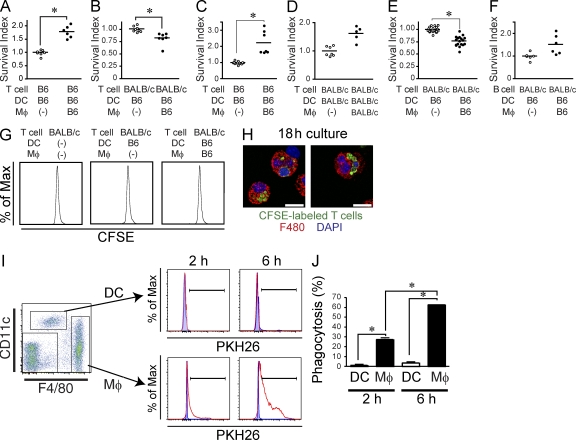
Host macrophages protect against graft-versus-host disease in part by engulfing donor T cells and inhibiting their proliferation.
Abstract
Acute graft-versus-host disease (GVHD) results from the attack of host tissues by donor allogeneic T cells and is the most serious limitation of allogeneic hematopoietic cell transplantation (allo-HCT). Host antigen-presenting cells are thought to control the priming of alloreactive T cells and the induction of acute GVHD after allo-HCT. However, whereas the role of host DC in GVHD has been established, the contribution of host macrophages to GVHD has not been clearly addressed. We show that, in contrast to DC, reducing of the host macrophage pool in recipient mice increased donor T cell expansion and aggravated GVHD mortality after allo-HCT. We also show that host macrophages that persist after allo-HCT engulf donor allogeneic T cells and inhibit their proliferation. Conversely, administration of the cytokine CSF-1 before transplant expanded the host macrophage pool, reduced donor T cell expansion, and improved GVHD morbidity and mortality after allo-HCT. This study establishes the unexpected key role of host macrophages in inhibiting GVHD and identifies CSF-1 as a potential prophylactic therapy to limit acute GVHD after allo-HCT in the clinic.
Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative treatment for many patients with high-risk hematological malignancies (Rabinowe et al., 1993; van Besien et al., 1998; Pavletic et al., 2000; Alyea et al., 2001; Bishop et al., 2003; Dreger et al., 2003; Maris et al., 2004; Peggs et al., 2005; Sorror et al., 2005). The success of allo-HCT is largely based on immunological graft-versus-tumor effects mediated by allogeneic T lymphocytes present in the graft (Collin et al., 2007). Unfortunately, this beneficial effect is counterbalanced by the occurrence of graft-versus-host reactions directed against normal host tissues resulting in graft-versus-host disease (GVHD), a potentially life-threatening complication which limits the success of allo-HCT. GVHD may manifest as inflammation of the host tissue including, but not limited to, the skin, liver, and gut. Depending on the degree of genetic disparity between allogeneic donor and recipient, GVHD may occur in up to 75% and may lead to death in up to 20% of transplant recipients (Mielcarek et al., 2003). Therefore, a major objective in allo-HCT is the prevention of GVHD.
Studies in the last decade have led to the concept that tissue injuries induced by the transplantation regimen activate host APCs, which in turn control the priming of donor T cells to host tissue antigens and the induction of GVH reactions. This concept is based on a series of studies pioneered in experimental mouse models of GVHD showing that BM chimeric mice in which host hematopoietic cells are unable to prime donor T cells are protected from GVHD after allo-HCT (Shlomchik et al., 1999), whereas alloantigen expression on host target epithelium is not essential for alloreactive T cell attack of the skin, liver, and intestine of recipient animals (Teshima et al., 2002). Previous studies, including ours, have shown that DCs are potent initiators of GVHD (Duffner et al., 2004; Merad et al., 2004; Koyama et al., 2009). Consistently, the use of liposomal clodronate (Lip-Clod) to deplete both host macrophages and DC limited GVHD and improved survival after transplant (Zhang et al., 2002b).
Similar to other adaptive immune responses (Miller et al., 2002; Mempel et al., 2004), GVHD is initiated upon priming of alloreactive T cells by host APC in secondary lymphoid organs during the first days after allo-HCT (Panoskaltsis-Mortari et al., 2004; Beilhack et al., 2005; Na et al., 2010). Therefore, host APCs that survive the conditioning regimen and remain in lymphoid organs during the first days that follow the injection of alloreactive T cells are uniquely capable of shaping donor T cell immune responses to host antigens (Zhang et al., 2002a). We have recently shown that recipient macrophages resist the conditioning regimen and persist in patients for many weeks after allo-HCT (Haniffa et al., 2009), providing ample opportunity to modulate donor T cell immunity. However, although the role of DC in GVHD has been established, the exact role of host macrophages in the induction of alloimmune responses has not been clearly addressed.
In this study, we examined the contribution of host macrophages to acute GVHD using an experimental mouse model of allo-HCT. Unexpectedly, we found that in contrast to host DC, host macrophages that resist the conditioning regimen play a key role in modulating the induction of alloreactive T cell immune responses and limit the severity of GVH reactions after allo-HCT.
RESULTS
Macrophages persist in lymphoid tissues after lethal irradiation
Remaining host APCs that resist the conditioning regimen play a key role in shaping donor T cell immunity (Collin et al., 2007). To examine whether host lymphoid tissue macrophages influence donor T cell immunity after allo-HCT, C57BL/6 mice (H-2b) were exposed to total body irradiation (TBI; 13 Gy). Recipient mice were then sacrificed at different time points after TBI to examine the rate of macrophage decline in the spleen and LN. The number of spleen red pulp macrophages and LN medullary and subcapsular sinus macrophages was unchanged at 48 h and significant numbers of macrophages persisted at 96 h after TBI (Fig. 1 A). In contrast, DC and B cells were strongly reduced by 48 h after TBI (Fig. 1, B and C). Neutrophils, T cells, and NK cells were also quickly eliminated after TBI (Fig. S1, A–C).
Figure 1.
Macrophages persist in lymphoid tissues for several days after TBI. C57BL/6 mice were sacrificed 48 h (gray bars, n = 6) and 96 h (black bars, n = 6) after TBI (13 Gy). Nonirradiated C57BL/6 mice were used as controls (white bars, n = 4–8). The number of remaining host macrophages (A), DCs (B), and B cells (C) in the spleen (top) and mesenteric LN (bottom) are shown as mean ± SEM. Pooled data from two independent experiments are shown. Mϕ, macrophage.
Anti–CSF-1 receptor (R) blocking mAb eliminates host macrophages that persist in lethally irradiated mice
The CSF-1R is expressed on all monocytes and tissue macrophages and is thought to play a key role in the homeostasis of these cells (Chitu and Stanley, 2006). CSF-1R has two ligands that include the cytokine CSF-1 and a newly identified cytokine called IL-34 (Lin et al., 2008). IL-34−/− mice are not yet available, but csf-1op/op mice (Cecchini et al., 1994) that carry a natural null mutation in the gene encoding the CSF-1 protein (Felix et al., 1990; Wiktor-Jedrzejczak et al., 1990; Yoshida et al., 1990) and csf-1r−/− mice lack tissue macrophages (Cecchini et al., 1994; Dai et al., 2002; Fig. S2 A). Circulating monocytes, including the Gr-1low and Gr-1high subsets, are also reduced in csf-1r−/− and csf-1op/op mice, although the Gr-1low subset is more strongly affected in these mice (Fig. S2 B and not depicted). Although we found that CSF-1R is expressed on a subset of DCs that express high levels of the CD11b integrin in lymphoid and nonlymphoid tissues (Ginhoux et al., 2009), csf-1r−/− mice only lack CD11b+ DC in nonlymphoid tissues whereas lymphoid organ DC remained intact in these mice (Ginhoux et al., 2006, 2009).
Based on these findings, we thought to use a blocking anti–CSF-1R mAb to determine whether CSF-1R blockade in mice could be used to eliminate lymphoid tissue macrophages without affecting lymphoid tissue DC. We found that systemic administration of anti–CSF-1R mAb in C57BL/6 mice strongly reduced red pulp spleen macrophages as well as LN medullary and subcapsular sinus macrophages (Fig. 2, A and D). Circulating monocytes were also significantly reduced upon systemic administration of anti–CSF-1R mAb treatment (Fig. 2, C and F). Strikingly, the Gr-1low monocytes subset was much more affected than the Gr-1high monocyte subset, suggesting that CSF-1 signaling controls the differentiation of Gr-1high into Gr-1low monocytes in vivo (Fig. 2, C and F). In contrast, spleen and LN DCs, T cells, B cells, NK cells, and neutrophils were not affected by the mAb treatment (Fig. 2, B and E; and Fig. S3).
Figure 2.
Pretransplant anti–CSF-1R mAb (αCSF-1R) administration depletes remaining host lymphoid tissue macrophages. (A–F) Mice were injected with αCSF-1R (black bar, n = 11) or rat IgG (white bar, n = 9) for 3 d and were sacrificed 5 d after the last injection. The numbers of spleen and LN macrophages (A and D), spleen and LN DCs (B and E), and circulating neutrophils and monocytes (C and F) are shown. In the LN panel shown in A, dot plots show the percentage of LN macrophages among CD11b+ gated cells. Other dot plots depicted in A and B show percentage of DCs or macrophages among total spleen or LN cells. Data from three independent experiments are pooled and shown as mean ± SEM. *, P < 0.05; **, P < 0.001. (G–K) C57BL/6 mice were injected i.p. with αCSF-1R (black bar, n = 10) or isotype IgG (white bar, n = 7–11) from days −5 to −3, were lethally irradiated and reconstituted with allogeneic BM and splenocytes on day 0, and were then sacrificed 2 d later. The relative (G) and absolute (H) numbers of remaining host spleen and LN macrophages and the absolute numbers of spleen and LN DCs (I) and spleen neutrophils (J) are shown. Data from three independent experiments are combined and shown as mean ± SEM. *, P < 0.05; **, P < 0.001. (K) Images show frozen sections of spleens stained for CD169+ marginal zone metallophilic macrophages. Bars, 100 µm.
To examine whether anti–CSF-1R mAb could also be used to eliminate host macrophages that remain in lymphoid tissues after allo-HCT, recipient mice received three consecutive injections of anti–CSF-1R mAb or isotype Ab control starting 5 d before lethal irradiation and allo-HCT. Mice were sacrificed 2 d after transplant to measure the effect of the anti–CSF-1R mAb on the remaining host macrophage pool. Consistent with the results obtained in naive mice, anti–CSF-1R mAb injections before transplant strongly reduced the spleen and LN macrophage pool (Fig. 2, G, H, and K), whereas spleen and LN DCs, as well as neutrophils, remained unaffected by the treatment (Fig. 2, I and J).
Anti–CSF-1R mAb–induced macrophage depletion could potentially be mediated via different mechanisms that include complement-dependent cytotoxicity, which requires the cleavage of the C3 complement component (Ehlenberger and Nussenzweig, 1977), antibody-dependent cell-mediated cytotoxicity (ADCC), which requires the engagement of an intact FcγR chain on recipient macrophages, or the blockade of c-fms signaling. The AFS98 anti–CSF-1R mAb clone used in this study depleted host macrophages as efficiently in mice deficient in C3 (Fig. S4 A) or FcγR (Fig. S4 B) as in wild-type mice, suggesting that macrophage depletion occurred independently of complement-dependent cytotoxicity or ADCC and was likely mainly a result of the inhibition of CSF-1 signaling. Consistently, we found that GW2580, a small molecule which inhibits c-fms signaling, had a similar effect to AFS98 on macrophages in vivo (Fig. S4 C). Based on these findings, we concluded that AFS98-mediated macrophage depletion is dependent on c-fms blockade.
Anti–CSF-1R mAb administration before allo-HCT exacerbates GVHD
The ability of anti–CSF-1R mAb to eliminate lymphoid tissue macrophages but not lymphoid tissue DC provides a tool to specifically assess the role of host macrophages in allo-HCT outcome. To examine whether elimination of conditioning-resistant host macrophages could affect GVHD outcome after allo-HCT, recipient C57BL/6 mice were treated with anti–CSF-1R mAb or rat IgG control from days −5 to −3, lethally irradiated on day 0, and injected with BM cells together with 2.5 or 10 × 106 splenocytes isolated from MHC-mismatch allogeneic BALB/c (H-2d) mice or syngeneic C57BL/6 mice. Severe GVHD occurred in recipient mice injected with 10 × 106 splenocytes, leading to the death of all animals by day 65 after transplant, whereas 40% of the recipient mice injected with 2.5 × 106 splenocytes survived >90 d after transplant. Unexpectedly, anti–CSF-1R administration exacerbated GVHD morbidity (Fig. 3 B) and mortality after allo-HCT, leading to the death of all recipient mice including those injected with low-dose allogeneic T cells by day 30 after transplant (Fig. 3, A, C, and D). In contrast, anti–CSF-1R mAb did not affect the outcome of lethally irradiated C57BL/6 mice reconstituted with syngeneic hematopoietic cells (Fig. 3, A and B), indicating that the adverse effect of anti–CSF-1R mAb occurs only in the context of allogeneic transplantation. In addition, anti–CSF-1R mAb treatment did not compromise donor hematopoietic cell engraftment after transplant, as lethally irradiated mice that received allogeneic hematopoietic cells were fully chimeric by day 11 (Fig. 3 E). The white blood cell count in peripheral blood and the number of myeloid cells were also equivalent in anti–CSF-1R mAb-treated and control recipients (Fig. 3, F and G). Aggravation of GVHD by the anti–CSF-1R mAb was not strain dependent, as similar results were obtained when BALB/c recipient mice were reconstituted with allogeneic hematopoietic progenitors and alloreactive T cells isolated from C57BL/6 mice (unpublished data). Altogether these results suggest that CSF-1R–expressing cells play a key role in limiting GVHD after allo-HCT.
Figure 3.
Anti–CSF-1R (αCSF-1R) mAb treatment exacerbates GVHD without impairing donor hematopoietic cell engraftment. (A and B) C57BL/6 mice (n = 5–6/group) were injected i.p. with αCSF-1R (solid lines, diamond) or control rat IgG (dashed lines, triangle) on days −5 to −3, lethally irradiated (13 Gy), and injected i.v. on day 0 with 2.5 (open symbols) or 10 (closed symbols) × 106 splenocytes plus 5 × 106 BM cells isolated from BALB/c mice. As controls, syngeneic C57BL/6 CD45.2+ mice (open circle, n = 3/group) were treated with anti–CSF-1R (solid lines) or rat IgG (dashed lines) on days −5 to −3, lethally irradiated (13 Gy), and i.v. injected on day 0 with 2.5 × 106 splenocytes plus 5 × 106 BM cells isolated from C57BL/6 CD45.1+ congenic mice. Graphs show survival curves after transplant (A) and clinical GVHD scores reported as mean ± SEM (B). *, P < 0.005; **, P < 0.05 versus allogeneic rat IgG-treated groups. (C) H&E staining of liver (i, ii, and iii) and colon (iv, v, and vi) sections harvested 20 d after transplant from mice treated with αCSF-1R before syngeneic HCT (i and iv) or mice treated with rat IgG (ii and v) or αCSF-1R (iii and vi) before allo-HCT. Arrowheads show inflammatory infiltrates accumulating in liver tissue sections. Bars, 100 µm. (D) Pathological GVHD scores from syngeneic recipients (white bar, n = 6), allogeneic recipients treated with rat IgG (gray bar, n = 6), and allogeneic recipients treated with αCSF-1R (black bar, n = 6) are shown as mean ± SEM. Scores were analyzed on day 20 after transplant and calculated as the sum of the scores of liver, small intestine, and colon. *, P < 0.005. (E–G) White blood cells in peripheral blood were counted and analyzed by flow cytometry 11 d after allo-HCT. Percent donor chimerism among myeloid and lymphoid subsets (E), absolute numbers of total blood leukocytes (F), and circulating CD11b+ myeloid cells (G) are shown. Horizontal bars indicate mean. Data from one of two similar experiments were shown.
Anti–CSF-1R mAb administration dramatically increases donor T cell expansion and cytokine release after allo-HCT
Alloreactive T cells are responsible for the induction of acute graft-versus-host reactions (Collin et al., 2007). In this study, we measured the effect of anti–CSF-1R mAb on the fate of donor allogeneic T cells in recipient animals. The numbers of donor CD4+ and CD8+ T cells were dramatically enhanced in the spleen, LN, and liver of mice treated with anti–CSF-1R mAb compared with control mice (Fig. 4, A–C). IFN-γ and TNF, two cytokines shown to play a role in the efferent and afferent phases of acute GVHD (Jenq and van den Brink, 2010), were elevated in the sera of mice treated with anti–CSF-1R mAb before allo-HCT compared with the control animals (Fig. 4, D and E). Th2 cytokines, such as IL-13 and IL-4, were either slightly decreased or below detection levels in both groups (Fig. 4 F and not depicted). Importantly, administration of anti–CSF-1R mAb did not affect the differentiation of donor Foxp3+ T cells after allo-HCT, suggesting that donor T cell expansion in these mice was not a result of the modulation of donor T regulatory cell differentiation in vivo (Fig. S5, A and B).
Figure 4.
Pretransplant anti–CSF-1R mAb (αCSF1R) injection enhances donor T cell expansion through its effect on host CSF-1R–expressing cells. (A–F) C57BL/6 mice were treated with αCSF-1R (black bars, n = 10) or control rat IgG (gray bars, n = 13) on days −5 to −3, lethally irradiated, and i.v. injected on day 0 with 5 × 106 splenocytes plus 5 × 106 BM cells isolated from BALB/c mice. Absolute numbers of spleen, LN, and liver donor T cells identified as H2Kd+TCRβ+CD4+ or H2Kd+TCRβ+CD8+ cells (A–C) and serum cytokine levels (pg/ml; D–F) measured on day 6 after transplant are shown as mean ± SEM. Data were pooled from three independent experiments. (G–L) C57BL/6 CD45.2+ mice were injected i.p. with αCSF-1R mAb on days −5 to −3, lethally irradiated, and injected on day 0 with 2 × 106 purified T cells isolated from congenic C57BL/6 CD45.1+ mice (G–I) or allogeneic BALB/c mice (J–L). Absolute numbers of donor T cells in the spleen, mesenteric LN, and liver analyzed on day 5 after transfer are shown as mean ± SEM. Data were pooled from two independent experiments. *, P < 0.05; **, P < 0.005.
Remaining host CSF-1R–positive cells modulate GVHD after allo-HCT
Anti–CSF-1R mAb persists in the circulation for many days after transplant (unpublished data). Therefore, aggravation of GVHD by anti–CSF-1R mAb could potentially be mediated by donor CSF-1R–expressing cells. To address this hypothesis, recipient C57BL/6 mice treated with anti–CSF-1R mAb were lethally irradiated and injected with highly purified donor T cells without additional donor BM cells and splenocytes to avoid injecting donor CSF-1R–expressing cells. Similar to the results in the previous section, anti–CSF-1R mAb enhanced the expansion of adoptively transferred allogeneic BALB/c T cells but not congenic C57BL/6 CD45.1+ T cells injected into lethally irradiated C57BL/6 CD45.2+ mice (Fig. 4, G–L). Because naive and activated T cells lack CSF-1R expression (Fig. S5 C), these results suggest that anti–CSF-1R mAb modulates GVHD through its effect on host and not donor CSF-1R–expressing cells.
Low-dose Lip-Clod treatment exacerbates GVHD when administered 10 d before allo-HCT
Because CSF-1R expression is not limited to myeloid cells (Dai et al., 2002; Menke et al., 2009), it is possible that GVHD aggravation by CSF-1R mAb is independent of its effect on macrophages. To better assess the role of host macrophages in GVHD, we used Lip-Clod which has a deleting effect that is limited to DC and macrophages in vivo (Van Rooijen and Sanders, 1994). To specifically examine the contribution of host macrophages in the pathogenesis of GVHD and to circumvent the depletion of DC, we took advantage of the faster turnover of lymphoid tissue DC compared with macrophages. Consistent with a previous study (Okamoto et al., 2008), we found that 10 d after Lip-Clod injection, spleen DCs, as well as circulating monocytes, recovered to normal levels (Fig. 5, A and B; and not depicted), whereas macrophages remained significantly reduced (Fig. 5, A and B). Similarly, nonlymphoid tissue DCs, including cutaneous, lung, liver, and intestinal DCs, were present in similar numbers in mice treated with Lip-Clod or Liposomal PBS (Fig. S6), enabling us to assess the contribution of macrophages and CSF-1R+ DC in modulating GVHD outcome after allo-HCT. Similar to the results observed using anti–CSF-1R mAb, Lip-Clod administered 10 d before transplant significantly increased GVHD mortality (Fig. 5 C) and morbidity (Fig. 5 D), confirming the key role of host macrophages in modulating GVHD after allo-HCT.
Figure 5.
Lip-Clod administered 10 d before allo-HCT exacerbates acute GVHD through the specific depletion of host macrophages. (A) Dot plots show the relative number of DC and macrophages among total splenocytes in C57BL/6 mice that were untreated or injected with 100 µl Lip-Clod and analyzed 2 or 10 d after injection. (B) The absolute numbers of splenic macrophages and DC in Lip-Clod–treated mice (black bars, n = 5) and control mice (white bar, n = 5) are shown as mean ± SEM. Data from one of two similar experiments were shown. (C and D) C57BL/6 mice were injected with 100 µl Lip-Clod (solid lines, diamonds) or PBS (dashed lines, triangles) 10 d before allo-HCT. Graphs show survival curves (C) and clinical GVHD scores presented as mean ± SEM (D). *, P < 0.05. Data are pooled from two independent experiments.
Host macrophages reduce alloreactive T cell proliferation in vitro
The results in the previous section demonstrate that in contrast to host DC, the depletion of host macrophages before allo-HCT aggravates GVHD clinical outcome, enhances donor T cell expansion, and increases the release of Th1 cytokines. Next, we explored the mechanisms by which host macrophages could potentially control donor T cell expansion induced by host DC after allo-HCT. Therefore, purified allogeneic BALB/c T cells and host C57BL/6 spleen DC were co-cultured in the presence or absence of host C57BL/6 macrophages purified from allogeneic recipient mice 2 d after transplant to mimic host APC–donor T cell interactions that occur in the recipient lymphoid tissues after allo-HCT. We found that host macrophages, but not host B cells, inhibited the proliferation of alloreactive T cells cultured in the presence of host DC in a dose-dependent manner (Fig. S7, A and C–E) and were more efficient at inhibiting allogeneic CD4+ T cell proliferation than CD8+ T cells. The use of a transwell co-culture system to prevent macrophages/T cell–cell contact partly reversed the suppression of T cell proliferation, suggesting that soluble factors secreted by host macrophages inhibited T cells proliferation. Importantly, we found that anti–TGF-β blocking mAb significantly reduced macrophage ability to suppress allogeneic proliferation without fully restoring T cell proliferation levels, suggesting that molecules other than TGF-β control macrophages ability to suppress allogeneic T cell proliferation or that the blocking mAb used in this culture does not fully block TGF-β activity (Fig. S7, F and G). However, addition to inducible nitric oxide synthase (iNOS) inhibitor (L-NMMA), Arginase 1 inhibitor (nor-NOHA), IDO inhibitor (1-MT), and IL-10 blocking mAb failed to reverse the suppressive function of macrophages in these cultures (Fig. S7, F and G). We also found that macrophages isolated from IFN-γ receptor knockout and iNOS knockout mice suppressed donor T cells as efficiently as macrophages isolated from wild-type mice (Fig. S7 F).
Remaining host macrophages engulf donor allogeneic T cell in a CD47-dependent manner
Importantly, using the same in vitro culture system described in the previous section, we found that macrophages reduced the number of allogeneic T cells, but not allogeneic B cells or syngenic T cells, long before the initiation of T cell proliferation (Fig. 6, A–G). Consistently, we found that host macrophages engulfed alloreactive T cells between 2 and 18 h of in vitro culture (Fig. 6, H–J) and, thus, much more efficiently than host DC (Fig. 6, I and J). To examine whether host macrophages can also engulf donor T cells in vivo, we traced the fate of alloreactive T cells during the 18 h after their injection in lethally irradiated recipient mice. Alloreactive T cells accumulated near the spleen marginal zone shortly after adoptive transfer and gradually shifted toward the T cell area (Fig. 7, A and B). A large number of donor T cells were trapped in the red pulp in close contact with host macrophages at early time points after their transfer (Fig. 7, A and B). Consistent with results obtained in cultures, CFSE-labeled donor T cells were engulfed by splenic macrophages during the first day of transplant (Fig. 7, C–E) and before the initiation of donor T cell proliferation in vivo (not depicted). Strikingly, 18 h after allo-HCT, the number of donor T cells accumulating in the recipient spleen and mesenteric LN were significantly higher in mice treated with anti–CSF-1R mAb compared with the control groups, whereas mAb treatment did not affect the numbers of donor B cell in the spleen (Fig. 7 F). Altogether, these results suggest that host macrophages limit the expansion of donor T cells partly through their ability to engulf donor allogeneic T cells. Because high CD47 expression protects cells from being captured by macrophages, we compared CD47 expression levels on naive T and B lymphocytes isolated from spleen. We found that CD47 was expressed at lower levels on donor T cells compared with B cells (Fig. S8 A) but was up-regulated on proliferating T cells stimulated with anti-CD3ε mAb or allogeneic DC (Fig. S8, B and C; and not depicted). To examine whether CD47 plays a role in donor T cell homeostasis after allo-HCT, we coinjected 106 T cells isolated from CD47+/+ and CD47−/− mice into lethally irradiated allogeneic recipients. As expected, CD47−/− donor T cells were quickly eliminated from the host and a higher number of wild-type T cells survived, resulting in a higher CD47+/+/CD47−/− T cell ratio in recipients (Fig. 7, G and I–K). The skewed ratio was observed as early as 18 h after transfer and before the initiation of T cell proliferation (Fig. 7 I). Depletion of macrophages before injection of CD47+/+ and CD47−/− mixed T cells dramatically prolonged the survival of CD47−/− T cells and, to a lesser extent, CD47+/+ T cells (Fig. 7 K), correcting the skewed CD47+/+/CD47−/− T cell ratio observed in recipient mice (Fig. 7, G and I–K). These data establish that CD47 expression on donor T cells partly control macrophage ability to capture donor T cells after allo-HCT.
Figure 6.
Macrophages reduce the survival of allogeneic T cells before the initiation of T cell proliferation (A–F) C57BL/6 T cells (A and C), BALB/c T cells (B, D, and E), or BALB/c B cells (F) were co-cultured with C57BL/6 DC in the presence or absence of splenic (A, B, and F) or peritoneal (C and E) macrophages from C57BL/6 mice. In D, DC and macrophages were purified from BALB/c mice. Graphs show the survival index of remaining live T cells or B cells after 44 h of culture in the presence of macrophages. The survival index was calculated as the ratio of remaining live lymphocytes in each well to the mean of numbers of live lymphocytes in the wells without macrophages. Horizontal bars indicate mean. (G) BALB/c T cells were labeled with 5 µM CFSE before co-culture with C57BL/6 DC in the presence or absence of C57BL/6 macrophages. CFSE dilution within TCR-β–positive cells was analyzed after 48 h of culture. (H) Images show macrophages isolated after 18 h of in vitro co-culture (as described in G) contained CFSE+ material. Bars, 10 µm. (I and J) DCs and macrophages purified from C57BL/6 mice were cultured with or without PKH26-labeled BALB/c T cells. Phagocytosis of PKH26-labeled T cells by DC and macrophages were evaluated after 2 and 6 h of co-culture. (I) Dot plot shows the gating strategy for DC and macrophages. Doublets and dead cells were excluded from the analysis. Histograms show the presence of PKH26+ material in gated DC (top) and macrophages (bottom) cultured with (red lines) or without (blue shaded area) PKH26-labeled T cells. (J) The percentage of PKH26-positive cells among DCs (white bar) and macrophages (black bar) after 2 and 6 h of co-culture are shown as mean ± SEM. B6, C57BL/6. *, P < 0.05. These results were representative of three independent experiments.
Figure 7.
Host macrophages reduce the donor T cell pool partly though their ability to clear donor allogeneic T cells after allo-HCT. (A and B) Lethally irradiated C57BL/6 mice were injected with 6 × 106 CFSE-labeled BALB/c T cells. Sequential lengthwise frozen sections of whole spleens were made at the indicated time points after transfer. The section with maximal area was chosen and stained with anti-F4/80 (blue) and anti-CD3 (red) mAb. (A) Representative images at each time points are shown. The far right panel indicates that the F4/80-positive area and CD3ε-positive area are defined as red pulp and T cell zone, respectively and the unstained area is defined as marginal zone or B cell follicle. Bars, 500 µm. (B) Images of the whole sections were captured using an automatic motorized stage. CFSE+ T cells in each area were counted separately. The percentage of CFSE-labeled T cells in T cell zone (red), red pulp (blue), and marginal zone or B cell follicle (green) are shown as mean ± SEM. (C and D) Lethally irradiated C57BL/6 mice were injected with 3 × 106 CFSE-labeled BALB/c T cells and sacrificed 1–2 h after transfer. Dot plots (C) show the gating strategy for recipient red pulp macrophages. The percentages of host macrophages that phagocytosed CFSE+ cells (D) upon adoptive transfer of CFSE+ T cells (filled circle) or noninjected controls (open circle) are shown. Data from one out of two separate experiments are shown. (E) Representative images of F4/80+ macrophages that engulfed CFSE-labeled T cells at 18 h after the transfer are shown. Bars, 10 µm. (F) B6 mice were treated with αCSF-1R (filled circles) or rat IgG (open circles) from days −5 to −3 and lethally irradiated and injected with 5 × 106 CFSE-labeled splenocytes and 5 × 106 CFSE-labeled BM cells on day 0. The absolute numbers of donor cells that accumulate in the recipient spleen 18 h after transfer are shown. Pooled data from three independent experiments were combined. Horizontal bars indicate mean. (G–K) BALB/c mice were treated with αCSF-1R or rat IgG from days −5 to −3. On day 0, the recipient mice were lethally irradiated and injected with 3 × 106 wild-type T cell–depleted BM cells together with 106 CD45.2+ B6 CD47 knockout (KO) T cells and 106 CD45.1+ B6 WT T cells. (G) Dot plot show the percentage of donor allogeneic CD47 KO T cells identified as CD45.1−H2-Kb+TCR-β+ cells and donor WT T cells identified as CD45.1+H2-Kb+TCR-β+ cells in allogeneic recipient mice on day 6 after allo-HCT. (H–J) The percentages of CD47 KO T cells (red) and WT T cells (blue) among donor allogeneic T cells in recipient mice sacrificed at 2 h (H, n = 2/group), 18 h (I, n = 4/group), or 6 d (J, n = 5/group) after allo-HCT are shown as mean ± SEM. (K) Absolute number of donor T cells in allogeneic recipients analyzed 6 d after transfer are shown as mean ± SEM. *, P < 0.05. The results are representative of two independent experiments. MZ, marginal zone; RPM, red pulp macrophage. *, P < 0.05.
Pretransplant CSF-1 treatment expands host macrophages and improves GVHD after allo-HCT
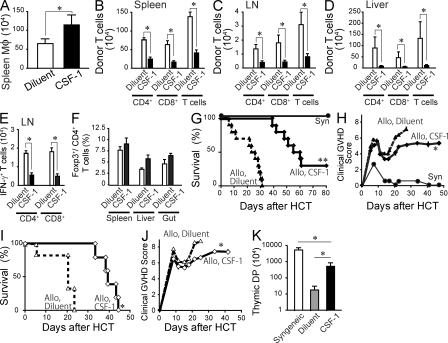
Human CSF-1 has been shown to expand macrophages in mice (Hume et al., 1988; Cecchini et al., 1994). To examine whether pretransplant CSF-1 administration could be used to improve GVHD after allo-HCT, recipient C57BL/6 mice were injected daily for 5 d with high-dose human CSF-1 before lethal irradiation and allo-HCT. CSF-1 injections significantly increased the number of host spleen macrophages that persisted after lethal irradiation (Fig. 8 A). Strikingly, CSF-1 injections also reduced the expansion of donor alloreactive T cells in the spleen, LN, and liver and the differentiation of IFN-γ–producing effector cells (Fig. 8, B–E) but did not alter the induction of donor Foxp3+ regulatory T cells (Fig. 8 F). Importantly, pretransplant CSF-1 treatment significantly improved the clinical GVHD score and survival of recipient mice injected with 10 or 20 × 106 splenocytes (Fig. 8, G–J). Consistently, the loss of CD4+CD8+ double-positive T cells in the thymus, a surrogate marker of thymic GVHD (Krenger et al., 2000), was significantly improved by CSF-1 administration (Fig. 8 K).
Figure 8.
CSF-1 administration reduces donor T cell expansion and GVHD severity. (A) C57BL/6 mice were injected i.p. with 106 U human CSF-1 (black bar, n = 11) or diluent (white bar, n = 11) daily for 5 d and transplanted with 5 × 106 splenocytes and 5 × 106 BM cells 1 d after the last injection. Splenic macrophages were enumerated 24 h after the transplant and shown as mean ± SEM. Data from three separate experiments were combined. (B–F) C57BL/6 mice were treated with CSF-1 (black bar, n = 12) or diluent (white bar, n = 12) and transplanted as in A. Bar graphs show the number of donor T cells in the spleen (B), mesenteric LN (C), and liver (D) and the number of IFN-γ–producing T cells (E) and Foxp3+ T regulatory cells in the mesenteric LN (F) on day 6 after transplant. Data are shown as mean ± SEM. (G–K) C57BL/6 mice were treated with CSF-1 (diamonds, solid lines) or diluent (triangles, dashed lines) for 5 d and injected with 10 × 106 (G, H, and K, n = 10/group) or 20 × 106 (I and J, n = 5–6/group) splenocytes plus 5 × 106 BM cells isolated from BALB/c donors 1 d after the last CSF-1 injection. As controls, recipient mice were reconstituted with syngeneic BM cells and splenocytes (G, filled circles, n = 3). Survival curves (G and I) and clinical GVHD scores (H and J) are shown. (K) The numbers of thymic CD4+ CD8+ double-positive cells from diluent-treated (gray bar) and CSF-1–treated (black bar) recipients were enumerated on day 22 and shown as mean ± SEM. As control, the data from syngeneic recipients (white bar, n = 3) were shown. Data were pooled from two independent experiments. *, P < 0. 05; *, P < 0.0001 versus diluent-treated controls
DISCUSSION
Our study identifies the unexpected key role of host macrophages in modulating GVHD morbidity and mortality after allo-HCT. In this paper, we show that host macrophages persist in lymphoid organs for several days after allo-HCT and are critical to limit host tissue damage by donor alloreactive T cells. We also establish that pretransplant CSF-1 administration improves GVHD in transplanted animals through the expansion of the host macrophage pool.
These results came as a surprise because the current dogma suggests that host APCs, including DCs and macrophages, contribute to the induction of GVHD. This concept is based on experiments showing that the pretransplant conditioning regimen leads to the release of inflammatory cytokines by host macrophages (Hill et al., 1997) and that the concomitant depletion of DC and macrophages improves GVHD (Zhang et al., 2002b). In this study, we revisited the role of macrophages in GVHD by developing means to target host macrophages while sparing host DC before allo-HCT. To this end, we targeted CSF-1R to reduce macrophages, but not DC numbers, in lymphoid organs. CSF-1 is required for macrophage development, survival, and proliferation in vivo, and mice that lack CSF-1 or the CSF-1R also lack macrophages in lymphoid tissues (Cecchini et al., 1994). We have shown in a series of studies (Helft et al., 2010) that although CSF-1R controls the homeostasis of specific DC subsets in nonlymphoid tissues (Bogunovic et al., 2009; Ginhoux et al., 2009), it does not control the maintenance of lymphoid organ DC, and csf-1r−/− mice have intact lymphoid organ DC populations (Ginhoux et al., 2006, 2009). In this paper, we show that CSF-1R blockade before transplant eliminates macrophages, but not DC, in lymphoid organs and, unexpectedly, enhanced donor T cell expansion and exacerbated GVHD morbidity and mortality after allo-HCT.
To further establish the role of macrophages in GVHD, we administered low-dose Lip-Clod 10 d before transplant to deplete host macrophages, whereas host DC, which has a half-life in lymphoid tissues that does not exceed 3 d (Merad and Manz, 2009), would have completely recovered at the time of transplant. Our results revealed that, in contrast to a previous study in which higher Lip-Clod doses administered 7 and 2 d before transplant led to the depletion of both DC and macrophages and improved GVHD (Zhang et al., 2002b), low-dose Lip-Clod administered 10 d before transplant depleted host macrophages, but not DC, and aggravated GVHD.
Anti–CSF-1R mAb administration also reduced the number of circulating monocytes and affected the Gr-1low monocyte subset more dramatically, suggesting that CSF-1R controls the differentiation of Gr-1high into Gr-1low monocytes in vivo. Because monocytes also limit T cell expansion after organ transplant (Garcia et al., 2010), they could potentially also modulate GVHD outcome in mice treated with anti–CSF-1R mAb. The role of circulating monocytes in GVHD, however, appears to be unlikely, as circulating monocytes are sensitive to radiation and nearly absent from the host at the time of transplant. In addition, mice that received Lip-Clod 10 d before allo-HCT and have recovered the number of monocytes to normal levels (Tacke et al., 2006) at the time of transplant developed more severe GVHD compared with control groups (unpublished data).
Our data also suggest that host macrophages improve GVHD by limiting the expansion of donor alloreactive T cells. The immunomodulatory role of macrophages has already been reported in several settings. In tumors, for example, macrophages modulate T cell function through several mechanisms that include, but are not limited to, the production of iNOS, arginase1, and IDO (Pollard, 2004; Allavena et al., 2008). Although these molecules have been shown to modulate GVHD after allo-HCT (Drobyski et al., 1994; Krenger et al., 1996; Blazar et al., 2003; Banovic et al., 2005; Jasperson et al., 2009), blockade of IL-10, IDO, iNOS, or arginase 1 at least individually failed to interfere with macrophage ability to suppress donor T cell expansion in vitro. Blockade of TGF-β was able to partly restore donor T cell proliferation, suggesting its potential role in regulating recipient macrophage ability to control donor T cell expansion in vivo.
The aggravating effect of CSF-1R blockade identified in this paper is consistent with a recently published study showing that an antibody to CSF-1R (M279 clone), distinct from the AFS98 clone used in our study, also aggravated GVHD outcome after allo-HCT in mice (MacDonald et al., 2010). Although this study did not identify the cellular target that control GVHD aggravation upon injection of the M279 Ab clone, it is likely that it differs from the cells targeted by the AFS98 clone. In contrast to AFS98, injection of the M279 Ab clone did not deplete spleen red pulp and LN medullary macrophages and affected mainly LN subcapsular macrophages, perifollicular macrophages in the spleen, and tissue-resident macrophages. The different target cell populations between the clones might explain the differential fate of donor T cells observed in the two studies. Whereas we found that AFS treatment significantly increased the donor T cell pool as early as 18 h after transplant and did not affect donor T regulatory cell differentiation in vivo, the M279 Ab clone failed to modulate the numbers of donor allogeneic T cells but affected Th1 differentiation and reduced donor T regulatory cell expansion in recipient mice.
Our results also suggest that host macrophages limit the donor T cell pool directly through their ability to engulf and clear donor T cells. CD47 is an integrin-associated protein ubiquitously expressed on all hematopoietic cells (Matozaki et al., 2009) and its receptor called SIRPα (signal regulatory protein α) is highly expressed on macrophages (unpublished data) and on a subset of DC (Ginhoux et al., 2009). We found that naive T cells express lower CD47 levels compared with other cell types. We also found that whereas naive allogeneic CD47−/− T cells are rapidly eliminated upon injection into lethally irradiated recipient animals compared with wild-type T cells, the survival of allogeneic CD47−/− T cells is dramatically prolonged when recipient mice are depleted of their macrophage content before transplant. These results strongly support CD47 having a key role in controlling macrophage ability to modulate the donor allogeneic T cell pool and are consistent with prior studies showing that CD47 expression by donor hematopoietic cells determine their ability to survive in recipient animals (Fraser et al., 1995; Blazar et al., 2001; Abe et al., 2002; Rozemuller et al., 2004; Ide et al., 2007; Takenaka et al., 2007).
Further supporting the importance of host macrophages in regulating allo-HCT outcome, we found that pretransplant injections of the cytokine CSF-1 increased the host macrophage pool, limited the expansion of donor alloreactive T cells, and improved GVHD morbidity and mortality. Because the half-life of CSF-1 in the circulation does not exceed 10 min (Bartocci et al., 1987), these results strongly suggest that CSF-1 pretransplant treatment improved the survival of mice after allo-HCT by promoting the survival of host macrophages and their ability to modulate donor T cell–mediated tissue damage. In contrast to a previous report showing that CSF-1 administration after allo-HCT interferes with donor cell engraftment in recipient mice injected with T cell–depleted allografts (Blazar et al., 1992), pretransplant use of CSF-1 did not affect the engraftment of donor hematopoietic cells in our study (unpublished data). CSF-1 after transplant administration was also found to prevent fungal infection in patients after allo-HCT (Nemunaitis et al., 1993), although CSF-1 posttransplant therapy to treat GVHD should be used with caution, as CSF-1 should also be able to prolong the survival of macrophages that infiltrate GVHD target tissue and promote tissue inflammation and fibrosis (Facon et al., 1995; Namba et al., 2007; Wynn and Barron, 2010).
In conclusion, our study establishes for the first time the differential role of host APC in allo-HCT. In this paper, we show that although both host DCs and macrophages survive the conditioning regimen, they have opposite contribution to GVHD outcome. Host DCs prime donor T cells against host tissue antigens and initiate GVH reactions. In contrast, remaining host macrophages reduce the donor T cell pool through their ability to engulf alloreactive T cells and to modulate their proliferation and, consequently, limit the severity of GVHD. Our study also identifies pretransplant CSF-1 therapy as a novel clinical strategy for the modulation of GVHD severity in patients undergoing allo-HCT.
MATERIALS AND METHODS
Mice.
Female C57BL/6 (H-2b, CD45.2+), C57BL/6-Ly5a (C57BL/6-CD45.1, H-2b, CD45.1+), BALB/c (H-2d), C57BL/6J-Tg (Csf1r-GFP, NGFR/FKBP12; MaFIA; Burnett et al., 2004), C57BL/6-csf-1op/op, B6.129S4-C3tm1Crr/J (C3-deficient mice), B6.129S7-CD47tmFpl/J (CD47-deficient mice), B6.129P2-Nos2tm1Lau/J (iNOS-deficient mice), and B6.129S7-Ifngtm1Ts/J (IFN-γR–deficient mice) were purchased from The Jackson Laboratory. B6.129P2-Fcer1gtm1Rav N12 mice (mice deficient in the Fcγ chain subunit of the Fcγ receptor I (FcgRI), FcgRIII, and FceRI receptors, as described in Takai et al. (1994), were purchased from Taconic. FVB/NJ-Csf1r−/− and littermate FVB/NJ-Csf1r+/− mice were produced as previously described (Dai et al., 2002). csf1op/op and csf1r−/− mice were 3–4 wk old, whereas all other mice used were 8–12 wk old. Mice were maintained in specific pathogen-free condition and received normal chow and hyperchlorinated drinking water for the first 3 wk after allo-HCT. All animal experiments were performed according to protocols approved by the Institutional Committee on Animal Welfare of the Mount Sinai School of Medicine.
allo-HCT.
Mice were exposed to TBI split into two doses separated by 4 h to minimize gastrointestinal toxicity. C57BL/6 mice received 13 Gy TBI, whereas BALB/c mice received 8.5 Gy TBI. 2 h after irradiation, mice were injected i.v. with 5 × 106 BM cells in addition to 2.5–20 × 106 splenocytes isolated from donor mice as indicated in the text. In some experiments, purified T cells were injected into lethally irradiated animals with or without T cell–depleted BM cells. T cells were purified by negative selection from the lymph nodes of donors, using a pan T cell isolation kit and the AutoMACS Pro Separator (Miltenyi Biotec) according to the manufacturer’s instructions to achieve 99% purity. T cell depletion was done with CD90-microbeads (Miltenyi Biotec) and AutoMACS Pro. Some recipient animals were injected with anti–CSF-1R mAb (αCSF-1R, clone AFS98) at doses of 2 mg/mouse on day −5 and 0.5 mg/mouse on days −4 and −3. αCSF-1R mAb was purified from culture supernatant of AFS98 hybridoma (Sudo et al., 1995), grown in a CELLine Flask (BD) in serum-free medium (PFHM-II; Invitrogen). Clodronate (Roche) was encapsulated in liposomes by N. van Rooijen. 100 µl Lip-Clod was administered at day −10 before allo-HCT. In some experiments, 106 U/d of human CSF-1 (Kyowa Hakko Kirin Co., Ltd.) was injected i.p. daily from day −5 to −1 before allo-HCT. Survival after allo-HCT was monitored daily and the clinical GVHD scores were assessed weekly using a scoring system that sums the changes in five clinical parameters that include the weight loss, posture, activity, fur texture, and skin integrity (maximum index = 10) as previously described (Cooke et al., 1996). To evaluate the histological GVHD scores, formalin-preserved livers and small and large bowels were embedded in paraffin, cut into 5-µm-thick sections, and stained with H&E for histological examination. Slides were examined in a blinded fashion by a pathologist (C. Liu). A semiquantitative scoring system was used to assess the severity of histological GVHD (Hill et al., 1998).
GW2580.
GW2580 (LC Laboratories) was suspended in 0.5% methylcellulose and 0.1% Tween 20 by using multiple strokes with a Teflon-glass homogenizer. C57BL/6 mice were injected per os with 1.6 mg twice daily for 6 d and analyzed 12 h after the last injection.
Preparation of single cell suspension and flow cytometry.
Spleen, mesenteric LN, liver, and lung were cut into small pieces, incubated in RPMI1640 containing 10% FBS and 0.2 mg/ml collagenase type IV (working activity of 770 U/mg; Sigma-Aldrich) for 20 min (spleen and LN) or 60 min (liver and lung) and then passed trough a 70-µm cell strainer (BD) to obtain a homogeneous cell suspension. Epidermal sheets were prepared using 2.4 mg/ml Dispase (Invitrogen) and further digested with collagenase as previously described (Ginhoux et al., 2007). Liver DC subsets were prepared as previously described (Ginhoux et al., 2009). Isolation of gut DC subsets was performed as previously described (Bogunovic et al., 2009). Red blood cells were lysed by incubating with RBC Lysis buffer (eBioscience) for 2 min. Fluorochrome or biotin-conjugated mAb specific to mouse F4/80 (BM-8), B220 (RA3-6B2), TCR-β (H57-597), CD4 (L3T4), CD8a (53–6.7), IA/IE (M5/114.15.2), CD11b (M1/70), CD11c (N418), CD45 (30F11), CD45.1 (A20), CD45.2 (104), Gr-1 (Rb6-8C5), anti-Foxp3 (FJK-16s), CD47 (clone miap301), and PE- or PE-Cy7–conjugated streptavidin were purchased from eBioscience. Biotinylated and Alexa Fluor 647–conjugated anti–mouse F4/80 (CI:A3-1) and FITC-conjugated anti–mouse CD169 mAb (3D6.112) were purchased from AbD Serotec. FITC- or PE-conjugated anti–H-2Kd (SF1-1.1) was purchased from BD. Blocking of FcγR was performed by incubating the cells with anti–mouse CD16/32 mAb (clone 93 or 2.4G2). DAPI (Vector Laboratories)-positive cells were excluded from analysis as dead cells. For intracellular IFN-γ staining, the splenocytes were incubated for 4 h with 500 ng/ml ionomycin and 50 ng/ml phorbol ester (Sigma-Aldrich), and 2 µg/ml brefeldin A (Sigma-Aldrich) at 37°C before permeabilization with a Cytofix/Cytoperm solution (BD). The cells were then stained with APC-conjugated anti–IFN-γ mAb (clone XMG1.2; eBioscience). Multiparameter analyses were performed on an LSR II (BD) and sorting was performed on FACS Aria II cell sorter (BD).
Cytokine bead array (CBA) analysis.
Blood was collected from the retroorbital sinus on day 6 after transplant, and the concentrations of Th1 cytokines (IFN-γ and TNF) and Th2 cytokines (IL-4 and IL-13) were measured using the CBA kit according to the manufacturer’s protocol.
In vitro co-culture assays.
Splenic macrophages were purified as F4/80highCD11blowMHC-IIlow cells, peritoneal macrophages were purified as F4/80high cells, DCs were purified as CD11chighMHC-IIhigh cells, and B cells were purified as B220+MHC-II+CD11c− cells from the spleen of naive or transplanted C57BL/6 or BALB/c. T cells were purified from the LN of naive C57BL/6 or BALB/c mice using magnetic negative selection. 5 × 104 T cells were stimulated with 5 × 103 DCs in the presence or absence of purified macrophages as indicated in the text. Cells were cultured in 96-well plates in complete DME (Invitrogen) supplemented with 10% FCS, 50 U/ml penicillin, 50 µg/ml streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 50 µM 2-ME, and 10 mM Hepes for 72 h. In some cases, macrophages were separated from the DC/T cell co-cultures by membrane with 1 µm pore (Corning). As indicated in the text, the following inhibitors were added: iNOS inhibitor, L-NMMA (NG-Methyl-l-arginine acetate; EMD) at 500 µM; ARG1 inhibitor, nor-NOHA (Nw-hydroxy-nor-Arginine; EMD) at 500 µM; iNOS inhibitor, 1-MT (1-methyl-dl-tryptophan; Sigma-Aldrich) at 20 µM; anti–IL-10 neutralizing Ab (clone: JES5-2A5; eBioscience) at 10 µg/ml; and anti–TGF-β neutralizing Ab (clone: 1D11; R&D Systems) at 30 µg/ml. In some experiments, T cells were stimulated with 5 µg/ml of plate-bound anti-CD3ε mAb (eBioscience) and 2 µg/ml anti-CD28 mAb (eBioscience). Cultured wells were pulsed with [3H]-Thymidine (0.5 µCi/well; GE Healthcare) for the final 16 h of culture, and [3H]-Thymidine-uptake was determined, using a Wallac 1450 MicroBeta Counter (Perkin Elmer). When indicated, purified T cells were labeled with 5 µM CFSE (Invitrogen) or 2 µM PKH26 before being incubated with DCs and macrophages for 5 d. CFSE expression levels on gated T cells were determined by LSRII.
Microscopy analysis.
For immunofluorescent analysis, isolated spleens were fixed with 4% PFA and 10% sucrose solution, frozen in O.C.T. compound (Sakura), and cut into 6-μm tissue section slides. Tissue sections were incubated with 10% goat serum for 30 min, followed by anti–mouse CD3ε (clone 500A2; BD) mAb and biotinylated anti–mouse F4/80 (CI:A3-1) or CD169 (clone MOMA-1; Abcam) mAb. Endogenous biotin was blocked using Avidin/Biotin blocking kit (Vector Laboratories). The primary mAbs were detected by incubating the slides with Cy3-conjugated anti-syrian hamster IgG and Cy5-conjugated streptavidin (Jackson ImmunoResearch Laboratories). To determine whether macrophages engulfed allogeneic T cells, splenocytes isolated from recipient mice 18 h after transfer of CFSE-labeled alloreactive T cells and in vitro–cultured cells (as described in the previous section) isolated after 18 h of co-culture were stained with biotinylated F4/80 antibody, followed by Cy-5–conjugated streptavidin, fixed with 2% PFA, cytospun onto slides, and analyzed by microscopy. The images were captured using a microscope (Axioplan 2IE; Carl Zeiss) equipped with a camera (AxioCam MR; Carl Zeiss) and an encoded motorized stage that automates image acquisition of large areas. The images were analyzed using ImageJ software (National Institutes of Health) and Photoshop CS3 (Adobe) and integrated with other figures using Illustrator CS3 (Adobe).
Statistical analysis.
Data analysis was performed with FlowJo v8 software (Tree Star). Data are presented as mean ± SEM. The statistical significance of differences between group means was determined using Mann-Whitney U test. Survival curves were plotted by the Kaplan-Meier method. The level of significance was set at P < 0.05.
Online supplemental material.
Fig. S1 shows that neutrophils, T cells, and NK cells are rapidly eliminated after TBI. Fig. S2 shows that lymphoid tissue macrophages are strongly reduced in CSF-1– and CSF-1R–deficient mice. Fig. S3 shows that anti–CSF-1R mAb treatment does not affect neutrophils and lymphoid cell homeostasis. Fig. S4 shows that anti–CSF-1R mAb treatment depletes macrophages independently of ADCC or complement-dependent cytotoxicity. Fig. S5 shows that anti–CSF-1R mAb injection acts on host cells to limit donor T cell pools without affecting donor foxp3+ T cells. Fig. S6 shows that nonlymphoid tissue-resident DCs are not affected in mice that received Lip-Clod 10 d before analysis. Fig. S7 shows that host macrophages suppress T cell proliferation in vitro. Fig. S8 shows that CD47 is expressed at lower levels on naive T lymphocytes compared with B cells and is up-regulated on activated T cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101709/DC1.
Acknowledgments
This work was supported by National Institutes of Health grants CA112100, HL086899, and AI080884 to M. Merad, CA32551 and CA26504 to E.R. Stanley, R01 AI 071185 to P.S. Heeger, and R01 DK056638 and R01 HL69438 to P.S. Frenette.
Footnotes
Abbreviations used:
- ADCC
- antibody-dependent cell-mediated cytotoxicity
- allo-HCT
- allogeneic hematopoietic cell transplantation
- GVHD
- graft-versus-host disease
- iNOS
- inducible nitric oxide synthase
- Lip-Clod
- liposomal clodronate
- TBI
- total body irradiation
References
- Abe M., Cheng J., Qi J., Glaser R.M., Thall A.D., Sykes M., Yang Y.-G. 2002. Elimination of porcine hemopoietic cells by macrophages in mice. J. Immunol. 168:621–628 [DOI] [PubMed] [Google Scholar]
- Allavena P., Sica A., Garlanda C., Mantovani A. 2008. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 222:155–161 10.1111/j.1600-065X.2008.00607.x [DOI] [PubMed] [Google Scholar]
- Alyea E., Weller E., Schlossman R., Canning C., Webb I., Doss D., Mauch P., Marcus K., Fisher D., Freeman A., et al. 2001. T-cell—depleted allogeneic bone marrow transplantation followed by donor lymphocyte infusion in patients with multiple myeloma: induction of graft-versus-myeloma effect. Blood. 98:934–939 10.1182/blood.V98.4.934 [DOI] [PubMed] [Google Scholar]
- Banovic T., MacDonald K.P., Morris E.S., Rowe V., Kuns R., Don A., Kelly J., Ledbetter S., Clouston A.D., Hill G.R. 2005. TGF-beta in allogeneic stem cell transplantation: friend or foe? Blood. 106:2206–2214 10.1182/blood-2005-01-0062 [DOI] [PubMed] [Google Scholar]
- Bartocci A., Mastrogiannis D.S., Migliorati G., Stockert R.J., Wolkoff A.W., Stanley E.R. 1987. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc. Natl. Acad. Sci. USA. 84:6179–6183 10.1073/pnas.84.17.6179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilhack A., Schulz S., Baker J., Beilhack G.F., Wieland C.B., Herman E.I., Baker E.M., Cao Y.A., Contag C.H., Negrin R.S. 2005. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 106:1113–1122 10.1182/blood-2005-02-0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop M.R., Hou J.W., Wilson W.H., Steinberg S.M., Odom J., Castro K., Kasten-Sportes C., Gea-Banacloche J., Marchigiani D., Gress R., Fowler D.H. 2003. Establishment of early donor engraftment after reduced-intensity allogeneic hematopoietic stem cell transplantation to potentiate the graft-versus-lymphoma effect against refractory lymphomas. Biol. Blood Marrow Transplant. 9:162–169 10.1016/S1083-8791(03)70005-6 [DOI] [PubMed] [Google Scholar]
- Blazar B.R., Aukerman S.L., Vallera D.A. 1992. Effect of recombinant human macrophage colony-stimulating factor in irradiated murine recipients of T-cell-depleted allogeneic or non-depleted syngeneic bone marrow transplants. Blood. 79:1636–1642 [PubMed] [Google Scholar]
- Blazar B.R., Lindberg F.P., Ingulli E., Panoskaltsis-Mortari A., Oldenborg P.A., Iizuka K., Yokoyama W.M., Taylor P.A. 2001. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J. Exp. Med. 194:541–549 10.1084/jem.194.4.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazar B.R., Carreno B.M., Panoskaltsis-Mortari A., Carter L., Iwai Y., Yagita H., Nishimura H., Taylor P.A. 2003. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J. Immunol. 171:1272–1277 [DOI] [PubMed] [Google Scholar]
- Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M.A., Leboeuf M., et al. 2009. Origin of the lamina propria dendritic cell network. Immunity. 31:513–525 10.1016/j.immuni.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S.H., Kershen E.J., Zhang J., Zeng L., Straley S.C., Kaplan A.M., Cohen D.A. 2004. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J. Leukoc. Biol. 75:612–623 10.1189/jlb.0903442 [DOI] [PubMed] [Google Scholar]
- Cecchini M.G., Dominguez M.G., Mocci S., Wetterwald A., Felix R., Fleisch H., Chisholm O., Hofstetter W., Pollard J.W., Stanley E.R. 1994. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 120:1357–1372 [DOI] [PubMed] [Google Scholar]
- Chitu V., Stanley E.R. 2006. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18:39–48 10.1016/j.coi.2005.11.006 [DOI] [PubMed] [Google Scholar]
- Collin M.P., Bogunovic M., Merad M. 2007. DC homeostasis in hematopoietic stem cell transplantation. Cytotherapy. 9:521–531 10.1080/14653240701507314 [DOI] [PubMed] [Google Scholar]
- Cooke K.R., Kobzik L., Martin T.R., Brewer J., Delmonte J., Jr, Crawford J.M., Ferrara J.L. 1996. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 88:3230–3239 [PubMed] [Google Scholar]
- Dai X.M., Ryan G.R., Hapel A.J., Dominguez M.G., Russell R.G., Kapp S., Sylvestre V., Stanley E.R. 2002. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 99:111–120 10.1182/blood.V99.1.111 [DOI] [PubMed] [Google Scholar]
- Dreger P., Brand R., Hansz J., Milligan D., Corradini P., Finke J., Deliliers G.L., Martino R., Russell N., Van Biezen A., et al. ; Chronic Leukemia Working Party of the EBMT 2003. Treatment-related mortality and graft-versus-leukemia activity after allogeneic stem cell transplantation for chronic lymphocytic leukemia using intensity-reduced conditioning. Leukemia. 17:841–848 10.1038/sj.leu.2402905 [DOI] [PubMed] [Google Scholar]
- Drobyski W.R., Keever C.A., Hanson G.A., McAuliffe T., Griffith O.W. 1994. Inhibition of nitric oxide production is associated with enhanced weight loss, decreased survival, and impaired alloengraftment in mice undergoing graft-versus-host disease after bone marrow transplantation. Blood. 84:2363–2373 [PubMed] [Google Scholar]
- Duffner U.A., Maeda Y., Cooke K.R., Reddy P., Ordemann R., Liu C., Ferrara J.L., Teshima T. 2004. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J. Immunol. 172:7393–7398 [DOI] [PubMed] [Google Scholar]
- Ehlenberger A.G., Nussenzweig V. 1977. The role of membrane receptors for C3b and C3d in phagocytosis. J. Exp. Med. 145:357–371 10.1084/jem.145.2.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facon T., Janin A., Noel M.P., Jouet J.P. 1995. Involvement of macrophages in lethal forms of graft-versus-host disease. Lancet. 345:392 10.1016/S0140-6736(95)90382-8 [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M.G., Fleisch H. 1990. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 127:2592–2594 10.1210/endo-127-5-2592 [DOI] [PubMed] [Google Scholar]
- Fraser C.C., Chen B.P., Webb S., van Rooijen N., Kraal G. 1995. Circulation of human hematopoietic cells in severe combined immunodeficient mice after Cl2MDP-liposome-mediated macrophage depletion. Blood. 86:183–192 [PubMed] [Google Scholar]
- Garcia M.R., Ledgerwood L., Yang Y., Xu J., Lal G., Burrell B., Ma G., Hashimoto D., Li Y., Boros P., et al. 2010. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J. Clin. Invest. 120:2486–2496 10.1172/JCI41628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Tacke F., Angeli V., Bogunovic M., Loubeau M., Dai X.M., Stanley E.R., Randolph G.J., Merad M. 2006. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7:265–273 10.1038/ni1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Collin M.P., Bogunovic M., Abel M., Leboeuf M., Helft J., Ochando J., Kissenpfennig A., Malissen B., Grisotto M., et al. 2007. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 204:3133–3146 10.1084/jem.20071733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Liu K., Helft J., Bogunovic M., Greter M., Hashimoto D., Price J., Yin N., Bromberg J., Lira S.A., et al. 2009. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206:3115–3130 10.1084/jem.20091756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa M., Ginhoux F., Wang X.N., Bigley V., Abel M., Dimmick I., Bullock S., Grisotto M., Booth T., Taub P., et al. 2009. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J. Exp. Med. 206:371–385 10.1084/jem.20081633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J., Ginhoux F., Bogunovic M., Merad M. 2010. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol. Rev. 234:55–75 10.1111/j.0105-2896.2009.00885.x [DOI] [PubMed] [Google Scholar]
- Hill G.R., Crawford J.M., Cooke K.R., Brinson Y.S., Pan L., Ferrara J.L. 1997. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 90:3204–3213 [PubMed] [Google Scholar]
- Hill G.R., Cooke K.R., Teshima T., Crawford J.M., Keith J.C., Jr, Brinson Y.S., Bungard D., Ferrara J.L. 1998. Interleukin-11 promotes T cell polarization and prevents acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Clin. Invest. 102:115–123 10.1172/JCI3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D.A., Pavli P., Donahue R.E., Fidler I.J. 1988. The effect of human recombinant macrophage colony-stimulating factor (CSF-1) on the murine mononuclear phagocyte system in vivo. J. Immunol. 141:3405–3409 [PubMed] [Google Scholar]
- Ide K., Wang H., Tahara H., Liu J., Wang X., Asahara T., Sykes M., Yang Y.-G., Ohdan H. 2007. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc. Natl. Acad. Sci. USA. 104:5062–5066 10.1073/pnas.0609661104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasperson L.K., Bucher C., Panoskaltsis-Mortari A., Mellor A.L., Munn D.H., Blazar B.R. 2009. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 114:5062–5070 10.1182/blood-2009-06-227587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq R.R., van den Brink M.R.M. 2010. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat. Rev. Cancer. 10:213–221 10.1038/nrc2804 [DOI] [PubMed] [Google Scholar]
- Koyama M., Hashimoto D., Aoyama K., Matsuoka K.-I., Karube K., Niiro H., Harada M., Tanimoto M., Akashi K., Teshima T. 2009. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 113:2088–2095 10.1182/blood-2008-07-168609 [DOI] [PubMed] [Google Scholar]
- Krenger W., Falzarano G., Delmonte J., Jr, Snyder K.M., Byon J.C., Ferrara J.L. 1996. Interferon-gamma suppresses T-cell proliferation to mitogen via the nitric oxide pathway during experimental acute graft-versus-host disease. Blood. 88:1113–1121 [PubMed] [Google Scholar]
- Krenger W., Rossi S., Piali L., Holländer G.A. 2000. Thymic atrophy in murine acute graft-versus-host disease is effected by impaired cell cycle progression of host pro-T and pre-T cells. Blood. 96:347–354 [PubMed] [Google Scholar]
- Lin H., Lee E., Hestir K., Leo C., Huang M., Bosch E., Halenbeck R., Wu G., Zhou A., Behrens D., et al. 2008. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 320:807–811 10.1126/science.1154370 [DOI] [PubMed] [Google Scholar]
- MacDonald K.P.A., Palmer J.S., Cronau S., Seppanen E., Olver S., Raffelt N.C., Kuns R., Pettit A.R., Clouston A., Wainwright B., et al. 2010. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 116:3955–3963 10.1182/blood-2010-02-266296 [DOI] [PubMed] [Google Scholar]
- Maris M.B., Sandmaier B.M., Storer B.E., Chauncey T., Stuart M.J., Maziarz R.T., Agura E., Langston A.A., Pulsipher M., Storb R., Maloney D.G. 2004. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 104:3535–3542 10.1182/blood-2004-06-2275 [DOI] [PubMed] [Google Scholar]
- Matozaki T., Murata Y., Okazawa H., Ohnishi H. 2009. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 19:72–80 10.1016/j.tcb.2008.12.001 [DOI] [PubMed] [Google Scholar]
- Mempel T.R., Henrickson S.E., Von Andrian U.H. 2004. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 427:154–159 10.1038/nature02238 [DOI] [PubMed] [Google Scholar]
- Menke J., Iwata Y., Rabacal W.A., Basu R., Yeung Y.G., Humphreys B.D., Wada T., Schwarting A., Stanley E.R., Kelley V.R. 2009. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J. Clin. Invest. 119:2330–2342 10.1172/JCI39087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Manz M.G. 2009. Dendritic cell homeostasis. Blood. 113:3418–3427 10.1182/blood-2008-12-180646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merad M., Hoffmann P., Ranheim E., Slaymaker S., Manz M.G., Lira S.A., Charo I., Cook D.N., Weissman I.L., Strober S., Engleman E.G. 2004. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat. Med. 10:510–517 10.1038/nm1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarek M., Martin P.J., Leisenring W., Flowers M.E., Maloney D.G., Sandmaier B.M., Maris M.B., Storb R. 2003. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 102:756–762 10.1182/blood-2002-08-2628 [DOI] [PubMed] [Google Scholar]
- Miller M.J., Wei S.H., Parker I., Cahalan M.D. 2002. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 296:1869–1873 10.1126/science.1070051 [DOI] [PubMed] [Google Scholar]
- Na I.K., Markley J.C., Tsai J.J., Yim N.L., Beattie B.J., Klose A.D., Holland A.M., Ghosh A., Rao U.K., Stephan M.T., et al. 2010. Concurrent visualization of trafficking, expansion, and activation of T lymphocytes and T-cell precursors in vivo. Blood. 116:e18–e25 10.1182/blood-2009-12-259432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba N., Shinagawa K., Fujii N., Maeda Y., Ishimaru F., Ikeda K., Matsui T., Tanimoto M., Katayama Y. 2007. Predominant infiltration of monocytes in chronic graft-versus-host disease. Transplantation. 83:220–224 10.1097/01.tp.0000245080.71722.87 [DOI] [PubMed] [Google Scholar]
- Nemunaitis J., Shannon-Dorcy K., Appelbaum F.R., Meyers J., Owens A., Day R., Ando D., O’Neill C., Buckner D., Singer J. 1993. Long-term follow-up of patients with invasive fungal disease who received adjunctive therapy with recombinant human macrophage colony-stimulating factor. Blood. 82:1422–1427 [PubMed] [Google Scholar]
- Okamoto A., Fujio K., van Rooijen N., Tsuno N.H., Takahashi K., Tsurui H., Hirose S., Elkon K.B., Yamamoto K. 2008. Splenic phagocytes promote responses to nucleosomes in (NZB x NZW) F1 mice. J. Immunol. 181:5264–5271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panoskaltsis-Mortari A., Price A., Hermanson J.R., Taras E., Lees C., Serody J.S., Blazar B.R. 2004. In vivo imaging of graft-versus-host-disease in mice. Blood. 103:3590–3598 10.1182/blood-2003-08-2827 [DOI] [PubMed] [Google Scholar]
- Pavletic Z.S., Arrowsmith E.R., Bierman P.J., Goodman S.A., Vose J.M., Tarantolo S.R., Stein R.S., Bociek G., Greer J.P., Wu C.D., et al. 2000. Outcome of allogeneic stem cell transplantation for B cell chronic lymphocytic leukemia. Bone Marrow Transplant. 25:717–722 10.1038/sj.bmt.1702237 [DOI] [PubMed] [Google Scholar]
- Peggs K.S., Mackinnon S., Linch D.C. 2005. The role of allogeneic transplantation in non-Hodgkin’s lymphoma. Br. J. Haematol. 128:153–168 10.1111/j.1365-2141.2004.05251.x [DOI] [PubMed] [Google Scholar]
- Pollard J.W. 2004. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer. 4:71–78 10.1038/nrc1256 [DOI] [PubMed] [Google Scholar]
- Rabinowe S.N., Soiffer R.J., Gribben J.G., Daley H., Freedman A.S., Daley J., Pesek K., Neuberg D., Pinkus G., Leavitt P.R., et al. 1993. Autologous and allogeneic bone marrow transplantation for poor prognosis patients with B-cell chronic lymphocytic leukemia. Blood. 82:1366–1376 [PubMed] [Google Scholar]
- Rozemuller H., Knaän-Shanzer S., Hagenbeek A., van Bloois L., Storm G., Martens A.C.M. 2004. Enhanced engraftment of human cells in RAG2/gammac double-knockout mice after treatment with CL2MDP liposomes. Exp. Hematol. 32:1118–1125 10.1016/j.exphem.2004.08.002 [DOI] [PubMed] [Google Scholar]
- Shlomchik W.D., Couzens M.S., Tang C.B., McNiff J., Robert M.E., Liu J., Shlomchik M.J., Emerson S.G. 1999. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 285:412–415 10.1126/science.285.5426.412 [DOI] [PubMed] [Google Scholar]
- Sorror M.L., Maris M.B., Sandmaier B.M., Storer B.E., Stuart M.J., Hegenbart U., Agura E., Chauncey T.R., Leis J., Pulsipher M., et al. 2005. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J. Clin. Oncol. 23:3819–3829 10.1200/JCO.2005.04.569 [DOI] [PubMed] [Google Scholar]
- Sudo T., Nishikawa S., Ogawa M., Kataoka H., Ohno N., Izawa A., Hayashi S., Nishikawa S. 1995. Functional hierarchy of c-kit and c-fms in intramarrow production of CFU-M. Oncogene. 11:2469–2476 [PubMed] [Google Scholar]
- Tacke F., Ginhoux F., Jakubzick C., van Rooijen N., Merad M., Randolph G.J. 2006. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J. Exp. Med. 203:583–597 10.1084/jem.20052119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T., Li M., Sylvestre D., Clynes R., Ravetch J.V. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 76:519–529 10.1016/0092-8674(94)90115-5 [DOI] [PubMed] [Google Scholar]
- Takenaka K., Prasolava T.K., Wang J.C.Y., Mortin-Toth S.M., Khalouei S., Gan O.I., Dick J.E., Danska J.S. 2007. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat. Immunol. 8:1313–1323 10.1038/ni1527 [DOI] [PubMed] [Google Scholar]
- Teshima T., Ordemann R., Reddy P., Gagin S., Liu C., Cooke K.R., Ferrara J.L. 2002. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat. Med. 8:575–581 10.1038/nm0602-575 [DOI] [PubMed] [Google Scholar]
- van Besien K., Sobocinski K.A., Rowlings P.A., Murphy S.C., Armitage J.O., Bishop M.R., Chaekal O.K., Gale R.P., Klein J.P., Lazarus H.M., et al. 1998. Allogeneic bone marrow transplantation for low-grade lymphoma. Blood. 92:1832–1836 [PubMed] [Google Scholar]
- Van Rooijen N., Sanders A. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods. 174:83–93 10.1016/0022-1759(94)90012-4 [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A.W., Jr, Ahmed-Ansari A., Sell K.W., Pollard J.W., Stanley E.R. 1990. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA. 87:4828–4832 10.1073/pnas.87.12.4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A., Barron L. 2010. Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 30:245–257 10.1055/s-0030-1255354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L.D., Nishikawa S. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 345:442–444 10.1038/345442a0 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Louboutin J.-P., Zhu J., Rivera A.J., Emerson S.G. 2002a. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J. Clin. Invest. 109:1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Shlomchik W.D., Joe G., Louboutin J.P., Zhu J., Rivera A., Giannola D., Emerson S.G. 2002b. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J. Immunol. 169:7111–7118 [DOI] [PubMed] [Google Scholar]