Persistent virus infection drives follicular T helper cell differentiation.
Abstract
CD4 T cell responses are crucial to prevent and control viral infection; however, virus-specific CD4 T cell activity is considered to be rapidly lost during many persistent viral infections. This is largely caused by the fact that during viral persistence CD4 T cells do not produce the classical Th1 cytokines associated with control of acute viral infections. Considering that CD4 T cell help is critical for both CD8 T cell and B cell functions, it is unclear how CD4 T cells can lose responsiveness but continue to sustain long-term control of persistent viral replication. We now demonstrate that CD4 T cell function is not extinguished as a result of viral persistence. Instead, viral persistence and prolonged T cell receptor stimulation progressively redirects CD4 T cell development away from the Th1 response induced during an acute infection toward T follicular helper cells. Importantly, this sustained CD4 T cell functionality is critical to maintain immunity and ultimately aid in the control of persistent viral infection.
During persistent infection, virus-specific T cells are either physically deleted or persist in an “exhausted” state, which is characterized by the decreased ability to lyse virally infected cells, proliferate, and produce the antiviral and immune stimulatory cytokines IL-2, TNF, and IFN-γ (Gallimore et al., 1998; Zajac et al., 1998; Wherry et al., 2003; Brooks et al., 2005). It is the culmination of these deficiencies that ultimately prevents viral clearance, leading to persistent infection. T cell exhaustion is observed during many persistent viral infections, including HIV and hepatitis B and hepatitis C virus infection in humans and lymphocytic choriomeningitis virus (LCMV) infection in mice, indicating that prolonged viral replication institutes a similar T cell differentiation program (Klenerman and Hill, 2005). Interestingly, persistent LCMV infection is eventually controlled from the periphery ∼60–80 d after infection via CD8 T cell– and B cell–dependent mechanisms, both of which are regulated by CD4 T cells (Battegay et al., 1994; Matloubian et al., 1994; Zajac et al., 1998; Ciurea et al., 2001; Bergthaler et al., 2009). Consequently, effective CD4 T cell responses are required for the ultimate resolution of persistent LCMV infection (Battegay et al., 1994; Matloubian et al., 1994). However, it is unclear how exhausted CD4 T cells are able to sustain diverse immune cell types.
Depending on the level of TCR stimulation and the composition of co-stimulatory and inflammatory signals, CD4 T cells differentiate into a variety of helper subsets that in turn orchestrate diverse immune responses. We have previously demonstrated that priming and initial CD4 T cell activation is similar after acute and persistent LCMV infection, indicating that exhaustion is not a programmed event, but rather a continual response to the antigenic environment (Brooks et al., 2006a), and raising the question as to how differentiation is altered during persistent infection. CD4 T cells continue to help CD8 T cells during persistent viral infection, allowing continued control over virus replication (Elsaesser et al., 2009; Fröhlich et al., 2009; Yi et al., 2009). Although the role of CD8 T cells and their differentiation pathways have been extensively analyzed in acute and persistent viral infection (Kaech et al., 2002; Wherry et al., 2007), CD4 T cell differentiation and the mechanisms that govern it are largely uncharacterized. Recently, we and others identified IL-21 as a critical component of CD4 T cell help during viral persistence (Elsaesser et al., 2009; Fröhlich et al., 2009; Yi et al., 2009). CD8 T cells require IL-21 to maintain residual levels of immunological function, avoid deletion, and resolve persistent infection. IL-21 is also important for stimulating germinal center (GC) B cells and antibody production (Ozaki et al., 2002; Linterman et al., 2010; Zotos et al., 2010), suggesting that CD4 T cells differentiate into a subset capable of providing help to both B cells and CD8 T cells during persistent viral infection.
Given that ongoing antiviral immune responses continually exert control over virus replication throughout persistent infection and that CD4 T cells play an integral role in this process, we sought to define how viral persistence impacts CD4 T cell differentiation such that it is capable of maintaining antiviral immunity in the face of prolonged periods of viral replication. Herein, we establish that the prolonged antigenic stimulation during viral persistence drives a specific CD4 T cell developmental program leading to the progressive differentiation of T follicular helper cells. These CD4 T cells continue to function during viral persistence to sustain antiviral antibody production and to ultimately control persistent virus replication. Thus, we demonstrate that CD4 T cells are not without function during viral persistence, but instead that their developmental program is redirected from promoting memory T and B cell differentiation after acute viral infection and toward a T helper subset that sustains multiple immune parameters to fight persistent viral infection.
RESULTS
Persistent viral infection drives progressive CD4 T follicular helper (Tfh) cell differentiation
Different environmental cues institute distinct CD4 T helper cell developmental programs. To delineate how viral persistence affects CD4 T helper cell differentiation, C57BL6 mice were infected with either LCMV-Armstrong (Arm) or LCMV-Clone 13 (Cl 13). LCMV-Arm generates an acute infection that is cleared primarily by CD8 T cells within 8–10 d and generates memory T cells. LCMV-Cl 13 induces a persistent infection characterized by the attenuation of CD4 and CD8 T cells, but is eventually resolved from most peripheral tissues via CD4 T cell, CD8 T cell, and B cell dependent mechanisms. Notably, LCMV-Arm and Cl 13 contain identical T cell epitopes, allowing for a direct comparison of virus-specific T cell responses. To specifically identify and analyze virus-specific CD4 T cells, LCMV-specific CD4 TCR transgenic (SMARTA) cells, which recognize the LCMV-derived peptide GP61–80 in the context of I-Ab (Oxenius et al., 1998), were transferred into mice before infection. Importantly, the LCMV-specific SMARTA cells behave similarly to their endogenous (host-derived) CD4 T cell counterparts (Brooks et al., 2005, 2006a; Elsaesser et al., 2009).
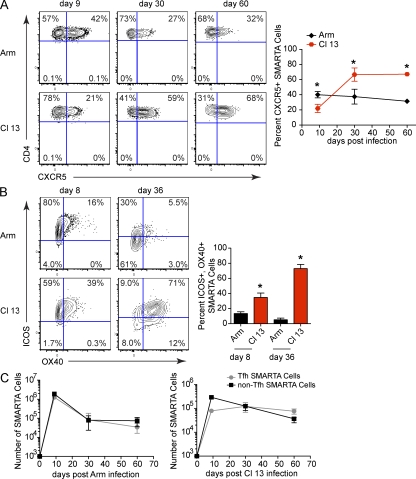
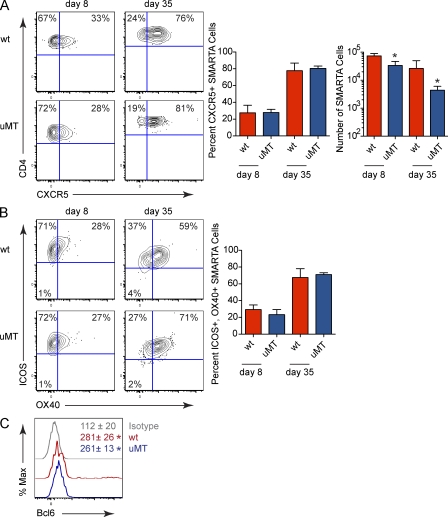
Early after infection (day 8 and 9), both LCMV-Arm and Cl 13 induced the up-regulation of the Tfh cell–associated effector molecules CXC chemokine receptor 5 (CXCR5), inducible T cell co-stimulator (ICOS), and OX40 on virus-specific CD4 T cells (Fig. 1, A and B). Tfh cells are recognized as a distinct CD4 T cell subset (CXCR5+, ICOS+, OX40+, and SLAMlow) and play a critical role in providing help to B cells (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009). High expression of CXCR5 promotes the localization of CD4 T cells to B cell areas in lymphoid follicles, whereas ICOS and OX40 are effector molecules that augment Tfh cell differentiation, function, and survival (Linterman and Vinuesa, 2010). The expression of Tfh effector molecules decreased after resolution of LCMV-Arm infection (Fig. 1, A and B). Interestingly, as persistent infection progressed, the expression of CXCR5, ICOS, and OX40 increased, whereas the expression of SLAM decreased, which is indicative of enhanced Tfh development (Fig. 1, A and B, and Fig. S1 A). Notably, the vast majority of virus-specific CXCR5 expressing CD4 T cells coexpressed ICOS and OX40 (Fig. S1 B). Additionally, PD-1 has been used to define Tfh cells (Haynes et al., 2007). However, it is not a useful indicator after LCMV infection because >98% of virus-specific CD4 T cells express PD-1 after both an acute and a persistent LCMV infection (Brooks et al., 2006b) and >99% of virus-specific CD4 T cells continue to express PD-1 throughout persistent LCMV infection (unpublished data). Thus, after LCMV infection, essentially all virus-specific CD4 T cells express PD-1, precluding its use as a marker of Tfh. Comparable Tfh development was also observed in the endogenous virus-specific CD4 T cell population after LCMV infection (unpublished data), suggesting that prolonged viral replication leads to Tfh programming.
Figure 1.
Tfh differentiation during viral persistence. (A) Virus-specific (SMARTA) CD4 T cells were isolated from the spleen on day 9, 30, and 60 after LCMV-Arm or Cl 13 infection and were analyzed for the surface expression of CXCR5. The line graph indicates the percentage ± SD of CXCR5+ SMARTA cells during LCMV-Arm (black, diamond) and Cl 13 (red, octagons) infection on day 9, 30, and 60. (B) SMARTA cells were isolated from the spleen on day 8 and 36 after LCMV-Arm or Cl 13 infection and were analyzed for the surface expression of ICOS and OX40. The bar graph depicts the percentage ± SD of ICOS+, OX40+ SMARTA cells during LCMV-Arm (black) and Cl 13 (red) infection on the indicated days. (C) The line graph indicates the number of Tfh SMARTA cells (CXCR5+; gray circles) and non-Tfh SMARTA cells (CXCR5−; black squares) during LCMV-Arm (left) and Cl 13 (right) infection. Data are representative of three to five mice per group and of four individual experiments (*, P < 0.05).
Similar to the percentage of cells expressing Tfh-associated molecules, the number of virus-specific Tfh cells (CD4+ CXCR5+) was higher in LCMV-Arm than Cl 13 on day 9 after infection (Fig. 1 C). With the resolution of acute LCMV-Arm infection, the number of virus-specific Tfh cells and non-Tfh cells (CD4+, CXCR5−) decreased with the same kinetic. In contrast, as the persistent viral infection progressed the number of virus-specific Tfh cells slightly increased and was sustained, whereas the number of virus-specific non-Tfh cells steadily decreased compared with day 9 after infection (Fig. 1 C). The decline in virus-specific non-Tfh cells during persistent infection was likely not the result of non-Tfh cell egress from lymphoid organs to nonlymphoid organs because a consistent number of virus-specific Tfh and non-Tfh cells was observed in the liver over the duration of the infection (Fig. S1 C). Collectively, these data are consistent with our previous finding that IL-21 protein expression is initially lower in LCMV-Cl 13 compared with Arm infection, but increases as persistent infection progresses (Elsaesser et al., 2009; Yi et al., 2009) and suggests that ongoing viral replication drives a distinct Th developmental program.
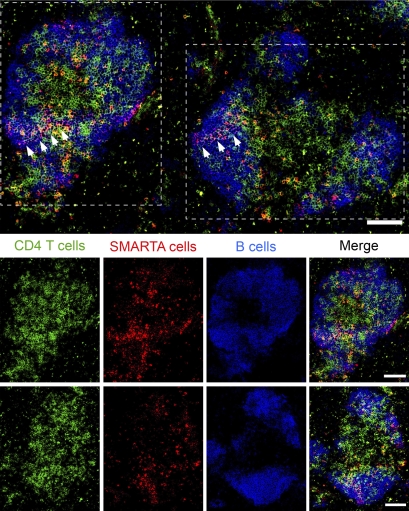
In addition to assessing the expression of Tfh effector molecules, we examined the location of virus-specific CD4 T cells within the spleen during persistent viral infection. 30 d after LCMV-Cl 13 infection, we observed CD4 T cells localized to B cell areas in follicular-like structures with the virus-specific CD4 T cells in close proximity and intermingled with the B cells (Fig. 2). Thus, in accordance with the expression of Tfh-associated molecules, virus-specific CD4 T cells are present in juxtaposition with B cells, consistent with their need to provide ongoing help to B cells.
Figure 2.
Virus-specific CD4 T cells traffic to B cell areas during persistent viral infection. Day 30 LCMV-Cl 13–infected spleen sections were stained with antibodies to CD4 (green), CD45.1 (SMARTA cells; red), and B220 (B cells; blue). The top row is a merged image illustrating the location of all the three cell types. The white arrows in the image indicate SMARTA cells within B cell areas. The bottom two rows depict the two follicular-like structures outlined with white dotted lines in the top image. The last row depicting a follicular-like structure is rotated 90 degrees relative to the top image. The first column represents total CD4 T cells, the second column represents virus-specific SMARTA cells, the third column represents total B cells, and the last column is a merged image. The scale bar within each image corresponds to 100 µm. The image is representative of 5–13 different CD4 T cells cell areas per spleen and 5 individual spleens.
To determine if Tfh cell differentiation occurs in other organs during persistent viral infection, we assessed the effector molecule profile of virus-specific CD4 T cells within the liver and mesenteric LN. Early after infection (day 8), virus-specific CD4 T cells in the liver (a nonlymphoid organ not associated with B cell GCs), and mesenteric LN expressed similar levels of CXCR5, ICOS, and OX40 (Fig. S2). As viral replication persisted, expression of CXCR5 on virus-specific CD4 T cells in the liver was maintained at a low level (although it did not increase as observed in the spleen) and the expression of ICOS and OX40 was largely absent. In the mesenteric LN, the vast majority of virus-specific CD4 T cells expressed CXCR5, ICOS, and OX40 (Fig. S2) at levels similar to those observed in the spleen (Fig. 1, A and B). Thus, progressive Tfh development is characteristic of CD4 T cells in multiple lymphoid organs during viral persistence.
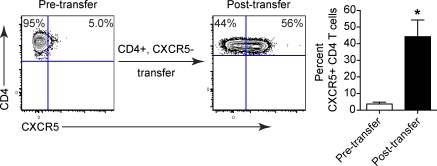
To differentiate whether viral persistence progressively drives Tfh development or preferentially sustains Tfh cells present early on in infection, we transferred CD4+, CXCR5− (non-Tfh) SMARTA cells into LCMV-Cl 13–infected mice that had not initially received SMARTA cells. Importantly, the transfer was performed early (day 9) after infection and before the extensive Tfh differentiation observed later during infection. As persistent infection progressed, the transferred non-Tfh SMARTA cells increased CXCR5 expression (Fig. 3), indicating that Tfh development is a progressive response to persistent viral replication.
Figure 3.
Persistent viral replication drives non-Tfh cells to differentiate into Tfh cells. SMARTA cells were transferred into WT mice and subsequently infected with LCMV-Cl 13. CD4+, CXCR5− (non-Tfh) cells were isolated on day 9 after infection and transferred into WT mice that had been infected with LCMV-Cl 13 (but that had not received SMARTA cells). The contour plots depict CXCR5 expression on sorted CD4 T cells pretransfer (left) and SMARTA cells in the spleen 14 d after transfer (i.e., 23 d after infection) into LCMV-Cl 13–infected recipients. The bar graph depicts the percentage ± SD of CXCR5+, CD4 T cells before transfer (left) and after transfer (right). These data are representative of two individual experiments containing four mice each (*, P < 0.05).
Tfh-associated master regulatory transcription factors are increased during viral persistence
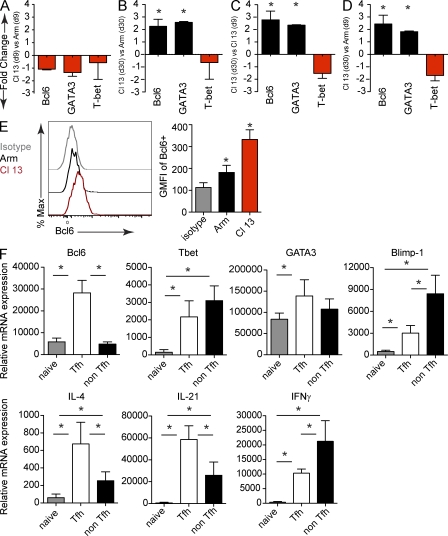
To molecularly assess progressive virus-specific CD4 T cell differentiation during acute versus persistent viral infection, we quantified messenger RNA (mRNA) levels of the following master transcription factors, which are known to regulate distinct Th fates: T-bet (Tbx21; Th1), GATA3-binding protein (GATA3; Th2), retinoid-related orphan receptor γt (RORγt; Th17), B cell lymphoma 6 protein (Bcl6; Tfh), and forkhead box P3 (FoxP3; T reg). Early after infection (day 9), virus-specific CD4 T cells in LCMV-Cl 13–infected mice expressed slightly lower levels of Bcl6, GATA3, and T-bet mRNA compared with LCMV-Arm–infected mice (Fig. 4 A), which is consistent with an attenuated phenotype (Brooks et al., 2005; Elsaesser et al., 2009). As persistent infection progressed (day 30), virus-specific CD4 T cells expressed two- to threefold more Bcl6 and GATA3-binding protein (GATA3) mRNA and one- to twofold less T-bet mRNA compared with virus-specific CD4 T cells 30 d after LCMV-Arm infection (Fig. 4 B). Consistent with the progressive differentiation toward CXCR5+, ICOS+, OX40+, SLAMlow–expressing Tfh cells during persistent infection, expression of the Tfh-associated master regulator, Bcl6, increased two to threefold between day 9 and 30 after LCMV-Cl 13 infection (Fig. 4 C). Interestingly, the molecular development of effector virus–specific CD4 T cells during persistent infection (day 30) is distinct from the effector response observed during acute LCMV-Arm infection (day 9; Fig. 4 D), indicating that persistent infection does not delay the effector response observed during acute infection but instead institutes a distinct developmental program. Further, virus-specific CD4 T cells expressed significantly higher levels of Bcl6 protein during persistent versus acute infection (Fig. 4 E). Increased levels of GATA3 mRNA were also observed during persistent infection (Fig. 4, A–D), consistent with a recent finding that Tfh cells can express both Bcl6 and GATA3 (Zaretsky et al., 2009). RORγt mRNA or mRNA of the Th17-defining cytokines, IL-17a and IL-22, was not detected in virus-specific CD4 T cells during acute or persistent infection (unpublished data). Additionally, minimal FoxP3 protein expression was detectable in virus-specific CD4 T cells after either LCMV-Arm or Cl 13 (Fig. S3), collectively indicating that virus-specific CD4 T cells were not differentiating into Th17 or T reg cells.
Figure 4.
Persistent viral infection induces Tfh transcriptional master regulators. SMARTA cells were FACS isolated from splenocytes on day 9 and 30 after LCMV-Arm or Cl 13 infection. mRNA expression was analyzed by quantitative RT-PCR. The results are presented as mean fold change in expression ± SD between SMARTA cells isolated from (A) LCMV-Cl 13 versus LCMV-Arm on day 9 after infection, (B) LCMV-Cl 13 versus LCMV-Arm on day 30 after infection, (C) LCMV-Cl 13 on day 30 versus day 9 after infection, or (D) LCMV-Cl 13 on day 30 versus LCMV-Arm on day 9 after infection. Data are representative of four mice per group and two individual experiments (*, P < 0.05). (E) The histogram illustrates Bcl6 expression in SMARTA cells on day 30 after LCMV-Arm (black) and Cl 13 infection (red). The gray line represents the isotype control. The bar graph on the right depicts the GMFI ± SD of Bcl6 in SMARTA cells. Data are representative of four to five mice per group and three separate experiments (*, P < 0.05). (F) mRNA expression was analyzed by quantitative RT-PCR from naive virus-specific CD4 T cells (SMARTA), splenic Tfh (CD4+, Ly5.1+, CXCR5+), and non-Tfh (CD4+, Ly5.1+, CXCR5) SMARTA cells sorted 30 d after LCMV-Cl 13 infection. The results are presented as the mean relative mRNA expression ± SD. Data are representative of four to five groups (each group consisting of sorted cells from five to six mice) and two to three separate experiments. Note, IL-4 data are presented from one experiment containing five groups (each group consisting of sorted cells from five mice; *, P < 0.05).
Because virus-specific CD4 T cells expressed the Th2-associated transcriptional regulator, GATA3, during persistent infection, we measured the expression of Th2-associated cytokines. Virus-specific CD4 T cells from LCMV-Cl 13–infected mice did not express mRNA for IL-5, IL-9, or IL-13 (unpublished data). Although the expression of IL-4 mRNA (a hallmark of Th2 and Tfh cells) was significantly increased (albeit at very low levels) during persistent infection, IL-4 protein expression was not detected after ex-vivo peptide stimulation and intracellular cytokine staining. Like the expression of ICOS, OX40, and Bcl6, mRNA for the transcription factor c-Maf, which is known to regulate IL-21 expression (Pot et al., 2009; Hiramatsu et al., 2010), was elevated during persistent infection and increased fourfold as infection persisted (unpublished data), further substantiating progressive Tfh differentiation.
The previous analyses were performed using the total population of virus-specific CD4 T cells (i.e., containing both Tfh and non-Tfh populations). To specifically delineate the contribution of virus-specific Tfh versus non-Tfh cells toward the production of Th regulatory and effector molecules during persistent infection, we sorted each population and performed mRNA analysis. Virus-specific Tfh cells expressed significantly higher levels of Bcl6 in comparison to virus-specific non-Tfh cells and naive virus-specific CD4 T cells, with the latter two populations expressing the same levels of Bcl6 (Fig. 4 F). In contrast, virus-specific non-Tfh cells expressed higher levels of T-bet, although both virus-specific populations expressed significantly more T-bet than naive virus-specific CD4 T cells (Fig. 4 F). Interestingly, GATA3 mRNA levels were comparable between virus-specific Tfh and non-Tfh populations, although the overall increase compared with naive was not as great as observed for Tbet or for Bcl6 by Tfh cells (Fig. 4 F). Like that of Tbet, non-Tfh cells expressed greater levels of the Bcl6 antagonist, Blimp-1 (Prdm1), than Tfh cells, but both subsets expressed more Blimp-1 than naive virus-specific CD4 T cells. Although CD4 T cell subsets expressed extremely low levels of IL-4 RNA, virus-specific Tfh cells expressed significantly more IL-4 than non-Tfh cells. However, both virus-specific populations expressed significantly higher levels of IL-4 than naive virus-specific CD4 T cells (Fig. 4 F). IL-21 was expressed significantly higher in virus-specific Tfh cells compared with non-Tfh cells. Yet, unlike Bcl6 expression both virus-specific Tfh and non-Tfh expressed significantly more IL-21 than naive virus-specific CD4 T cells (Fig. 4 F), potentially explaining how IL-21 mediated help can be provided to CD8 T cells despite the extensive progressive differentiation of CD4 T cells into Tfh cells. IFN-γ expression was higher in both virus-specific Tfh cells and non-Tfh cells compared with naive virus-specific CD4 T cells, but was highest in virus-specific non-Tfh cells compared with virus-specific Tfh cells. Thus, both subsets express Tbet, GATA3, Blimp-1, IL-21, IFN-γ, and slight levels of IL-4 during persistent infection; however, virus-specific Tfh cells produce more Bcl6 and IL-21 in vivo, whereas non-Tfh cells express greater levels of IFN-γ, Blimp-1, and slightly more Tbet, providing molecular evidence that the Th subsets are developmentally and functionally distinct.
B cells are not required for Tfh development
We observed that the progressive differentiation of Tfh cells in lymphoid organs during persistent LCMV infection corresponded with GC B cell development (Fig. S4). Based on this correlation and the reported need of B cell interaction for Tfh development (Haynes et al., 2007; Johnston et al., 2009), we assessed the contribution of B cells toward Tfh differentiation during viral persistence. Numerically, the amount of SMARTA cells was significantly less in persistently infected B cell–deficient (μMT) mice compared with WT mice (Fig. 5 A), suggesting that virus-specific CD4 T cells require B cells for survival during persistent infection. Yet, virus-specific CD4 T cells from both WT and μMT mice expressed comparable levels of CXCR5, ICOS, OX40, and Bcl6 during persistent infection (Fig. 5), indicating similar Tfh differentiation of the remaining virus-specific cells. On the other hand, B cells were required for Tfh development after acute LCMV-Arm infection (unpublished data) confirming previous findings (Johnston et al., 2009). Thus, mechanisms present during persistent infection bypass the B cell requirement for Tfh differentiation.
Figure 5.
B cell–independent Tfh development during persistent viral infection. (A) The flow plots illustrate CXCR5 expression on SMARTA cells from LCMV-Cl 13–infected WT or μMT mice. The left bar graphs indicate the percentage ± SD of CXCR5 + SMARTA cells and the right bar graph depicts the absolute number ± SD of SMARTA cells. (B) The flow plots show ICOS and OX40 expression on SMARTA cells from LCMV-Cl 13–infected WT or μMT mice. Bar graphs indicate the percentage ± SD of ICOS+, OX40+ SMARTA cells. (C) The histogram depicts Bcl6 expression within SMARTA cells isolated from WT (red) and μMT (blue) mice 30 d after LCMV-Cl 13 infection. The gray line represents the isotype control. Numbers indicate the mean GMFI ± SD of Bcl6. Data are representative of three to four mice per group and three individual experiments (*, P < 0.05).
TCR stimulation drives Tfh cell responses during persistent viral infection
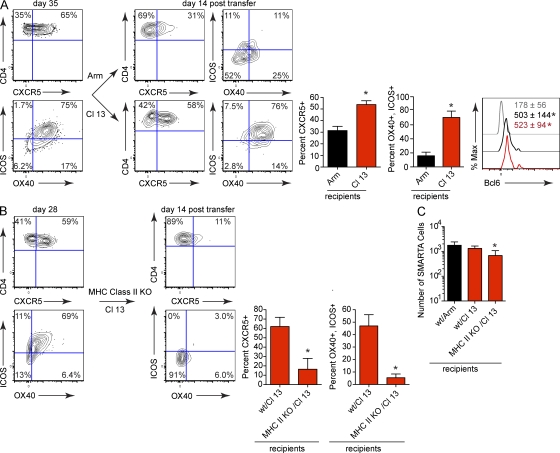
Considering that B cells are not required for Tfh differentiation during persistent infection, we next sought to define the mechanism that enhances the development of Tfh cells during viral persistence. One of the main differences between acute and persistent viral infection is elevated and prolonged antigen stimulation and inflammatory signals (Hahm et al., 2005; Shin et al., 2007). To determine how these factors contribute to Tfh development, we transferred SMARTA cells into WT mice and subsequently infected them with LCMV-Cl 13. On day 35 after infection (a time when viral replication is still high), T cells were harvested from these donor mice and transferred into either LCMV-immune or LCMV-Cl 13–infected WT mice that had been infected in parallel but had not received SMARTA cells. LCMV-immune mice are WT mice that cleared LCMV-Arm infection and contain LCMV-specific memory T cells. LCMV-immune mice were chosen instead of naive mice to prevent infection from any virus potentially carried over with the transfer. SMARTA cells transferred into LCMV-Cl 13–infected WT recipients expressed comparable levels of CXCR5, ICOS, and OX40, as observed before transfer, indicating the validity of the transfer system (Fig. 6 A). In contrast, the SMARTA cells transferred into LCMV-immune recipients displayed significantly decreased expression of CXCR5, ICOS, and OX40 (Fig. 6 A). Interestingly, Bcl6 expression was similar in virus-specific CD4 T cells after adoptive transfer into LCMV-Arm or LCMV-Cl 13–infected mice (Fig. 6 A), suggesting that persistent viral infection institutes a prolonged molecular program of Tfh differentiation, whereas constant immune-activating signals sustain Tfh effector molecules.
Figure 6.
Sustained TCR stimulation drives Tfh differentiation during persistent viral infection. (A) SMARTA cells from LCMV-Cl 13–infected WT mice (left) were isolated on day 35 after infection and transferred into WT mice that had been infected with LCMV-Arm or Cl 13 35 d earlier (but that had not received SMARTA cells). The contour plots depict CXCR5, ICOS, and OX40 expression on SMARTA cells pretransfer (left) and 14 d after transfer into LCMV-Arm immune (top right) or LCMV-Cl 13–infected (bottom right) recipients. The bar graphs indicate the percentage ± SD of CXCR5+ or ICOS+, OX40+ SMARTA cells. The histogram illustrates Bcl6 expression in SMARTA cells 14 d after transfer into LCMV-Arm (black) and Cl 13 infection (red). The gray line represents the isotype control. Numbers indicate the mean GMFI ± SD of Bcl6 in each population. These data are representative of 4–6 mice per group and three individual experiments (*, P < 0.05). (B) SMARTA cells from LCMV-Cl 13–infected WT mice (left) were isolated on day 27 after infection and transferred into LCMV-Cl 13–infected WT C57BL6 or LCMV-Cl 13–infected MHC class II KO mice (all recipient mice were infected in parallel but had not received SMARTA cells). The contour plots illustrate CXCR5, ICOS and OX40 expression on SMARTA cells pretransfer (left panel) and fourteen days after transfer into LCMV-Cl 13–infected MHC Class II KO recipients (right). Bar graphs indicate the percentage ± SD of CXCR5+ or ICOS+, OX40+ SMARTA cells (*, P < 0.05). Data are representative of 3–5 mice per group and two individual experiments (C) The bar graph indicates the number ± SD of SMARTA cells in the spleen fourteen days after transfer. Data are representative of three to five mice per group and two individual experiments (*, P < 0.05).
High-affinity TCR binding and strong TCR signals are associated with Tfh development (Fazilleau et al., 2009). A major difference between acute and persistent infection is prolonged antigen presentation and, consequently, TCR signaling. To specifically assess the contribution of prolonged TCR signaling toward Tfh cell development during viral persistence, virus-specific CD4 T cells were isolated from LCMV-Cl 13 persistently infected mice and transferred into LCMV-Cl 13–infected WT mice or LCMV-Cl 13–infected MHC class II–deficient mice. Importantly, LCMV-Cl 13–infected MHC class II–deficient recipient mice had significantly higher levels of virus replication than LCMV-Cl 13–infected WT recipient mice (MHC class II–deficient mice, 1.2 × 105 ± 3.2 × 104 PFU/ml serum; WT mice, 9.6 × 103 ± 7.3 × 103 PFU/ml serum; P = 0.008) in the periphery 14 d after cell transfer. Thus, MHC class II–deficient mice have other components of the immune activating environment, but cannot provide TCR stimulation to CD4 T cells. Despite the high levels of virus replication in the MHC class II–deficient mice, virus-specific CD4 T cells decreased the expression of the Tfh effector molecules (Fig. 6b), indicating that TCR signals are necessary to sustain Tfh effector differentiation during viral persistence. Notably, the data are likely not a result of Tfh selection after transfer into LCMV-Cl 13 recipients because the number of SMARTA cells in the spleen on day 14 after transfer was similar in both LCMV-Arm and Cl 13 recipients, and although there was less SMARTA cells in MHC class II–deficient recipients, this was likely caused by the lack of sustaining signals (Viret et al., 1999; Fig. 6 C).
CD4 T cells retain B cell helping function during viral persistence
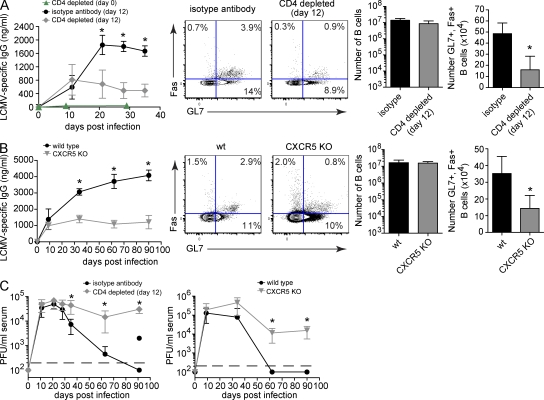
Considering the important role of B cells in resolving persistent LCMV infection (Recher et al., 2007; Bergthaler et al., 2009) and the specific development of CD4 T cells into Tfh cells, we sought to define whether CD4 T cells retained specific B cell helping function during persistent infection. Ex vivo Tfh cells retained the ability to provide help to B cells during persistent infection (Fig. S5 A), suggesting that in vivo helper function remains. To further evaluate sustained in vivo helper function, we depleted CD4 T cells both before and during infection and subsequently assessed antiviral B cell responses. Antibody depletion of CD4 T cells before infection almost entirely abrogated LCMV-specific IgG antibody production (Fig. 7 A) because of the need for CD4 T cell help to generate antiviral B cell responses. To define the ongoing need for CD4 T cell help, CD4 T cells were depleted 12 d after LCMV-Cl 13 infection (i.e., allowing for the initial programming and generation of B cell responses but before extensive Tfh differentiation) at a time when these cells are considered to be exhausted. Consistent with the initial provision of CD4 T cell help, LCMV-specific antibody production increased before depletion between isotype antibody and day 12 CD4-depleted mice (Fig. 7 A; day 11). However, progressive infection in the absence of sustained CD4 T cell help resulted in a threefold decrease in LCMV-specific antibody titers and a 3–4 fold decrease in the frequency and number of GC B cells compared with isotype antibody-treated controls (Fig. 7 A). Notably, the total number of B cells was not affected by CD4 depletion (Fig. 7 A), demonstrating that virus-specific CD4 T cells remain functional and specifically sustain antiviral B cell activity as viral infection progresses. On the contrary, in response to CD4 T cell depletion 12 d after LCMV-Arm infection (a time point when the virus is cleared), LCMV-specific antibody levels continued to increase and CD4 depletion led to only a minimal long-term effect on virus-specific antibody production (Fig. S5 B). No reemergence of viral replication was observed. Further, although the level of virus-specific antibody was decreased during persistent infection, the distribution of virus-specific IgG antibody isotypes (IgG1, IgG2a, IgG2b, and IgG3) was similar (unpublished data). These data demonstrate that virus-specific CD4 T cells retain function during viral persistence and exhibit a unique role in sustaining B cell responses not required after an acute infection.
Figure 7.
Tfh cell development is required to sustain LCMV-specific B cell responses during persistent viral infection. (A) Antiviral B cell responses during LCMV-Cl 13 infection in isotype antibody control treated mice versus day 0 or 12 CD4-depleted WT mice. The line graph depicts the serum concentration (nanogram/milliliter) ± SD of LCMV-specific IgG produced by mice injected with isotype control antibody (black circles), or mice depleted of CD4 T cells on day 0 (green triangles) or day 12 (gray diamonds) on the indicated day after infection. Flow plots illustrate the frequency of GC B cells (GL7+, Fas+ B cells) on day thirty-five after infection. The bar graphs indicate the number ± SD of total B cells (left bar graph) and GC B cells (right bar graph) during persistent LCMV-Cl 13 infection. (B) Antiviral B cell responses during LCMV-Cl 13 infection in WT (black, circles) versus CXCR5 KO mice (gray, inverted triangles). The same analyses were performed as described in A. (C) Serum viral titers after LCMV-Cl 13 infection of isotype antibody control (black, circles) versus day 12 CD4-depleted (gray, diamonds) mice (left) or WT (black, circles) versus CXCR5 KO (gray, inverted triangles) mice (right). Data are expressed as PFU per milliliter serum ± SD. The black, dashed line indicates the lower limit of detection (200 PFU/ml). Data are representative of three to six mice per group and three individual experiments (*, P < 0.05).
To investigate the importance of the progressive Tfh differentiation and their interaction with B cells without depleting CD4 T cells, we infected CXCR5 KO mice with LCMV-Cl 13. CXCR5 is expressed on Tfh cells and mature B cells (Förster et al., 1996), and is required for proper follicular organization (Förster et al., 1996; Voigt et al., 2000), efficient GC responses, and T cell homing into the B cell follicle (Förster et al., 1996; Haynes et al., 2007). Additionally, CXCR5 is essential for the coordinated interaction of virus-specific CD4 T cells and B cells in the follicle (Junt et al., 2005). Therefore, in using CXCR5 KO mice, we can assess the requirement of follicular Tfh: B cell interactions during persistent viral infection. Interestingly, similar to CD4 T cell depletion on day 12 after persistent infection, no significant difference in LCMV-specific antibody production was initially observed between WT and CXCR5 KO mice (day 9) after LCMV-Cl 13 infection (Fig. 7 B), suggesting that the early interaction of CD4 T cells with B cells is less dependent on CXCR5-mediated homing to the follicle, likely because of the splenic disorganization that occurs early after infection (Odermatt et al., 1991). As persistent infection progresses and splenic architecture is restored, LCMV-specific antibody titers continued to increase in WT mice, whereas they failed to do so in CXCR5 KO mice (Fig. 7 B). This failure to progressively increase antibody production occurred despite similar numbers of virus-specific CD4 T cells (Fig. S6 A), suggesting that organized follicular CD4 T cell–B cell interactions are required during persistent viral infection to sustain antiviral immunity. Again, although the total number of B cells was unaffected, the frequency and number of GC B cells was decreased in CXCR5 KO mice compared with WT controls (Fig. 7 B), which is likely a consequence of reduced virus-specific CD4 T cell–B cell interactions (Junt et al., 2005). Conversely, LCMV antibody production continued to increase and was similar in WT and CXCR5 KO mice infected acutely with LCMV-Arm (Fig. S5 C), indicating the unique need for ongoing CD4 T cell function and CD4 T cell–B cell interaction during persistent infection.
Importantly, persistent virus replication was eventually resolved in isotype antibody-treated and in WT mice; whereas, virus titers remained high in the absence of ongoing CD4 T cell help (i.e., day 12 CD4 T cell depletion) or lack of organized interactions with B cells (Fig. 7 C). Interestingly, like virus-specific CD4 T cells, the number and function of virus-specific CD8 T cells was not decreased in CXCR5 KO mice (Fig. S6 B), again highlighting the importance of the ongoing interactions between virus-specific CD4 T cells with B cells to resolve persistent virus infection.
One caveat in using the CXCR5 KO mice is that B cells also do not express CXCR5, and as a result do not efficiently localize to or form follicles (Förster et al., 1996; Voigt et al., 2000). To specifically delineate the impact of the progressive increase of CXCR5 expression on CD4 T cells (i.e., Tfh-mediated interactions) in an otherwise CXCR5-sufficient host, lethally irradiated WT mice were reconstituted with a mixture of bone marrow from CD4 KO (85%) and either WT (15%) or CXCR5 KO (15%) mice. In this manner, all hematopoietic cells are derived from the CD4 KO bone marrow, except for the CD4 T cells, which are consequently derived from the minority WT or CXCR5 KO bone marrow donor. As a result, mice in which CD4 T cells are CXCR5 sufficient (i.e., CD4 KO–WT chimeras) can directly be compared with mice in which only CD4 T cells lacked CXCR5 expression (i.e., CD4 KO–CXCR5 KO chimeras) to specifically define the role of Tfh interactions during viral persistence. In preliminary results, no difference in LCMV-specific antibody production was initially observed between CD4 KO–WT and CD4 KO–CXCR5 KO bone marrow chimera mice after persistent LCMV-Cl 13 infection (Fig. S7), similar to mice depleted of CD4 T cells 12 d after infection and CXCR5 KO mice. However, as the infection progressed there was a greater than fourfold decrease in LCMV-specific antibody titers and a significant decrease in the frequency and number of GC B cells when CD4 T cells were incapable of expressing CXCR5 (Fig. S7). Thus, in combination with the results from the CD4 depletion and the CXCR5 KO mouse infection studies, the finding that antiviral B cell responses are invoked, but not sustained when only CD4 T cells lack CXCR5 expression, demonstrates that CD4 T cells retain important functions during persistent infection and that their progressive Tfh interactions are critical to sustaining the antiviral B cell immunity through viral persistence.
DISCUSSION
The importance of CD4 T cell help during persistent infection has long been recognized; however, the mechanisms of CD4 T cell help have remained unclear. Collectively, our data demonstrate that CD4 T cells maintain important in vivo function during viral persistence. Instead of losing function, CD4 T helper cells differentiate toward Tfh responses that actively sustain immunity in the face of widespread immunosuppression and persistent viral replication. Increased Tfh responses during viral persistence are demonstrated by the up-regulation of phenotypic and effector molecules, location of virus-specific CD4 T cells within follicular structures and B cell areas and increased expression of master transcriptional regulators associated with Tfh cells. Further, the functionality and importance of these cells is illustrated ex vivo and in vivo by the finding that when physically depleted during infection or Tfh function is impaired, multiple parameters of antiviral B cell immunity are extinguished and the immune system is unable to gain control over virus replication.
The development of distinct CD4 T helper subsets is programmed by specific transcription factors; yet, multiple master transcription regulators have been observed within a given cell (Zaretsky et al., 2009; Hegazy et al., 2010). Thus, in addition to their presence, the balance between the expression of these transcription factors is likely important in instituting a developmental program. During persistent viral infection, the expression of Bcl6 (Tfh master regulator) and c-Maf increased, whereas the expression of T-bet (Th1 master regulator) decreased in virus-specific CD4 T cells as virus replication progressed, consistent with the observed progressive development of Tfh cells. Accordingly, the greatest levels of Bcl6 and IL-21 expression were observed in the Tfh subset of virus-specific CD4 T cells, whereas virus-specific non-Tfh cells expressed the greatest levels of Blimp-1, Tbet, and IFN-γ during persistent infection. Even though the virus-specific Th subsets appear to be developmentally and functionally distinct, based on the mRNA levels of transcription factors and cytokines, both virus-specific Tfh and non-Tfh cells continually express significant levels of IL-21 and IFN-γ during persistent infection, again demonstrating that virus-specific CD4 T subsets retain ongoing function in the face of prolonged viral replication. Because both virus-specific Tfh and non-Tfh express IL-21 in vivo, this may be an explanation of how IL-21-mediated help is provided to both B cells and CD8 T cells. Interestingly, the mRNA expression of the Th2 master regulator GATA3 also increased in the virus-specific CD4 T cell population during persistent viral replication, with comparable levels of GATA3 observed in each virus-specific subset, Tfh, and non-Tfh. Yet, other Th2-associated effector molecules were either absent or expressed at extremely low levels (i.e., IL-4) in virus-specific CD4 T cells. Thus, our results are consistent with several recent studies that indicate that Tfh cells can simultaneously express classically Th2-associated factors and with their role in helping B cells (Johnston et al., 2009; King and Mohrs, 2009; Reinhardt et al., 2009; Zaretsky et al., 2009). Such plasticity in CD4 T cells may be necessary to ensure that they can respond to changes in the antigenic environment and signal the effector arms of the immune response to respond accordingly.
In addition to dysfunctional CD8 T cell responses, impaired B cell and antibody responses can lead to viral persistence (Thomsen et al., 1996; Bergthaler et al., 2009). B cells are also important in regulating the generation of Tfh cells (Nurieva et al., 2008; Johnston et al., 2009). However, during persistent infection in both WT and B cell–deficient mice, similar virus-specific CD4 Tfh development is observed, suggesting that B cells may not be absolutely required for Tfh differentiation in the presence of prolonged antigenic stimulation. Similarly, B cell–independent Tfh development was recently demonstrated using a protein/adjuvant prime-boost strategy (Deenick et al., 2010). We now illustrate using an established viral model in which antigen presentation is prolonged, that B cell–mediated instruction of Tfh differentiation can be bypassed by viral persistence. Although B cells are not necessary for Tfh development during viral persistence, we demonstrate that the progressive CD4 T cell response is continually required to maintain GC B cells and importantly sustain antiviral antibody production in the face of prolonged high levels of virus replication and immunosuppression. In addition to LCMV-specific antibodies, an increase in non-LCMV–specific antibodies is associated with persistent viral infections (Hunziker et al., 2003). This non–virus-specific hypergammaglobulinemia is observed during many persistent infections, including HIV and hepatitis C virus infections, and could potentially lead to autoimmune disorders or hinder antibody responses to secondary infections (Hunziker et al., 2003; Recher et al., 2004). Thus, in addition to providing factors that sustain antiviral B cell and CD8 T cell responses (e.g., IL-21), the progressive differentiation of CD4 T cells may also have a detrimental effect of providing help to nonspecific B cells and, consequently, the hypergammaglobulinemia observed during persistent viral replication.
Mechanistically, prolonged TCR signaling is essential for the progressive Tfh differentiation during viral persistence. High-affinity TCR interactions (translating into heightened TCR signaling) are important components of Tfh differentiation (Fazilleau et al., 2009). Similar to high-affinity interactions, our data suggest that excessive and prolonged TCR stimulation also specifically drives robust Tfh development. Yet, the CD4 T cell response generated during a persistent infection is not merely a delayed version of the response initiated during an acute infection, but instead sustained TCR stimulation and potentially other factors supported by TCR–MHC II interactions drives a molecularly and functionally distinct CD4 T cell developmental program. This unique differentiation leads to the generation of CD4 Th cells capable of sustaining multiple immune cell populations (e.g., CD8 T cells and B cells) that likely work together to eventually overcome the persistent infection.
The early decreased expression of multiple Th lineage factors and cytokines by CD4 T cells (e.g., IFN γ, TNF, IL-2, and IL-21; Fuller and Zajac, 2003; Brooks et al., 2005; Elsaesser et al., 2009; Fröhlich et al., 2009) suggests that CD4 T cells may initially down-regulate their function during the height of viral replication, which is consistent with the observed exhaustion. However, as viral infection progresses, the continued TCR stimulation progressively institutes a distinct and functional developmental program. Thus, the altered CD4 T cell helper differentiation may be a means to initially limit more aggressive Th1 responses, and thus immunopathology (Barber et al., 2006), while progressively providing critical factors to sustain CD8 T cell and B cell effector responses to control virus replication (Elsaesser et al., 2009; Fröhlich et al., 2009; Yi et al., 2009). Thus, our study establishes that CD4 T cell function is not abolished but instead redirected by viral persistence. As a result of their sustained functionality, CD4 T cells may be targets for redirective immunotherapies that specifically enhance or alter T helper function to ultimately eliminate persistent viral infections.
MATERIALS AND METHODS
Mice and virus.
C57BL6 (WT) mice were purchased from The Jackson Laboratory or the rodent breeding colony at University of California, Los Angeles. μMT, CD4 KO, MHC class II KO, and CXCR5 KO mice were purchased from The Jackson Laboratory. LCMV-GP61-80–specific CD4+ TCR transgenic (SMARTA) mice have been described previously (Oxenius et al., 1998). All mice were housed under specific pathogen–free conditions. Mouse handling conformed to the experimental protocols approved by the University of California, Los Angeles Animal Research Committee (ARC). In all experiments the mice were infected i.v via the retroorbital sinus with 2 × 106 PFU of LCMV-Arm or LCMV-Cl 13. Virus stocks were prepared and viral titers were quantified as described previously (Brooks et al., 2005).
Isolation and adoptive transfer of virus-specific CD4 T cells.
To specifically identify and isolate virus-specific CD4 T cells, CD4+ T cells were purified from the spleens of naive Ly5.1+ SMARTA mice by negative selection (StemCell Technologies). After purification, 500–1000 cells were transferred i.v. into 6–8-wk-old Ly5.2+ C57BL6 mice. The transfers enabled identification and isolation of LCMV-specific CD4 T cells separately from endogenous responses. We avoided the problems associated with using large, nonphysiological numbers of transferred transgenic T cells (Marzo et al., 2005) by only transferring low numbers of transgenic T cells. Mice were then infected with LCMV-Arm or LCMV-Cl 13 1 d after cell transfer. For experiments in which intrahepatic lymphocytes were analyzed, mice were perfused with 25 ml of sterile PBS by cardiac injection to remove blood from all tissues. Intrahepatic lymphocytes were isolated by centrifugation in 35% Percoll (GE Healthcare). The absolute number of Tfh and non-Tfh SMARTA cells was determined by multiplying the frequency of CD4+, Ly5.1+, CXCR5+ cells or CD4+, Ly5.1+, CXCR5− cells, respectively, by the total number of cells in the organ.
To determine whether non-Tfh cells progressively differentiate into Tfh cells during persistent infection, SMARTA cells were adoptively transferred into C57BL6 mice that were subsequently infected with LCMV-Cl 13. Spleens from these mice were isolated on day 9 after infection, depleted of B cells (>98% removed) using anti-CD19 MACs beads (Miltenyi Biotec), FACS sorted based on CD4+, CXCR5−, and adoptively transferred into day 12 LCMV-Cl 13–infected C57BL6 mice that had not originally received SMARTA cells. We transferred into day 12 infected mice (after the start of CD4 T cell contraction) to avoid introducing large numbers CD4 T cells during the peak of the immune response, which may increase immunopathology and mortality. Spleens from recipient mice were analyzed 14 d after transfer (23 d after infection for the SMARTA cells).
To assess the contribution of immune activation and TCR stimulation toward virus-specific CD4 T cell differentiation, SMARTA cells were adoptively transferred into C57BL6 mice and subsequently infected with LCMV-Cl 13. Spleens from these mice were isolated on day 35 after infection, depleted of B cells (>98% removed) using anti-CD19 MACs beads, and then adoptively transferred into C57BL6 LCMV-Arm–immune, LCMV-Cl 13–infected mice or into LCMV-Cl 13–infected MHC class II–deficient mice that had been infected in parallel but had not originally received SMARTA cells. Spleens from recipient mice were analyzed 14 d after cell transfer.
In vivo CD4 depletion.
To deplete CD4 T cells before LCMV infection, mice were injected i.p. with 500 μg anti-CD4 antibody (clone GK1.5) and infected 1 d later. To deplete CD4 T cells during infection, C57BL6 mice were injected i.v. once daily for 3 consecutive days with either 1 mg of the isotype control antibody (LTF-2) or of anti-CD4 antibody (YTS 191; BioXCell). In all cases, CD4 T cell depletion was confirmed via flow cytometry.
Bone marrow chimera experiments.
To perform mixed bone marrow chimera experiments WT C57BL6 recipient mice were lethally irradiated with 950 rads. On the same day, the irradiated mice were injected with a mixture of bone marrow cells from CD4 KO (85%) and WT (15%) mice or CD4 KO (85%) and CXCR5 KO (15%) mice. Bone marrow cells were isolated from femurs and tibia of donor mice. 20 million total cells were transferred (i.v.) into the recipient mice. The recipient mice were treated with antibiotics (Sulfamethoxazole and Trimethoprim in the drinking water) for 3 wk to prevent infection and allow for immune reconstitution. Reconstitution was confirmed 8 wk after bone marrow transfer, and the mice were subsequently infected with LCMV-Cl 13.
Flow cytometry.
Splenocytes were stained ex vivo with LCMV-GP66-77 tetramers, LCMV-GP33-41 tetramers, and/or for the expression of CD4-APC-Cy7, CD8-FITC, CXCR5-biotin/streptavidin (SA)-APC, GL7-FITC, isotype controls (BD); CD4-Pacific blue, ICOS-FITC, OX40-PE, SLAM-Alexa Fluor 488, B220-Pacific blue (BioLegend); Ly5.1-PerCP-Cy5.5, Fas-PE (eBioscience); and Bcl6-PE (Santa Cruz Biotechnology, Inc.). LCMV GP66-77 tetramers were provided by the National Institutes of Health Tetramer Core Facility. Flow cytometric analysis was performed using the Digital LSR II (BD) at the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility.
Immunofluorescence microscopy.
Fresh spleen sections were embedded and frozen on dry ice in optimal cutting temperature (OCT; TissueTek). 6-µm frozen cryostat sections were cut, fixed in 4% paraformaldehyde for 10 min, blocked with an avidin/biotin blocking kit (Vector Laboratories), and stained with CD45.1-biotin (BioLegend), CD4-FITC (BD), or B220-APC (BioLegend). After the primary antibody incubation, sections were washed in PBS and stained with anti-APC (BioLegend) conjugated to Alexa Fluor 647 (Invitrogen) and SA-Rhodamine Red-X (Jackson ImmunoResearch Laboratories). After the second stain, the sections were washed in PBS, incubated with biotinylated anti-SA (Vector Laboratories), washed again in PBS, and then stained with SA-Rhodamine Red-X. Sections were visualized using an immunofluorescence microscope (Zeiss Axio Observe Z1; Carl Zeiss, Inc.), and images were captured with AxioCam MRm camera (Carl Zeiss, Inc.) using a 20× objective. Reconstructions were performed using the MosaiX function in AxioVision software (Carl Zeiss, Inc.). The images were prepared using AxioVision software and Photoshop (Adobe).
Quantitative RT-PCR.
Splenocytes were harvested on day 9 or 30 after infection. Virus-specific CD4 T cells were FACS isolated based on CD4+, Ly5.1+ staining (after sort purity was >97%). RNA from purified virus-specific CD4 T cells was isolated with the RNEasy extraction kit (QIAGEN) and analyzed using the RT2 Profiler PCR array mouse Th1-Th2-Th3 kit (SABiosciences). mRNA was detected using the iCycler (Bio-Rad Laboratories) and normalized to five housekeeping genes.
To specifically assess mRNA expression in naive, virus-specific Tfh and non-Tfh cells. Naive virus-specific CD4+ T cells were purified from spleens of naive Ly5.1+ SMARTA mice by negative selection (StemCell Technologies). Virus-specific Tfh and non-Tfh cells were FACS isolated from splenocytes harvested on day 32 after LCMV-Cl 13 infection. Virus-specific CD4 T cells were sorted based on CD4+, Ly5.1+, CXCR5+ (Tfh) or CD4+, Ly5.1+ CXCR5− (non-Tfh) expression. RNA from purified virus-specific CD4 T cells was isolated with the RNEasy extraction kit. Individual RNA was amplified using Applied Biosystems TaqMan expression assays and normalized to HPRT. mRNA was detected using the iCycler.
ELISA.
To quantify LCMV-specific antibodies, LCMV-Cl 13 was used to coat 96-well Maxisorp ELISA plates (Nunc) overnight. Plates were blocked with 3% BSA/PBS/0.05% Tween 20. Subsequently, serum isolated from the indicated mice was incubated on the LCMV-coated plates. Plates were washed and incubated with an HRP-labeled goat anti–mouse IgG antibody (Invitrogen), followed by the addition of o-phenylenediamine substrate in 0.05 M phosphate citrate buffer. The reaction was stopped with 2N H2SO4, and the optical density values were read using an ELISA plate reader (Synergy 2; BioTek) at 490 nm. The concentration of antibody was interpolated from a standard curve. The standard curve was generated from a serial dilution of purified mouse IgG (Invitrogen; 500–0.49 ng/ml) incubated on plates coated with goat anti–mouse IgGγ (Invitrogen). To assess specific antibody isotypes, LCMV-coated plates were incubated with serum from LCMV-Arm or Cl 13–infected mice, and then incubated with biotinylated goat anti–mouse IgG1, IgG2a, IgG2b, or IgG3 (BioLegend). The plates were subsequently incubated with a SA-HRP (BioLegend), and specific levels of each antibody isotype was measured as stated above.
In vitro B cell help assay.
Virus-specific Tfh and non-Tfh cells were FACS purified from splenocytes harvested on day 28 after LCMV-Cl 13 infection. Virus-specific CD4 T cells were sorted based on CD4+, Ly5.1+, CXCR5+ (Tfh) or CD4+, Ly5.1+ CXCR5− (non-Tfh) expression. Naive B cells were purified by positive selection using anti-CD19 MACs beads. Purified naive B cells (5 × 105) were co-cultured with or without virus-specific Tfh or non-Tfh CD4 T cells (5 × 104) in the presence of 50 U/ml recombinant mouse IL-2 and 50 µM β-mercaptoethanol for 7 d. Supernatant was collected from each culture and assayed for the presence of IgG by ELISA.
Statistical analysis.
Student’s t tests (two-tailed, unpaired) were performed using the GraphPad Prism 5 software (GraphPad Software, Inc.).
Online supplemental material.
Fig. S1 shows CD4 T cell development during viral peristence in the spleen and liver. Fig. S2 demonstrates that virus-specific CD4 T cells differentiate into Tfh cells in lymphoid organs, but not in nonlymphoid organs during persistent viral infection. Fig. S3 shows virus-specific CD4 T cells lack FoxP3 expression during LCMV infection. Fig. S4 depicts GC B cell development in lymphoid and nonlymphoid organs during LCMV infection. Fig. S5 shows ex vivo Tfh cells functionality. Fig. S6 illustrates similar amounts of virus-specific CD4 and CD8 T cells in WT and CXCR5 KO mice during persistent viral infection. Fig. S7 depicts B cell responses in CD4 KO–WT bone marrow chimera mice and CD4 KO–CXCR5 KO bone marrow chimera mice during persistent viral infection. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101773/DC1.
Acknowledgments
Our work was supported by the UCLA Center for AIDS Research and by National Institutes of Health Grants (AI 082975 to D.G. Brooks and AI 007323 to L.M. Fahey).
The authors declare no conflict of interest.
Footnotes
Abbreviations used:
- Arm
- Armstrong
- Bcl6
- B cell lymphoma 6 protein
- Cl 13
- clone 13
- CXCR5
- chemokine receptor 5
- GATA3
- GATA3-binding protein
- GC
- germinal center
- ICOS
- inducible T cell co-stimulator
- LCMV
- lymphocytic choriomeningitis virus
- mRNA
- messenger RNA
- SA
- streptavidin
- SMARTA
- LCMV-specific CD4 TCR transgenic
- Tfh
- T follicular helper
References
- Barber D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- Battegay M., Moskophidis D., Rahemtulla A., Hengartner H., Mak T.W., Zinkernagel R.M. 1994. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 68:4700–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergthaler A., Flatz L., Verschoor A., Hegazy A.N., Holdener M., Fink K., Eschli B., Merkler D., Sommerstein R., Horvath E., et al. 2009. Impaired antibody response causes persistence of prototypic T cell-contained virus. PLoS Biol. 7:e1000080 10.1371/journal.pbio.1000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D.G., Teyton L., Oldstone M.B., McGavern D.B. 2005. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 79:10514–10527 10.1128/JVI.79.16.10514-10527.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D.G., McGavern D.B., Oldstone M.B. 2006a. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 116:1675–1685 10.1172/JCI26856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D.G., Trifilo M.J., Edelmann K.H., Teyton L., McGavern D.B., Oldstone M.B. 2006b. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309 10.1038/nm1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciurea A., Hunziker L., Klenerman P., Hengartner H., Zinkernagel R.M. 2001. Impairment of CD4+ T cell responses during chronic virus infection prevents neutralizing antibody responses against virus escape mutants. J. Exp. Med. 193:297–305 10.1084/jem.193.3.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick E.K., Chan A., Ma C.S., Gatto D., Schwartzberg P.L., Brink R., Tangye S.G. 2010. Follicular Helper T Cell Differentiation Requires Continuous Antigen Presentation that Is Independent of Unique B Cell Signaling. Immunity. 33:1–13 10.1016/j.immuni.2010.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser H., Sauer K., Brooks D.G. 2009. IL-21 is required to control chronic viral infection. Science. 324:1569–1572 10.1126/science.1174182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazilleau N., McHeyzer-Williams L.J., Rosen H., McHeyzer-Williams M.G. 2009. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 10:375–384 10.1038/ni.1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster R., Mattis A.E., Kremmer E., Wolf E., Brem G., Lipp M. 1996. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 87:1037–1047 10.1016/S0092-8674(00)81798-5 [DOI] [PubMed] [Google Scholar]
- Fröhlich A., Kisielow J., Schmitz I., Freigang S., Shamshiev A.T., Weber J., Marsland B.J., Oxenius A., Kopf M. 2009. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 324:1576–1580 10.1126/science.1172815 [DOI] [PubMed] [Google Scholar]
- Fuller M.J., Zajac A.J. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477–486 [DOI] [PubMed] [Google Scholar]
- Gallimore A., Dumrese T., Hengartner H., Zinkernagel R.M., Rammensee H.G. 1998. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 187:1647–1657 10.1084/jem.187.10.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm B., Trifilo M.J., Zuniga E.I., Oldstone M.B. 2005. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 22:247–257 10.1016/j.immuni.2005.01.005 [DOI] [PubMed] [Google Scholar]
- Haynes N.M., Allen C.D., Lesley R., Ansel K.M., Killeen N., Cyster J.G. 2007. Role of CXCR5 and CCR7 in follicular Th cell positioning and appearance of a programmed cell death gene-1high germinal center-associated subpopulation. J. Immunol. 179:5099–5108 [DOI] [PubMed] [Google Scholar]
- Hegazy A.N., Peine M., Helmstetter C., Panse I., Fröhlich A., Bergthaler A., Flatz L., Pinschewer D.D., Radbruch A., Löhning M. 2010. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 32:116–128 10.1016/j.immuni.2009.12.004 [DOI] [PubMed] [Google Scholar]
- Hiramatsu Y., Suto A., Kashiwakuma D., Kanari H., Kagami S., Ikeda K., Hirose K., Watanabe N., Grusby M.J., Iwamoto I., Nakajima H. 2010. c-Maf activates the promoter and enhancer of the IL-21 gene, and TGF-beta inhibits c-Maf-induced IL-21 production in CD4+ T cells. J. Leukoc. Biol. 87:703–712 10.1189/jlb.0909639 [DOI] [PubMed] [Google Scholar]
- Hunziker L., Recher M., Macpherson A.J., Ciurea A., Freigang S., Hengartner H., Zinkernagel R.M. 2003. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat. Immunol. 4:343–349 10.1038/ni911 [DOI] [PubMed] [Google Scholar]
- Johnston R.J., Poholek A.C., DiToro D., Yusuf I., Eto D., Barnett B., Dent A.L., Craft J., Crotty S. 2009. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 325:1006–1010 10.1126/science.1175870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T., Fink K., Förster R., Senn B., Lipp M., Muramatsu M., Zinkernagel R.M., Ludewig B., Hengartner H. 2005. CXCR5-dependent seeding of follicular niches by B and Th cells augments antiviral B cell responses. J. Immunol. 175:7109–7116 [DOI] [PubMed] [Google Scholar]
- Kaech S.M., Hemby S., Kersh E., Ahmed R. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 111:837–851 10.1016/S0092-8674(02)01139-X [DOI] [PubMed] [Google Scholar]
- King I.L., Mohrs M. 2009. IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J. Exp. Med. 206:1001–1007 10.1084/jem.20090313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman P., Hill A. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6:873–879 10.1038/ni1241 [DOI] [PubMed] [Google Scholar]
- Linterman M.A., Vinuesa C.G. 2010. Signals that influence T follicular helper cell differentiation and function. Semin. Immunopathol. 32:183–196 10.1007/s00281-009-0194-z [DOI] [PubMed] [Google Scholar]
- Linterman M.A., Beaton L., Yu D., Ramiscal R.R., Srivastava M., Hogan J.J., Verma N.K., Smyth M.J., Rigby R.J., Vinuesa C.G. 2010. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J. Exp. Med. 207:353–363 10.1084/jem.20091738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo A.L., Klonowski K.D., Le Bon A., Borrow P., Tough D.F., Lefrançois L. 2005. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 6:793–799 10.1038/ni1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M., Concepcion R.J., Ahmed R. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056–8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Hwang D., Yang X.O., Kang H.S., Ma L., Wang Y.H., Watowich S.S., Jetten A.M., Tian Q., Dong C. 2008. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 29:138–149 10.1016/j.immuni.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R.I., Chung Y., Martinez G.J., Yang X.O., Tanaka S., Matskevitch T.D., Wang Y.H., Dong C. 2009. Bcl6 mediates the development of T follicular helper cells. Science. 325:1001–1005 10.1126/science.1176676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B., Eppler M., Leist T.P., Hengartner H., Zinkernagel R.M. 1991. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc. Natl. Acad. Sci. USA. 88:8252–8256 10.1073/pnas.88.18.8252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenius A., Bachmann M.F., Zinkernagel R.M., Hengartner H. 1998. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28:390–400 [DOI] [PubMed] [Google Scholar]
- Ozaki K., Spolski R., Feng C.G., Qi C.F., Cheng J., Sher A., Morse H.C., III, Liu C., Schwartzberg P.L., Leonard W.J. 2002. A critical role for IL-21 in regulating immunoglobulin production. Science. 298:1630–1634 10.1126/science.1077002 [DOI] [PubMed] [Google Scholar]
- Pot C., Jin H., Awasthi A., Liu S.M., Lai C.Y., Madan R., Sharpe A.H., Karp C.L., Miaw S.C., Ho I.C., Kuchroo V.K. 2009. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 183:797–801 10.4049/jimmunol.0901233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recher M., Hunziker L., Ciurea A., Harris N., Lang K.S. 2004. Public, private and non-specific antibodies induced by non-cytopathic viral infections. Curr. Opin. Microbiol. 7:426–433 10.1016/j.mib.2004.06.008 [DOI] [PubMed] [Google Scholar]
- Recher M., Lang K.S., Navarini A., Hunziker L., Lang P.A., Fink K., Freigang S., Georgiev P., Hangartner L., Zellweger R., et al. 2007. Extralymphatic virus sanctuaries as a consequence of potent T-cell activation. Nat. Med. 13:1316–1323 10.1038/nm1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt R.L., Liang H.E., Locksley R.M. 2009. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat. Immunol. 10:385–393 10.1038/ni.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Blackburn S.D., Blattman J.N., Wherry E.J. 2007. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204:941–949 10.1084/jem.20061937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen A.R., Johansen J., Marker O., Christensen J.P. 1996. Exhaustion of CTL memory and recrudescence of viremia in lymphocytic choriomeningitis virus-infected MHC class II-deficient mice and B cell-deficient mice. J. Immunol. 157:3074–3080 [PubMed] [Google Scholar]
- Viret C., Wong F.S., Janeway C.A., Jr 1999. Designing and maintaining the mature TCR repertoire: the continuum of self-peptide:self-MHC complex recognition. Immunity. 10:559–568 10.1016/S1074-7613(00)80055-2 [DOI] [PubMed] [Google Scholar]
- Voigt I., Camacho S.A., de Boer B.A., Lipp M., Förster R., Berek C. 2000. CXCR5-deficient mice develop functional germinal centers in the splenic T cell zone. Eur. J. Immunol. 30:560–567 [DOI] [PubMed] [Google Scholar]
- Wherry E.J., Blattman J.N., Murali-Krishna K., van der Most R., Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927 10.1128/JVI.77.8.4911-4927.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry E.J., Ha S.J., Kaech S.M., Haining W.N., Sarkar S., Kalia V., Subramaniam S., Blattman J.N., Barber D.L., Ahmed R. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 27:670–684 10.1016/j.immuni.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Yi J.S., Du M., Zajac A.J. 2009. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 324:1572–1576 10.1126/science.1175194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Rao S., Tsai L.M., Lee S.K., He Y., Sutcliffe E.L., Srivastava M., Linterman M., Zheng L., Simpson N., et al. 2009. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 31:457–468 10.1016/j.immuni.2009.07.002 [DOI] [PubMed] [Google Scholar]
- Zajac A.J., Blattman J.N., Murali-Krishna K., Sourdive D.J., Suresh M., Altman J.D., Ahmed R. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213 10.1084/jem.188.12.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky A.G., Taylor J.J., King I.L., Marshall F.A., Mohrs M., Pearce E.J. 2009. T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J. Exp. Med. 206:991–999 10.1084/jem.20090303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D., Coquet J.M., Zhang Y., Light A., D’Costa K., Kallies A., Corcoran L.M., Godfrey D.I., Toellner K.M., Smyth M.J., et al. 2010. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207:365–378 10.1084/jem.20091777 [DOI] [PMC free article] [PubMed] [Google Scholar]