after knockdown of 5-HT2B by siRNA in 5-HT–stimulated SSc dermal fibroblasts compared with mock-transfected cells (n = 5, two independent series). Error bars indicate SE.
As off-target effects of the chemical inhibitors cannot be fully excluded, SSc fibroblasts were transfected with small interfering RNAs (siRNAs) against 5-HT2B, which efficiently reduced mRNA and protein levels of 5-HT2B (Fig. 2 E). Moreover, silencing of 5-HT2B in the presence of 5-HT significantly decreased mRNA expression for col 1a1, col 1a2, and fibronectin-1 compared with SSc fibroblasts transfected with nontargeting control siRNA (Fig. 2, F–H), suggesting that 5-HT2B plays a crucial role for the synthesis of extracellular matrix proteins in dermal fibroblasts.
To exclude the possibility that the decreased synthesis of extracellular matrix proteins is counter-regulated by induction of matrix-degrading enzymes or their inhibitors, the expression of MMPs (matrix metalloproteinases) and TIMPs (tissue inhibitors of MMPs) was analyzed in fibroblasts treated with 5-HT, terguride, and SB 204741 and in fibroblasts transfected with 5-HT2B siRNA. The expression of MMP-1, MMP-2, MMP-3, and MMP-13 and TIMP-1, TIMP-2, TIMP-3, and TIMP-4 was not affected upon stimulation with 5-HT or inhibition of 5-HT2B (unpublished data).
5-HT2B is overexpressed in skin of SSc patients
After demonstrating that 5-HT exerts its profibrotic effects via 5-HT2B, we were interested whether 5-HT2B might be up-regulated in human fibrotic diseases. 5-HT2B was detectable by immunohistochemistry in the fibrotic skin of SSc patients as well as in normal skin of healthy individuals (Fig. 3). However, expression of 5-HT2B was strongly increased in fibrotic tissue compared with unaffected tissue from healthy controls (Fig. 3 A). Double staining for the fibroblast-specific marker prolyl-4-hydroxylase β and 5-HT2B demonstrated that almost all fibroblasts stained positive for 5-HT2B in fibrotic tissue but not in controls (Fig. S2 E). Of note, intensive staining for 5-HT2B was observed in α-SMA (α smooth muscle actin)–positive, SM22-α (smooth muscle protein 22 α)–negative myofibroblasts in skin of SSc patients (Fig. 3 B). Together with a previous report of an increased serum concentration of 5-HT in SSc (Biondi et al., 1988), these findings suggest that the 5-HT/5-HT2B axis is up-regulated in systemic fibrotic disease.
Figure 3.
5-HT2B is overexpressed in patients with SSc. (A) 5-HT2B was detectable by immunohistochemistry in fibroblasts, keratinocytes, and microvessels of SSc patients and controls. Two representative tissue sections and an isotype control are shown. (B) 5-HT2B expression in SM22-α–negative and α-SMA–positive myofibroblasts. A representative skin section of an SSc patient is shown. SM22-α as specific marker for vascular smooth muscle cells was stained with DAB, the myofibroblast marker α-SMA with Alexa Flour 594 (red), and 5-HT2B with Alexa Flour 488 (green; n = 8 each). The stainings were performed in three independent series. Bars: (A and B [top]) 50 µm; (B, bottom) 10 µm.
5-HT2B activates TGF-β/Smad signaling
TGF-β has been identified as a key player in the pathogenesis of fibrosis (Varga and Abraham, 2007). When we compared the effects of 5-HT and TGF-β in col 1a2 reporter assays, we observed different time kinetics. The stimulatory effects of 5-HT peaked after 24 h, whereas the maximal effects of TGF-β were observed after 6 h (Fig. S1 D). The delayed activation by 5-HT implicates that the profibrotic effects of 5-HT might be mediated indirectly by induction of a second messenger.
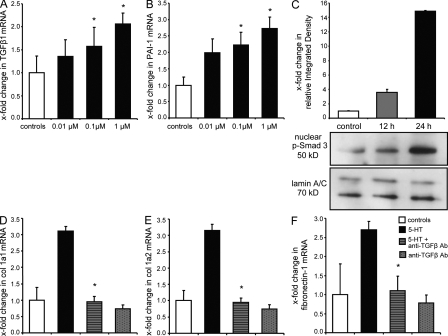
To investigate whether TGF-β itself might be this second mediator, we first analyzed the effects of 5-HT on the expression of TGF-β1 and of its target gene PAI-1 (plasminogen activator inhibitor 1). 5-HT dose-dependently increased the mRNA levels of TGF-β1 and PAI-1 in SSc fibroblasts (Fig. 4, A and B). Furthermore, 5-HT induced a time-dependent increase in nuclear levels of phospho-Smad3, the typical intracellular mediator of TGF-β signaling (Fig. 4 C). We next evaluated whether the profibrotic effects of 5-HT depend on TGF-β. Preincubation of SSc fibroblasts with neutralizing antibodies against TGF-β1 completely abrogated the profibrotic effects of 5-HT on mRNA expression of col 1a1, col 1a2, and fibronectin-1 (Fig. 4, D–F). The mRNA levels of col 1a1, col 1a2, and fibronectin-1 were only slightly reduced by neutralizing antibodies against TGF-β1 in unstimulated fibroblasts, suggesting that the observed effects of anti–TGF-β1 antibodies in 5-HT–stimulated fibroblasts were caused by blockade of the stimulatory effects of 5-HT rather than caused by effects on the basal synthesis (Fig. 4, D–F). Similar results were also obtained for healthy dermal fibroblasts (unpublished data).
Figure 4.
5-HT exerts its profibrotic effects via activation of TGF-β/Smad-dependent pathways. (A and B) mRNA levels of TGF-β1 (A) and PAI-1 (B) in 5-HT–stimulated SSc fibroblasts (n = 9, three independent series). (C) Nuclear accumulation of p-Smad3 protein complexes after 12 and 24 h of stimulation with 1 µM 5-HT (n = 3). (D–F) mRNA expression of col 1a1 (D), col 1a2 (E), and fibronectin-1 (F) upon incubation with 500 ng/ml of neutralizing antibodies (Ab) against TGF-β1 in 5-HT–stimulated SSc fibroblasts (n = 5 each, two independent series). *, P < 0.05 compared with 5-HT–stimulated (D–F) or nonstimulated (A and B) controls. Error bars indicate SE.
Targeting of 5-HT2B exerts potent antifibrotic effects
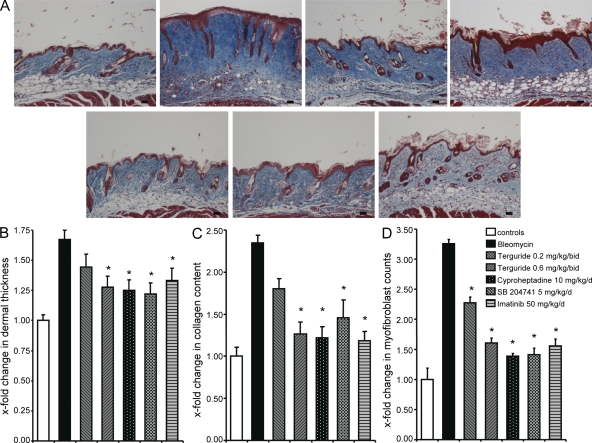
We then assessed different inhibitors of 5-HT receptor for their antifibrotic activity in the mouse model of bleomycin-induced dermal fibrosis. Imatinib, a tyrosine kinase inhibitor with well established antifibrotic effects in this model, served as positive control (Wang et al., 2005; Distler et al., 2007a). No signs of toxicity were observed upon treatment with terguride, cyproheptadine, SB 204741, and imatinib. Injection of bleomycin potently stimulated the expression of 5-HT2B and induced dermal fibrosis (Fig. 5 A and Fig. S3 A). However, the 5-HT2 inhibitors terguride and cyproheptadine as well as the selective 5-HT2B inhibitor SB 204741 (Goldberg et al., 1979; Bonhaus et al., 1995; Glusa and Pertz, 2000; Jähnichen et al., 2005) efficiently prevented bleomycin-induced dermal thickening (Fig. 5, A and B). Collagen content and myofibroblast counts were also dose-dependently reduced by the inhibition of 5-HT2B (Fig. 5, C and D).
Figure 5.
Inhibition of 5-HT signaling by the 5-HT2 inhibitors terguride, cyproheptadine, and SB 204741 prevents accumulation of extracellular matrix in experimental dermal fibrosis. (A) Representative trichrome-stained tissue sections are shown: control mice injected intracutaneously with NaCl (n = 10), bleomycin-challenged mice without antifibrotic treatment (n = 10), mice receiving bleomycin and terguride at a concentration of 0.2 mg/kg/bid (n = 8), mice treated with bleomycin and terguride at a concentration of 0.6 mg/kg/bid (n = 8), mice treated with bleomycin and cyproheptadine at a concentration of 10 mg/kg/d (n = 6), mice treated with bleomycin and SB 204741 at a concentration of 5 mg/kg/d (n = 6), and mice treated with bleomycin and imatinib at a concentration of 50 mg/kg/d (n = 6). Bars, 100 µm. (B) Dermal thickening upon challenge with bleomycin and treatment with terguride, cyproheptadine, SB 204741, or imatinib. (C) Collagen accumulation in lesional skin as analyzed with the SirCol collagen assay. (D) Myofibroblast counts after treatment of bleomycin-challenged mice with terguride, cyproheptadine, SB 204741, or imatinib. *, P < 0.05 compared with bleomycin-treated mice without antifibrotic treatment. The experiment was performed in two independent series. Error bars indicate SE.
The antifibrotic effects of the inhibition of 5-HT2 were further tested in a therapeutic approach in a modified bleomycin model. When the treatment with terguride was started after induction of fibrosis, terguride did not only prevent further progression, but induced regression of fibrosis (Fig. S3 B). Dermal thickening decreased by 78 ± 4% in mice treated with terguride for the last 3 wk compared with mice challenged with bleomycin for 6 wk (P = 0.003) and by 66 ± 4% compared with mice injected with bleomycin for 3 wk (P = 0.002; Fig. S3 C). The collagen content and the number of myofibroblasts were also efficiently reduced (Fig. S3, D and E).
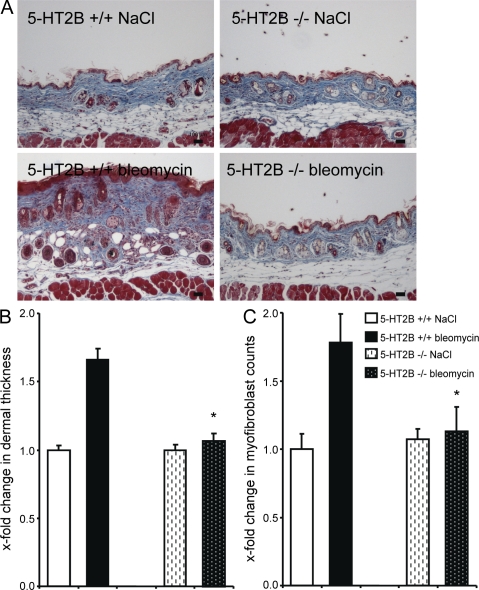
To confirm the role of 5-HT2B in experimental fibrosis by a genetic approach, we evaluated the effects of bleomycin in mice lacking 5-HT2B. No histological changes or differences in dermal thickness were observed in untreated 5-HT2B−/− mice (Fig. 6 A). However, 5-HT2B−/− mice were almost completely protected from bleomycin-induced fibrosis. Bleomycin induced only minor dermal thickening, and the hydroxyproline content and the myofibroblast numbers did not increase in 5-HT2B−/− mice upon bleomycin challenge (Fig. 6, A–C).
Figure 6.
Mice lacking 5-HT2B are protected from bleomycin-induced dermal fibrosis. (A) Trichrome-stained tissue sections of 5-HT2B−/− and wild-type mice. Representative tissue sections are shown: 5-HT2B+/+ mice injected with NaCl intracutaneously, 5-HT2B−/− mice receiving intracutaneous injections of NaCl, 5-HT2B+/+ mice receiving intracutaneous injections of bleomycin, and 5-HT2B−/− mice injected with bleomycin (n = 8 each). Bars, 100 µm. (B) Dermal thickening in bleomycin-challenged 5-HT2B−/− and wild-type mice. (C) Myofibroblast number in 5-HT2B–deficient and wild-type mice upon bleomycin challenge. *, P < 0.05 compared with bleomycin-injected wild-type mice. The experiment was performed in two independent series. Error bars indicate SE.
To assess the role of 5-HT2B in a less inflammation-dependent model of fibrosis, the Tsk-1 (tight skin 1) model was used. The Tsk-1 model is characterized by increased hypodermal thickness compared with matched wild-type mice because of a tandem duplication in the fibrillin-1 gene (Green et al., 1976). Consistent with the findings in human SSc and in bleomycin-induced fibrosis, 5-HT2B was overexpressed in skin sections of Tsk-1 mice (Fig. S4 A). Treatment with the 5-HT2B inhibitor SB 204741 normalized fibrotic changes observed in Tsk-1 mice (Fig. S4 B). Hypodermal thickening, collagen content, and the differentiation of resting fibroblasts into myofibroblasts in Tsk-1 mice were significantly reduced upon treatment with SB 204741 (Fig. S4, C–E).
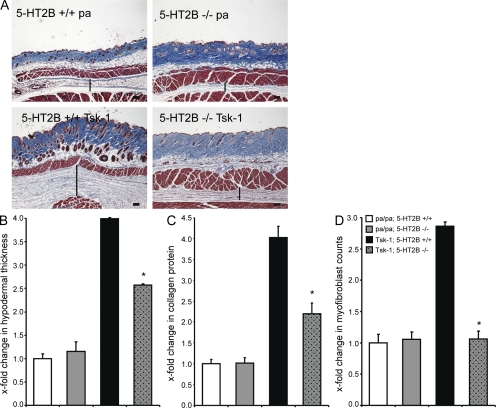
Moreover, when 5-HT2B−/− mice were crossed with Tsk-1 mice, hypodermal thickening was significantly reduced in 5-HT2B−/−/Tsk-1 mice compared with 5-HT2B+/+/Tsk-1 mice (Fig. 7, A and B). Furthermore, the collagen content and the myofibroblast numbers in the skin of 5-HT2B−/−/Tsk-1 mice were significantly decreased compared with 5-HT2B+/+/Tsk-1 mice (Fig. 7, C and D).
Figure 7.
Deficiency of 5-HT2B corrects the histological changes in Tsk-1 mice. (A) Representative sections of trichrome staining are shown: 5-HT2B+/+ mice without Tsk-1 allele (n = 5), 5-HT2B−/− mice without Tsk-1 allele (n = 8), 5-HT2B+/+/Tsk-1 mice (n = 5), and 5-HT2B−/−/Tsk-1 mice (n = 8). Vertical bars indicate the hypodermal thickness. Bars, 250 µm. (B) Hypodermal thickness in 5-HT2B–deficient Tsk-1 mice. (C) Collagen content in the skin of 5-HT2B−/−/Tsk-1 mice. (D) Myofibroblast counts in Tsk-1 mice after knockdown of 5-HT2B. *, P < 0.05 compared with 5-HT2B+/+/Tsk-1 mice. The measurements were performed twice. Error bars indicate SE.
Inhibition of platelet activation ameliorates bleomycin-induced skin fibrosis and the Tsk-1 phenotype
To confirm the link between increased platelet activation and increased 5-HT/5-HT2B signaling, we first measured the levels of 5-HT in fibrotic skin. The concentration of 5-HT in lesional skin increased to 5.8 ± 0.5 ng/mg in bleomycin-injected mice compared with 3.2 ± 0.2 ng/mg in control mice (P = 0.02). In the Tsk-1 model, the concentration of 5-HT was increased from 4.0 ± 0.4 ng/mg to 6.7 ± 1.0 ng/mg (P = 0.04). Thus, levels of 5-HT are up-regulated in different models of experimental fibrosis in accordance with the increased levels seen in human SSc (Stachów et al., 1979; Biondi et al., 1988).
Because the vast majority of 5-HT in nonneuronal tissues is derived from platelets, we hypothesized that inhibition of platelet activation might reduce the tissue levels of 5-HT and prevent experimental fibrosis. Indeed, treatment with the P2Y12 receptor inhibitor clopidogrel reduced the 5-HT content in the fibrotic skin of bleomycin-challenged mice by 58 ± 21% (P = 0.03). In parallel, clopidogrel decreased dermal thickening by 61 ± 13% compared with control mice (P = 0.001; Fig. S5, A and B). Consistently, the collagen content and the myofibroblast counts were significantly reduced (Fig. S5, C and D).
Hypodermal thickening in Tsk-1 mice was also reduced by 51 ± 16% (P = 0.009) upon treatment with clopidogrel (Fig. S5, E and F). Additionally, the collagen content and the number of activated myofibroblasts in skin were significantly decreased (Fig. S5, G and H).
Lack of 5-HT in platelets via knockout of TPH1 prevents experimental dermal fibrosis
Platelet granules do not only contain 5-HT but also other growth factors. To demonstrate that 5-HT is the major profibrotic mediator released by platelets, we investigated the outcome of TPH1-deficient (TPH1−/−) mice in experimental fibrosis. TPH1−/− mice do not completely lack 5-HT, but total blood levels of 5-HT are reduced to 5% as compared with wild-type animals (Walther et al., 2003b). Consistent with an important role of platelet-derived 5-HT in fibrosis, the profibrotic effects of bleomycin were markedly reduced in TPH1−/− mice (Fig. 8 A). Dermal thickening was decreased by 61 ± 6% (P = 0.004), and the collagen content as well as myofibroblast counts were significantly reduced in TPH1−/− mice as compared with TPH1+/+ mice (Fig. 8, B–D).
Figure 8.
Mice lacking TPH1 are protected from bleomycin-induced dermal fibrosis. (A) Trichrome-stained tissue sections are shown: TPH1+/+ mice injected with NaCl intracutaneously (n = 7), TPH1−/− mice with intracutaneous injections of NaCl (n = 5), bleomycin-injected TPH1+/+ mice (n = 7), and TPH1−/− mice challenged with bleomycin (n = 5). Bars, 100 µm. (B) Dermal thickness in bleomycin-challenged TPH1-deficient mice. (C) Collagen protein content in bleomycin-challenged TPH1−/− mice as analyzed by hydroxyproline assay. (D) Changes in the number of α-SMA–positive myofibroblasts upon bleomycin challenge in TPH1-deficient mice. *, P < 0.05 compared with bleomycin-injected wild-type mice. The experiment was performed in two independent series. Error bars indicate SE.
TPH1−/− mice were interbred with Tsk-1 mice to yield Tsk-1 mice lacking 5-HT in platelets. Fibrosis was also efficiently ameliorated in TPH1−/−/Tsk-1 with reduced hypodermal thickening, decreased hydroxyproline content, and lower myofibroblast counts mice as compared with TPH1+/+/Tsk-1 (Fig. S6, A–D).
DISCUSSION
Herein, we demonstrate that 5-HT2B signaling plays a central role for fibrosis. Several experimental levels such as 5-HT2B–null mice and treatment with small molecule inhibitors as well as in vitro and ex vivo studies in humans suggested that binding of 5-HT to its receptor 5-HT2B is a key step in tissue fibrosis. We believe that these findings have direct clinical implications: potent inhibitors of 5-HT2 receptors, including cyproheptadine and terguride used in our study as well as mianserin and lisuride, are already in clinical use and are well tolerated (Obeso et al., 1986; von Werner et al., 1989; Moertel et al., 1991; Szegedi and Schwertfeger, 2005). Furthermore, selective inhibitors of 5-HT2B are in clinical development and might be available in the near future. Thus, inhibition of 5-HT2B might be a promising novel therapeutic approach as efficient antifibrotic therapies are not yet available.
Patients affected with SSc develop a characteristic microangiopathy as the result of ongoing endothelial cell damage and endothelial cell activation early in the course of the disease, before tissue fibrosis becomes evident (Sgonc et al., 1996; Varga and Abraham, 2007). Loss of the anticoagulant properties of the endothelial cell layer results in platelet activation and the release of bioactive molecules such as β-thrombomodulin and vascular endothelial growth factor, which are increased in the serum of SSc patients (Soma et al., 1993; Mercié et al., 1995; Distler et al., 2002). Platelets are also the largest storage pool for 5-HT, and elevated plasma levels of 5-HT have been observed in systemic fibrosis (Biondi et al., 1988). Thus, microangiopathy with subsequent platelet activation and increased release of 5-HT might further activate 5-HT/5-HT2B signaling in addition to the 5-HT2B overexpression in fibrotic diseases. Indeed, inhibition of platelet activation or selective inhibition of the synthesis of 5-HT prevents experimental fibrosis in different mouse models, thereby highlighting the role of platelet activation in fibrosis.
Our study could also explain the molecular mechanism of the clinical association between carcinoid tumors and fibrosis. Carcinoid tumors are neuroendocrine tumors producing large amounts of 5-HT. Tissue fibrosis is often found in patients with carcinoid tumors, both locally and systemically (Modlin et al., 2004). Local tissue fibrosis in the peritumoral tissue is found in most patients. The accumulation of extracellular matrix might be substantial and by far exceed the actual tumor volume (Moertel et al., 1961; Makridis et al., 1996). The most common systemic fibrotic manifestation is carcinoid heart disease with fibrotic changes of the cardiac valves (Hallen, 1964). The lesions are located on the mural and valvular endocardium and consist of a matrix-rich stroma and interspersed myofibroblasts, which were also found in an animal model of carcinoid tumors (Gustafsson et al., 2005). Pleural, pulmonary, gastrointestinal, retroperitoneal, and skin fibrosis can occur. Skin involvement manifests as scleroderma-like disease with dermal fibrosis (Zarafonetis et al., 1958; Fries et al., 1973; Modlin et al., 2004). Of note, dermal fibrosis has been reported to regress after effective treatment of carcinoid tumors (Pavlovic et al., 1995). Despite these abundant studies, the molecular mechanism by which these tumors caused tissue fibrosis was unclear. Some authors suggested that tissue hypoxia caused by vasospasm or compression might trigger fibrosis (Modlin et al., 2004). Others speculated that tachykinins released from carcinoid tumors might mediate tissue fibrosis (Katayama and Nishioka, 1997; Ashour et al., 2006). Others proposed that 5-HT might be the cause of carcinoid-associated fibrosis (MacDonald et al., 1958; Hallen, 1964).
The ergot methysergide can also cause retroperitoneal fibrosis. The metabolism of the 5-HT2B antagonist methysergide to its active metabolite methylergonovine, which is a potent 5-HT2B agonist, provides an explanation for this paradoxical observation (Reimund, 1987). Similarly, 5-HT2B agonism has been implicated in the development of fibrotic changes by selected ergots such as pergolide or bromocriptine, anorexins like fenfluramine, or drugs of abuse as for example MDMA (3,4-methylenedioxymethamphetamine; also known as ecstasy; Rothman et al., 2000; Setola et al., 2003; Hofmann et al., 2006; Roth, 2007). As we demonstrate herein that activation of 5-HT2B potently stimulates the production of extracellular matrix proteins, it might be concluded that activation of 5-HT2B by the metabolite methylergonovine might be sufficient to promote the development of fibrosis. The importance of 5-HT signaling for fibrotic diseases is supported by recent studies with 5-HT receptor antagonists in experimental pulmonary or liver fibrosis (Ruddell et al., 2006; Fabre et al., 2008). Thus, our findings might have widespread implications, namely that inhibition of the 5-HT2B pathway might be a promising strategy for fibrotic disorders.
MATERIALS AND METHODS
Patients, mice, and fibroblast cultures.
Fibroblast cultures were obtained from skin biopsies of SSc patients and healthy volunteers as described previously (Akhmetshina et al., 2008). All patients fulfilled the criteria for SSc as suggested by LeRoy and Medsger (2001). Patient characteristics are summarized in Table S1. All patients and controls signed a consent form approved by the local institutional review boards. All human protocols were approved by the ethical board of the University of Erlangen-Nuremberg.
TPH1−/− mice and 5-HT2B−/− mice have been described previously (Nebigil et al., 2000; Walther et al., 2003b). All mouse experiments were approved by the Government of Mittelfranken.
ELISA.
4-mg skin biopsies of the injected area were analyzed by competitive ELISA for 5-HT using a serotonin ELISA kit (GenWay).
Stimulation with 5-HT and 5-HT receptor inhibition.
For stimulation experiments, a special FCS depleted of 5-HT was used (HyClone). Dermal fibroblasts were stimulated with 5-HT (Sigma-Aldrich) in concentrations from 0.01 to 1.0 µM.
Cells were incubated with the nonselective 5-HT2 inhibitor terguride (0.01–1.0 µM) or the selective inhibitors SB 224289 (5-HT1B), ketanserin (5-HT2A), and SB 204741 (5-HT2B) at concentrations of 0.1 and 1.0 µM (Kasho et al., 1998; Lawrie et al., 2005). The Ki values of all chemical inhibitors according to the National Institute of Mental Health Ki Database are given in Table S2. 5-HT was added 1 h after the inhibitors at a concentration of 1.0 µM.
Transfection with siRNAs against 5-HT2B.
SSc fibroblasts were transfected with 1.5 µg siRNA duplexes against 5-HT2B using the human dermal fibroblast Nucleofector kit (Lonza; Jüngel et al., 2007). Two different siRNA duplexes with the following sequences were used: siRNA duplex 1 sense, 5′-CCGCAUCCAUCAUGCAUCU-3′; and antisense, 5′-AGAUGCAUGAUGGAUGCGG-3′; and siRNA duplex 2 sense, 5′-GUGGUGUGGUUAAUUUCAA-3′; and antisense, 5′-UUGAAAUUAACCACACCAC-3′. Fibroblasts transfected with nontargeting control siRNAs (Invitrogen) were used as controls. Medium was changed after 6 h to remove the Nucleofector solution. Cells were harvested after 48 h.
Selective inhibition of TGF-β signaling.
Fibroblasts were stimulated with 1 µM 5-HT in the presence of neutralizing mouse anti–human TGF-β1 or IgG control antibodies (R&D Systems) at a concentration of 500 ng/ml as described previously (Distler et al., 2007b).
mRNA stability assay.
The mRNA stability assay was performed to investigate whether 5-HT stabilizes collagen or fibronectin mRNA as described previously (Distler et al., 2004). SSc fibroblasts were stimulated with 1 µM 5-HT. Actinomycin D was added at a concentration of 10 µg/ml 6 h later. Nonstimulated fibroblasts with and without actinomycin D served as controls. Fibroblasts were harvested 4, 8, 12, 18, and 24 h after the addition of actinomycin D and analyzed with real-time PCR.
Luciferase reporter assay.
The -353 COL1A2/LUC construct was provided by M. Trojanowska (Boston University School of Medicine, Boston, MA; Czuwara-Ladykowska et al., 2001). HEK293T cells were transfected in serum-free medium with 0.5 µg of reporter construct mixed with polyethylenimine. A common lacZ reporter vector was used as control. Cells were stimulated with 5-HT or TGF-β directly after transfection.
Quantitative real-time PCR.
Gene expression was quantified by TaqMan or by SYBR Green real-time PCR (ABI Prism 7300 sequence detection system; Applied Biosystems) as described previously (Akhmetshina et al., 2008). Total RNA was isolated with the NucleoSpin RNA II extraction system (Macherey-Nagel) according to the instructions of the manufacturer. RT into cDNA was performed as described previously (Akhmetshina et al., 2008) using random hexamers. Samples without enzyme in the RT reaction (non-RT controls) were used as negative controls. Unspecific signals caused by primer dimers were excluded by dissociation curve analysis and samples without cDNA (no template controls). A predeveloped 18S assay (Applied Biosystems) was used to normalize for the amounts of loaded cDNA. Differences were calculated with the comparative Ct method for relative quantification.
Collagen measurements.
Total soluble collagen in cell culture supernatants was quantified using the SirCol collagen assay (Biocolor; Akhmetshina et al., 2008). To detect also highly cross-linked collagen, the collagen content in skin samples was analyzed by determination of the hydroxyproline content (Woessner, 1961).
Western blot.
Nuclear and cytoplasmic extracts were prepared as previously described (Andrews and Faller, 1991; Khelifi et al., 2005). Polyvinylidene fluoride membranes were incubated with polyclonal rabbit anti–5-HT2B (GeneTex) or monoclonal rabbit anti–phospho-Smad3 antibodies (Cell Signaling Technology). Equal loading of proteins was confirmed by visualization of the nuclear protein lamin A/C (Cell Signaling Technology) and the cytoplasmic protein β-actin (Sigma-Aldrich). Quantification was performed with ImageJ software (version 1.41; National Institutes of Health).
Immunohistochemistry for 5-HT2B, α-SMA, and SM22-α.
Skin sections from SSc patients and controls were stained with polyclonal rabbit anti–5-HT2B antibodies. A subset of 5-HT2B–stained sections was double stained with the fibroblast marker prolyl-4-hydroxylase β (Acris Antibodies). To analyze the expression of 5-HT2B in myofibroblasts, we performed triple staining for 5-HT2B, the myofibroblast marker α-SMA (clone 1A4; Sigma-Aldrich), and SM22-α (Abcam) as specific marker for vascular smooth muscle cells to distinguish myofibroblasts from vessels. Antibodies labeled with horseradish peroxidase (Dako), alkaline phosphatase (Jackson ImmunoResearch Laboratories, Inc.), and Alexa Flour 488 or Alexa Flour 594 (both Invitrogen) were used as secondary antibodies. The expression of SM22-α and 5-HT2B in single- and double-stained sections was visualized with DAB (diaminobenzidine) peroxidase substrate solution (Sigma-Aldrich), and the expression of prolyl-4-hydroxylase β with BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium; Vector Laboratories). Isotype antibodies in the same concentration were used for controls. Myofibroblasts in mouse sections were identified by staining for α-SMA as described previously (Distler et al., 2007a).
MTT assay.
The metabolic activity of dermal fibroblasts incubated with inhibitors of 5-HT2B was measured using the MTT (3, [4, 5-dimethylthiazol-2-yl] 2, 5-diphenyl-tetrazolium bromide) method (Distler et al., 2005). In brief, SSc and healthy dermal fibroblasts were incubated with terguride or SB 204741 in concentrations of 0.1 and 1.0 µM in 96-well plates for 20 h. After removing 100 µl of the medium, MTT was added at a final concentration of 1 mg/ml, and the cells were incubated at 37°C for 4 h. After dilution with 300 µl of 0.04 N HCl in isopropanol, the cells were analyzed using the ELISA reader (Spectra MAX 190 microplate spectrophotometer; Molecular Devices) at a test wavelength of 570 nm with a control wavelength of 630 nm. Untreated fibroblasts were used as negative controls, and all other results were normalized to untreated cells. Fibroblasts incubated with 50% DMSO served as positive controls.
Quantification of apoptotic and necrotic cells.
Dermal fibroblasts were incubated with terguride or SB 204741 for 7 d. The number of apoptotic and necrotic cells was analyzed as described previously (Distler et al., 2007a).
Bleomycin-induced dermal fibrosis.
Skin fibrosis was induced in 6-wk-old, pathogen-free, female DBA/2 mice (Charles River) by local injections of bleomycin for 21 d. Seven groups received subcutaneous injections of 100 µl bleomycin dissolved in 0.9% NaCl at a concentration of 0.5 mg/ml in defined areas of the upper back every other day to induce dermal fibrosis. Six groups of bleomycin-challenged mice were treated with the P2Y12 receptor inhibitor clopidogrel (25 mg/kg/d) by oral gavage, terguride (0.2 mg/kg/bid and 0.6 mg/kg/bid) by intraperitoneal injections, cyproheptadine (10 mg/kg/d) by oral gavage, SB 204741 (5 mg/kg/d) subcutaneously, or imatinib (50 mg/kg/d) by intraperitoneal injections, which served as positive control. One group with subcutaneous injections of 100 µl of 0.9% NaCl served as control group. After 21 d, animals were sacrificed by cervical dislocation.
The mouse model of bleomycin-induced dermal fibrosis was also used to evaluate a potential protection from fibrosis in mice lacking TPH1 or 5-HT2B. Four groups of mice were analyzed. Two groups consisted of TPH1-deficient FVB mice (n = 5) or 129/PAS mice lacking 5-HT2B (n = 8), whereas the other two groups consisted of wild-type FVB (n = 7) and 129/PAS mice (n = 8). Dermal fibrosis was induced in one group each by repeated injections of bleomycin, whereas the other two groups received sham treatment with subcutaneous injections of NaCl and served as controls.
Treatment of established fibrosis.
For the treatment of established fibrosis, a modified model of bleomycin-induced dermal fibrosis was used. Six groups of mice were analyzed. Two groups of mice were challenged with bleomycin for 3 wk and for 6 wk, respectively. The third group received bleomycin injections for 3 wk followed by NaCl injections for the next 3 wk to control for spontaneous regression of fibrosis. To assess the effects of terguride for treatment of established fibrosis, mice were challenged with bleomycin for 6 wk and treated in parallel with terguride (0.6 mg/kg/bid) for the last 3 wk. Two groups of mice receiving intracutaneous injections of 100 µl of 0.9% NaCl for 3 wk and 6 wk, respectively, were used as controls.
Inhibition of platelet aggregation and 5-HT2B signaling in Tsk-1 mice.
B6.Cg-Fbn1tsk+/+Pldnpa/J (Tsk-1) mice and age-matched pa/pa (wild type) controls were purchased from the Jackson Laboratory (Charles River). Four groups of mice were analyzed. Three groups of Tsk-1 mice were treated with clopidogrel (25 mg/kg/d) by oral gavage, SB 204741 (5 mg/kg/d) subcutaneously, or the solvent DMSO subcutaneously. The fourth group consisted of pa/pa (control) mice, which also received subcutaneous injections of DMSO. The treatment was started at an age of 3 wk, and mice were sacrificed after 6 wk of treatment.
In addition, Tsk-1 mice were crossed with 5-HT2B−/− or TPH1−/− mice to yield Tsk-1 mice deficient for 5-HT2B or TPH1. The F2 generations consisting of 5-HT2B−/−/Tsk-1 (TPH1−/−/Tsk-1), 5-HT2B+/+/Tsk-1 (TPH1+/+/Tsk-1), 5-HT2B−/−/pa (TPH1−/−/pa), and 5-HT2B+/+/pa (TPH1+/+/pa) mice were sacrificed at an age of 10 wk for further analysis.
Genotyping of the mice was performed by PCR. The following primers were used: 5-HT2B+/+ (forward, 5′-CTGGTTATTCCTCGATGTTCTCTT-3′; and reverse, 5′-AACCATACCACTGTAATCTTGATGAAT-3′), 5-HT2B−/− (forward, 5′-AGACAATCGGCTGCTCTGAT-3′; and reverse, 5′-AGCCAACGCTATGTCCTGAT-3′), mutated fibrillin-1/Tsk-1 (forward, 5′-GTTGGCAACTATACCTGCAT-3′; and reverse, 5′-CCTTTCCTGGTAACATAGGA-3′), and TPH1 (forward, 5′-GCTTGCAGGAGTTGGTTCTC-3′; reverse 1, 5′-CGTTGGCTACCCGTGATATT-3′; and reverse 2, 5′-CAGCTCTGTGATGGACGGTA-3′).
Histological analysis.
Paraffin-embedded histological sections were stained with hematoxylin and eosin for the determination of dermal thickness as described previously (Distler et al., 2007a). For direct visualization of collagen fibers, trichrome staining was performed using the Masson’s Trichrome Staining kit (Sigma-Aldrich).
Statistics.
Data are expressed as mean ± SE. The Wilcoxon signed rank tests for related samples and the Mann-Whitney U test for nonrelated samples were used for statistical analyses. A p-value of <0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 shows that 5-HT activates collagen transcription. Fig. S2 demonstrates that the 5-HT2 inhibitor terguride reduces the profibrotic effects of 5-HT. Fig. S3 shows that 5-HT2B is overexpressed in bleomycin-induced skin fibrosis and that terguride induces regression of preestablished fibrosis. Fig. S4 shows increased expression of 5-HT2B in Tsk-1 mice and that selective inhibition of 5-HT2B ameliorates histological changes in the Tsk-1 mouse model. Fig. S5 demonstrates that inhibition of platelet activation reduces experimental dermal fibrosis. Fig. S6 shows reduced hypodermal thickness in TPH1-deficient Tsk-1 mice. Table S1 lists the patient characteristics at date of biopsy. Table S2 shows the Ki values of the chemical inhibitors used in this study. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101629/DC1.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DI 1537/2-1), grant A20 of the Interdisciplinary Center of Clinical Research in Erlangen, a Career Support Award of Medicine of the Ernst Jung Foundation, and a research grant from ErgoNex Pharma GmbH, Switzerland. L. Maroteaux was supported by grants from the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Medicale, and Fondation de France.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- 5-HT
- 5-hydroxytryptamine
- mRNA
- messenger RNA
- MTT
- microtiter tetrazolium
- siRNA
- small interfering RNA
- SSc
- systemic sclerosis
References
- Akhmetshina A., Dees C., Pileckyte M., Maurer B., Axmann R., Jüngel A., Zwerina J., Gay S., Schett G., Distler O., Distler J.H. 2008. Dual inhibition of c-abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis. FASEB J. 22:2214–2222 10.1096/fj.07-105627 [DOI] [PubMed] [Google Scholar]
- Andrews N.C., Faller D.V. 1991. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 19:2499 10.1093/nar/19.9.2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour K., Shan L., Lee J.H., Schlicher W., Wada K., Wada E., Sunday M.E. 2006. Bombesin inhibits alveolarization and promotes pulmonary fibrosis in newborn mice. Am. J. Respir. Crit. Care Med. 173:1377–1385 10.1164/rccm.200507-1014OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataller R., Brenner D.A. 2005. Liver fibrosis. J. Clin. Invest. 115:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi M.L., Marasini B., Bianchi E., Agostoni A. 1988. Plasma free and intraplatelet serotonin in patients with Raynaud’s phenomenon. Int. J. Cardiol. 19:335–339 10.1016/0167-5273(88)90238-0 [DOI] [PubMed] [Google Scholar]
- Bonhaus D.W., Bach C., DeSouza A., Salazar F.H., Matsuoka B.D., Zuppan P., Chan H.W., Eglen R.M. 1995. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 115:622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L., Krishnan E., Chakravarty E.F. 2007. Hospitalizations and mortality in systemic sclerosis: results from the Nationwide Inpatient Sample. Rheumatology (Oxford). 46:1808–1813 10.1093/rheumatology/kem273 [DOI] [PubMed] [Google Scholar]
- Czuwara-Ladykowska J., Shirasaki F., Jackers P., Watson D.K., Trojanowska M. 2001. Fli-1 inhibits collagen type I production in dermal fibroblasts via an Sp1-dependent pathway. J. Biol. Chem. 276:20839–20848 10.1074/jbc.M010133200 [DOI] [PubMed] [Google Scholar]
- Distler J.H., Hagen C., Hirth A., Müller-Ladner U., Lorenz H.M., del Rosso A., Michel B.A., Gay R.E., Nanagara R., Nishioka K., et al. 2004. Bucillamine induces the synthesis of vascular endothelial growth factor dose-dependently in systemic sclerosis fibroblasts via nuclear factor-kappaB and simian virus 40 promoter factor 1 pathways. Mol. Pharmacol. 65:389–399 10.1124/mol.65.2.389 [DOI] [PubMed] [Google Scholar]
- Distler J.H., Jüngel A., Huber L.C., Seemayer C.A., Reich C.F., III, Gay R.E., Michel B.A., Fontana A., Gay S., Pisetsky D.S., Distler O. 2005. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc. Natl. Acad. Sci. USA. 102:2892–2897 10.1073/pnas.0409781102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler J.H., Jüngel A., Huber L.C., Schulze-Horsel U., Zwerina J., Gay R.E., Michel B.A., Hauser T., Schett G., Gay S., Distler O. 2007a. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 56:311–322 10.1002/art.22314 [DOI] [PubMed] [Google Scholar]
- Distler J.H., Jüngel A., Pileckyte M., Zwerina J., Michel B.A., Gay R.E., Kowal-Bielecka O., Matucci-Cerinic M., Schett G., Marti H.H., et al. 2007b. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 56:4203–4215 10.1002/art.23074 [DOI] [PubMed] [Google Scholar]
- Distler O., Del Rosso A., Giacomelli R., Cipriani P., Conforti M.L., Guiducci S., Gay R.E., Michel B.A., Brühlmann P., Müller-Ladner U., et al. 2002. Angiogenic and angiostatic factors in systemic sclerosis: increased levels of vascular endothelial growth factor are a feature of the earliest disease stages and are associated with the absence of fingertip ulcers. Arthritis Res. 4:R11 10.1186/ar596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre A., Marchal-Sommé J., Marchand-Adam S., Quesnel C., Borie R., Dehoux M., Ruffié C., Callebert J., Launay J.M., Hénin D., et al. 2008. Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur. Respir. J. 32:426–436 10.1183/09031936.00126907 [DOI] [PubMed] [Google Scholar]
- Fries J.F., Lindgren J.A., Bull J.M. 1973. Scleroderma-like lesions and the carcinoid syndrome. Arch. Intern. Med. 131:550–553 [DOI] [PubMed] [Google Scholar]
- Gabrielli A., Avvedimento E.V., Krieg T. 2009. Scleroderma. N. Engl. J. Med. 360:1989–2003 10.1056/NEJMra0806188 [DOI] [PubMed] [Google Scholar]
- Glusa E., Pertz H.H. 2000. Further evidence that 5-HT-induced relaxation of pig pulmonary artery is mediated by endothelial 5-HT(2B) receptors. Br. J. Pharmacol. 130:692–698 10.1038/sj.bjp.0703341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S.C., Halmi K.A., Eckert E.D., Casper R.C., Davis J.M. 1979. Cyproheptadine in anorexia nervosa. Br. J. Psychiatry. 134:67–70 10.1192/bjp.134.1.67 [DOI] [PubMed] [Google Scholar]
- Green M.C., Sweet H.O., Bunker L.E. 1976. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am. J. Pathol. 82:493–512 [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B.I., Tømmerås K., Nordrum I., Loennechen J.P., Brunsvik A., Solligård E., Fossmark R., Bakke I., Syversen U., Waldum H. 2005. Long-term serotonin administration induces heart valve disease in rats. Circulation. 111:1517–1522 10.1161/01.CIR.0000159356.42064.48 [DOI] [PubMed] [Google Scholar]
- Hallen A. 1964. Fibrosis in the carcinoid syndrome. Lancet. 283:746–747 10.1016/S0140-6736(64)92853-3 [DOI] [PubMed] [Google Scholar]
- Hervé P., Launay J.M., Scrobohaci M.L., Brenot F., Simonneau G., Petitpretz P., Poubeau P., Cerrina J., Duroux P., Drouet L. 1995. Increased plasma serotonin in primary pulmonary hypertension. Am. J. Med. 99:249–254 10.1016/S0002-9343(99)80156-9 [DOI] [PubMed] [Google Scholar]
- Hofmann C., Penner U., Dorow R., Pertz H.H., Jähnichen S., Horowski R., Latté K.P., Palla D., Schurad B. 2006. Lisuride, a dopamine receptor agonist with 5-HT2B receptor antagonist properties: absence of cardiac valvulopathy adverse drug reaction reports supports the concept of a crucial role for 5-HT2B receptor agonism in cardiac valvular fibrosis. Clin. Neuropharmacol. 29:80–86 10.1097/00002826-200603000-00005 [DOI] [PubMed] [Google Scholar]
- Humphrey P.P., Hartig P., Hoyer D. 1993. A proposed new nomenclature for 5-HT receptors. Trends Pharmacol. Sci. 14:233–236 10.1016/0165-6147(93)90016-D [DOI] [PubMed] [Google Scholar]
- Jähnichen S., Horowski R., Pertz H.H. 2005. Agonism at 5-HT2B receptors is not a class effect of the ergolines. Eur. J. Pharmacol. 513:225–228 10.1016/j.ejphar.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Jüngel A., Distler O., Schulze-Horsel U., Huber L.C., Ha H.R., Simmen B., Kalden J.R., Pisetsky D.S., Gay S., Distler J.H. 2007. Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 56:3564–3574 10.1002/art.22980 [DOI] [PubMed] [Google Scholar]
- Kahaleh M.B., Osborn I., Leroy E.C. 1982. Elevated levels of circulating platelet aggregates and beta-thromboglobulin in scleroderma. Ann. Intern. Med. 96:610–613 [DOI] [PubMed] [Google Scholar]
- Kasho M., Sakai M., Sasahara T., Anami Y., Matsumura T., Takemura T., Matsuda H., Kobori S., Shichiri M. 1998. Serotonin enhances the production of type IV collagen by human mesangial cells. Kidney Int. 54:1083–1092 10.1046/j.1523-1755.1998.00114.x [DOI] [PubMed] [Google Scholar]
- Katayama I., Nishioka K. 1997. Substance P augments fibrogenic cytokine-induced fibroblast proliferation: possible involvement of neuropeptide in tissue fibrosis. J. Dermatol. Sci. 15:201–206 10.1016/S0923-1811(97)00608-7 [DOI] [PubMed] [Google Scholar]
- Khelifi A.F., D’Alcontres M.S., Salomoni P. 2005. Daxx is required for stress-induced cell death and JNK activation. Cell Death Differ. 12:724–733 10.1038/sj.cdd.4401559 [DOI] [PubMed] [Google Scholar]
- Lawrie A., Spiekerkoetter E., Martinez E.C., Ambartsumian N., Sheward W.J., MacLean M.R., Harmar A.J., Schmidt A.M., Lukanidin E., Rabinovitch M. 2005. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circ. Res. 97:227–235 10.1161/01.RES.0000176025.57706.1e [DOI] [PubMed] [Google Scholar]
- LeRoy E.C., Medsger T.A., Jr 2001. Criteria for the classification of early systemic sclerosis. J. Rheumatol. 28:1573–1576 [PubMed] [Google Scholar]
- MacDonald R.A., Robbins S.L., Mallory G.K. 1958. Dermal fibrosis following subcutaneous injections of serotonin creatinine sulphate. Proc. Soc. Exp. Biol. Med. 97:334–337 [DOI] [PubMed] [Google Scholar]
- Makridis C., Rastad J., Oberg K., Akerström G. 1996. Progression of metastases and symptom improvement from laparotomy in midgut carcinoid tumors. World J. Surg. 20:900–906 10.1007/s002689900137 [DOI] [PubMed] [Google Scholar]
- Mercié P., Seigneur M., Conri C. 1995. Plasma thrombomodulin as a marker of vascular damage in systemic sclerosis. J. Rheumatol. 22:1440–1441 [PubMed] [Google Scholar]
- Modlin I.M., Shapiro M.D., Kidd M. 2004. Carcinoid tumors and fibrosis: an association with no explanation. Am. J. Gastroenterol. 99:2466–2478 10.1111/j.1572-0241.2004.40507.x [DOI] [PubMed] [Google Scholar]
- Moertel C.G., Sauer W.G., Dockerty M.B., Baggenstoss A.H. 1961. Life history of the carcinoid tumor of the small intestine. Cancer. 14:901–912 [DOI] [PubMed] [Google Scholar]
- Moertel C.G., Kvols L.K., Rubin J. 1991. A study of cyproheptadine in the treatment of metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer. 67:33–36 [DOI] [PubMed] [Google Scholar]
- Nebigil C.G., Choi D.S., Dierich A., Hickel P., Le Meur M., Messaddeq N., Launay J.M., Maroteaux L. 2000. Serotonin 2B receptor is required for heart development. Proc. Natl. Acad. Sci. USA. 97:9508–9513 10.1073/pnas.97.17.9508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J.A., Rothwell J.C., Quinn N.P., Lang A.E., Artieda J., Marsden C.D. 1986. Lisuride in the treatment of myoclonus. Adv. Neurol. 43:191–196 [PubMed] [Google Scholar]
- Pavlovic M., Saiag P., Lotz J.P., Marinho E., Clerici T., Izrael V. 1995. Regression of sclerodermatous skin lesions in a patient with carcinoid syndrome treated by octreotide. Arch. Dermatol. 131:1207–1209 10.1001/archderm.131.10.1207 [DOI] [PubMed] [Google Scholar]
- Postlethwaite A.E., Chiang T.M. 2007. Platelet contributions to the pathogenesis of systemic sclerosis. Curr. Opin. Rheumatol. 19:574–579 10.1097/BOR.0b013e3282eeb3a4 [DOI] [PubMed] [Google Scholar]
- Reimund E. 1987. Methysergide and retroperitoneal fibrosis. Lancet. 392:443 10.1016/S0140-6736(87)90140-1 [DOI] [PubMed] [Google Scholar]
- Roth B.L. 2007. Drugs and valvular heart disease. N. Engl. J. Med. 356:6–9 10.1056/NEJMp068265 [DOI] [PubMed] [Google Scholar]
- Rothman R.B., Baumann M.H., Savage J.E., Rauser L., McBride A., Hufeisen S.J., Roth B.L. 2000. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 102:2836–2841 [DOI] [PubMed] [Google Scholar]
- Ruddell R.G., Oakley F., Hussain Z., Yeung I., Bryan-Lluka L.J., Ramm G.A., Mann D.A. 2006. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am. J. Pathol. 169:861–876 10.2353/ajpath.2006.050767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setola V., Hufeisen S.J., Grande-Allen K.J., Vesely I., Glennon R.A., Blough B., Rothman R.B., Roth B.L. 2003. 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol. Pharmacol. 63:1223–1229 10.1124/mol.63.6.1223 [DOI] [PubMed] [Google Scholar]
- Sgonc R., Gruschwitz M.S., Dietrich H., Recheis H., Gershwin M.E., Wick G. 1996. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J. Clin. Invest. 98:785–792 10.1172/JCI118851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A., Pisarchik A., Zbytek B., Tobin D.J., Kauser S., Wortsman J. 2003. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell. Physiol. 196:144–153 10.1002/jcp.10287 [DOI] [PubMed] [Google Scholar]
- Soma Y., Takehara K., Sato S., Ishibashi Y. 1993. Increase in plasma thrombomodulin in patients with systemic sclerosis. J. Rheumatol. 20:1444–1445 [PubMed] [Google Scholar]
- Stachów A., Jabłońska S., Skiendzielewska A. 1979. Biogenic amines derived from tryptophan in systemic and cutaneous scleroderma. Acta Derm. Venereol. 59:1–5 [PubMed] [Google Scholar]
- Steen V.D., Medsger T.A. 2007. Changes in causes of death in systemic sclerosis, 1972-2002. Ann. Rheum. Dis. 66:940–944 10.1136/ard.2006.066068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R.M., Mehrad B. 2009. New mechanisms of pulmonary fibrosis. Chest. 136:1364–1370 10.1378/chest.09-0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegedi A., Schwertfeger N. 2005. Mirtazapine: a review of its clinical efficacy and tolerability. Expert Opin. Pharmacother. 6:631–641 10.1517/14656566.6.4.631 [DOI] [PubMed] [Google Scholar]
- Varga J., Abraham D. 2007. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J. Clin. Invest. 117:557–567 10.1172/JCI31139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Werner K., Gräf K.-J., Scholz A., Horowski R. 1989. Therapy with terguride. Nichidoku-Iho. 33:189–200 [Google Scholar]
- Walther D.J., Peter J.U., Bashammakh S., Hörtnagl H., Voits M., Fink H., Bader M. 2003a. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 299:76 10.1126/science.1078197 [DOI] [PubMed] [Google Scholar]
- Walther D.J., Peter J.U., Winter S., Höltje M., Paulmann N., Grohmann M., Vowinckel J., Alamo-Bethencourt V., Wilhelm C.S., Ahnert-Hilger G., Bader M. 2003b. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell. 115:851–862 10.1016/S0092-8674(03)01014-6 [DOI] [PubMed] [Google Scholar]
- Wang S., Wilkes M.C., Leof E.B., Hirschberg R. 2005. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 19:1–11 10.1096/fj.04-2370com [DOI] [PubMed] [Google Scholar]
- Woessner J.F., Jr 1961. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch. Biochem. Biophys. 93:440–447 10.1016/0003-9861(61)90291-0 [DOI] [PubMed] [Google Scholar]
- Wouters M.M., Gibbons S.J., Roeder J.L., Distad M., Ou Y., Strege P.R., Szurszewski J.H., Farrugia G. 2007. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 133:897–906 10.1053/j.gastro.2007.06.017 [DOI] [PubMed] [Google Scholar]
- Zarafonetis C.J., Lorber S.H., Hanson S.M. 1958. Association of functioning carcinoid syndrome and scleroderma. I. Case report. Am. J. Med. Sci. 236:1–14 10.1097/00000441-195807000-00001 [DOI] [PubMed] [Google Scholar]