Abstract
We reported that left ventricular (LV) dilatation after four weeks of isolated mitral regurgitation (MR) in the dogs is marked by extracellular matrix (ECM) loss and an increase in adrenergic drive. Given that ECM proteins and their receptors integrins influence β-adrenergic receptor (β-AR) responses in-vitro, we tested whether β1-AR activation modulates focal adhesion (FA) signaling and LV remodeling in these same dogs with isolated MR. Normal dogs (NL) were compared with dogs with MR of 4-weeks duration and with MR dogs treated with β1-AR blockade (β1-RB) (extended-release metoprolol succinate, 100 mg QD) that was started 24-hours after MR induction. In MR LVs, a decrease in collagen accumulation compared to NL was associated with a decrease in FA kinase (FAK) tyrosine phosphorylation along with FAK interaction with adapter and cytoskeletal proteins, p130Cas and paxillin, respectively, as determined by immunoprecipitiation assays. There was increased phosphorylation of stress related molecules p38-MAPK and Hsp27 and survival signaling kinases ERK1/2 and AKT with no evidence of cardiomyocyte apoptosis. β1-RB attenuated FA signaling loss and prevented p38-MAPK, Hsp27, and AKT phosphorylation induced by MR and significantly increased LV epicardial collagen content. However, β1-RB did not improve LV endocardial collagen loss or LV dilatation induced by MR. Isolated myocytes from NL and MR hearts treated with β1- or β2-AR agonists demonstrated no difference in FAK, p38-MAPK, Hsp27, or AKT phosphorylation. These results showed that chronic stimulation of β1-AR during early compensated MR impairs FA signaling that may affect myocyte/fibroblast-ECM scaffolding necessary for LV remodeling.
Keywords: beta adrenergic receptors, focal adhesion, volume overload, mitral regurgitation, apoptosis
INTRODUCTION
Myocardial remodeling in response to pressure or volume overload (VO) is an adaptation of myocardial structure to accommodate chronic changes in myocardial demand. This remodeling is thought ultimately to drive a maladaptive process of ventricular dilatation and pump dysfunction that contributes to the pathogenesis of heart failure (HF). In contrast to pressure overload, the VO of isolated mitral regurgitation (MR) results in left ventricular (LV) dilatation, side-to-side slippage of cells, and degradation of extracellular matrix (ECM) proteins.1,2 In addition to these structural changes, there is development of cardiac hypertrophy and subsequent LV dysfunction in MR that are improved by β-adrenergic receptor blockade (β-RB)3 but not by angiotensin-converting enzyme inhibition or angiotensin type 1 receptor blockade,4,5 suggesting that the adrenergic system is more central to the pathophysiology of VO of isolated MR. Moreover, studies in humans and dogs have demonstrated an increase in adrenergic drive even in a mild compensated state of isolated MR,6,7 most likely due to the early achievement of preload reserve.
β-adrenergic receptors (β-AR) belong to the large family of G protein–coupled receptors (GPCRs) that is involved in positive inotropic, chronotropic, and lusitropic responses through activation of G protein (Gs).8 In the failing hearts, several defects in β-AR signaling have been detected including receptor down-regulation and uncoupling from the stimulatory Gs,9 increased level of inhibitory G-protein subunits (Gi),10 decreased adenylate cyclase activity and increased β-ARK1 expression and activity.11,12 β-AR signaling may also be affected by ECM proteins. Herein, plating myocytes on laminin substrate as opposed to glass selectively reduced β1-AR and enhanced β2-AR regulation of ICa.13 In addition, activation of integrin signaling blocks adult cardiomyocyte apoptosis induced by β1-AR activation.14
The ability of integrins as ECM receptors to regulate cytoskeletal architecture has been well characterized in-vitro and in-vivo.15,16 In addition, integrin signaling has been implicated in GPCRs, hormone, and growth factor-induced alterations in gene transcription in cardiac myocytes.15,16 Integrins are a family of heterodimeric transmembrane receptors (composed of α and β subunits) containing extracellular ligand binding domains that show binding specificity for ECM components and a short cytoplasmic domain that serves to couple integrins with the actin cytoskeleton.16 Binding of a matrix protein to an integrin heterodimer typically results in the activation of the non-receptor tyrosine kinase, focal adhesion kinase (FAK). Activated FAK, in turn, recruits the non-receptor tyrosine kinase c-Src, the multifunctional adapter molecule Grb2, p130Cas, paxillin and other signaling intermediates.15,16 Integrin β1 and FAK knockout result in defective heart development and early embryonic lethality.17,18 Interestingly, in mice with myocyte-restricted FAK inactivation, an eccentric cardiac hypertrophy develops with age and in response to pressure overload stimuli suggesting that downregulation of FA signaling is associated with the development of eccentric cardiac hypertrophy.19 In a similar study, persistent challenge of mice with myocyte-restricted FAK inactivation leads to enhanced cardiac fibrosis and cardiac dysfunction in comparison with challenged genetic controls.20
Pressure overload-induced cardiac hypertrophy is accompanied by enhanced expression of ECM proteins, integrins, and enhanced activation of FA signaling that correlates with advancement of hypertrophy.21–23 However, little is known about integrin signaling during VO-induced cardiac hypertrophy that is associated with a decrease in ECM accumulation as during isolated MR in the dog.1 We reported an increase in catecholamine release into the LV interstitial fluid space after 4-weeks of MR.7 We extended this work using samples from these same dogs and now show for the first time that increased adrenergic drive impairs FA signaling early in the course of LV remodeling in the VO of isolated MR.
MATERIALS & METHODS
Experimental Preparation
Mitral valve regurgitation was induced in conditioned mongrel dogs of either sex (19–26 kg) by chordal rupture using a fluoroscopic guided catheterization method previously described in our laboratory.1,7 Dogs were randomly assigned to one of 5 groups: (1) unoperated controls (n=6), (2) 2 and 4-weeks of MR (2W-MR and 4W-MR, n=6), and (3) 2 and 4-weeks of MR treated with β1-AR blocker (extended release metoprolol succinate, 100 mg PO, once daily; n=6) starting 24-hours after MR induction. In these same animals, we have previously reported the results of M-mode echocardiography which was performed in the conscious state at baseline and at the time of sacrifice.1 Animals were maintained at a deep plane of general anesthesia using isofluorane and were mechanically ventilated. At the end of the in-vivo experiments the heart was arrested with intracardiac KCl and quickly extirpated, placed in phosphate buffered ice slush, and the coronaries flushed with oxygenated Krebs solution. A portion of the LV (mid-myocardium) was cut and snap frozen in liquid nitrogen for subsequent biochemical studies. This study was approved by The Animal Services Committees at the University of Alabama at Birmingham and Auburn University.
An expanded Materials and Methods section is in the online data supplement.
RESULTS
Effects of β1-RB on LV remodeling and function following MR
Mean arterial pressure was significantly decreased compared with controls in both 4W-MR and 4W-MR+β1-RB dogs (Table 1). Heart rate, LV end diastolic pressure, and mean systemic vascular resistance did not differ from controls in 4W-MR and 4W-MR+β1-RB dogs. However, cardiac output was significantly decreased in 4W-MR+β1-RB dogs compared to controls. There was a trend toward increases in LV mass in 4W-MR and 4W-MR+β1-RB dogs compared to controls but this did not achieve statistical significance. We have previously shown in these same 4W-MR dogs a marked increase in catecholamine release into the LV interstitial fluid space that was significantly attenuated by β1-RB.7 The resting heart rate was measured under general anesthesia approximately 24-hours after the last dose of metoprolol and, thus, may not reflect the effect of β1-RB on heart rate in the conscious state. However, in these same dogs heart rate response to stellate stimulation was decreased in 4W-MR+β1-RB compared to 4W-MR dogs7 suggestive of a negative chronotropic effect of β1-RB.
TABLE 1.
LV hemodynamics and echocardiography in control, 4W-MR, and 4W-MR+ β1-RB dogs
| Control (n=6) |
4W-MR (n=6) |
4W-MR+β1-RB (n=6) |
|
|---|---|---|---|
| Heart rate, bpm | 94 ±6 | 86 ±6 | 83 ±5 |
| Mean arterial pressure (mmHg) | 84 ±3 | 65 ±4* | 62 ±3* |
| LV end diastolic pressure (mmHg) | 4 ±1 | 8 ±1 | 6 ±2 |
| Systemic vascular resistance, dyne.s.cm−5 | 2007 ±219 | 1606 ±240 | 2342 ±176 |
| Cardiac output, L/min | 3.6 ±0.4 | 3.8 ±0.6 | 2.1 ±0.1* |
| LV mass, g/kg | 4.4 ±0.2 | 5.0 ±0.3 | 5.0 ±0.2 |
| LV end-diastolic dimension, % change | 9.2 ±2.9† | 17.7 ±4.7† | |
| LV end-systolic dimension, % change | −7.4 ±5.8 | 0.1 ±3.6 | |
| LV end-diastolic wall thickness, % change | −12.0 ±8.5 | −10.2 ±5.8 | |
| Wall thickness/end-diastolic dimension, % change | −18.6 ±9.3† | −20.9 ±6.5† | |
| Fractional shortening, % change | 32.7 ±11.3† | 34.3 ±13.9† |
Values presented as mean±SEM.
P<0.05 vs control.
P<0.05, % change vs baseline study.
LV end-diastolic dimension increased similarly in both 4W-MR and 4W-MR+β1-RB compared to baseline (Table 1). In addition, cardiac function assessed by LV fractional shortening was significantly increased from baseline in 4W-MR group, as LV end-systolic dimension remained unchanged (Table 1). However, this increase was not affected by β1-RB. In a similar fashion, response of LV dP/dt to stellate stimulation was similar in 4W-MR and 4W-MR+β1-RB dogs, whereas stellate-stimulated heart rate change was attenuated in 4W-MR+β1-RB compared with control and 4W-MR dogs.7 There was a significant decrease in LV end-diastolic wall thickness decreased significantly in 4W-MR group compared to baseline along with a significant increase in LV end-diastolic dimension indicating eccentric LV remodeling. However, these changes were not affected by β1-RB treatment.
β1-RB reduces interstitial collagen degradation induced by MR
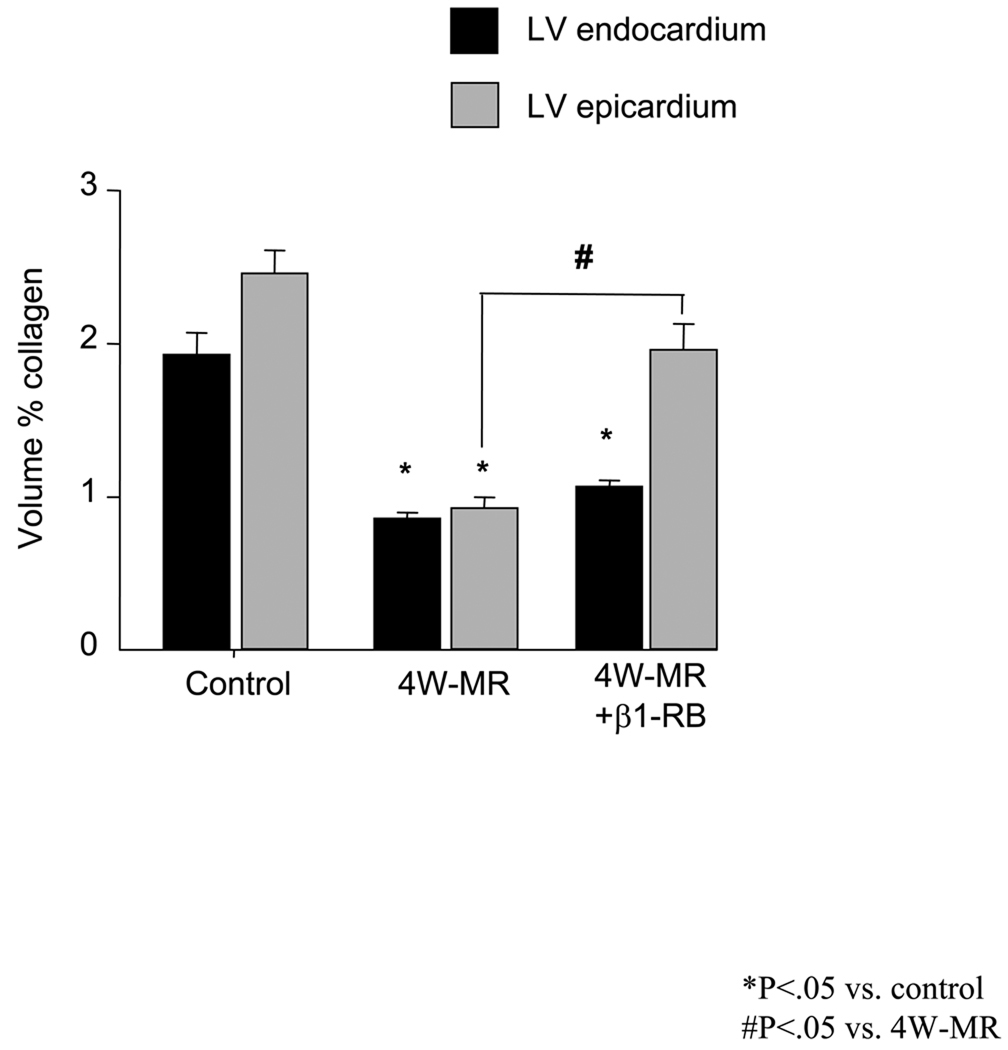
Quantitative evaluation of myocardial interstitial collagen revealed a significant decrease in volume percent collagen for untreated 4W-MR hearts compared to controls at both the endocardium and epicardium levels (Figure 1). Moreover, the decrease in collagen accumulation was evident at the endocardium at 2-weeks after MR (supplemental Figure S1) and was sustained for 4-months after MR (data not shown). β1-RB prevented collagen loss by ~68% in the epicardium induced by 4-weeks of MR but has no detectable effect on interstitial endocardium collagen.
Figure 1.
Volume percent collagen of LV endocardium and epicardium in control, 4W-MR, and 4W-MR+β1-RB dogs. *P<0.05 vs control; #P<0.05, epicardium in 4W-MR vs. 4W-MR+β1-RB dogs.
β1-RB prevents FAK signaling downregulation induced by MR
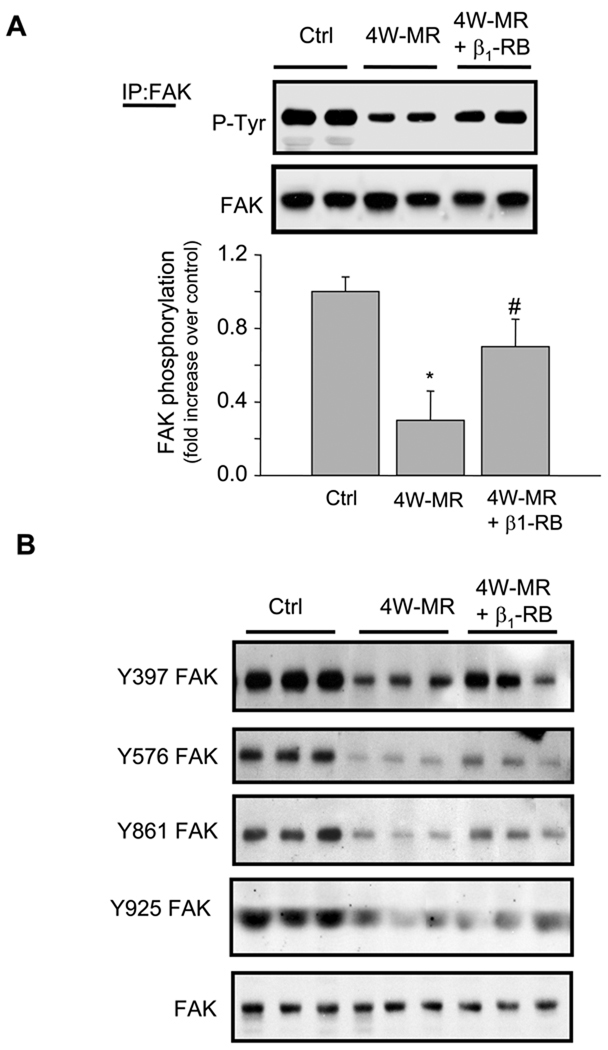
Several studies have shown that pressure overload-induced cardiac hypertrophy is associated with an increase in integrin signaling.22,23 However, these models of cardiac hypertrophy were associated with an increase rather than a decrease in ECM deposition. Since maximal cardiac hypertrophy requires ECM, we examined whether components of the integrin-signaling cascade were altered after 4W-MR. We examined tyrosine phosphorylation of FAK and correlated these with ECM changes. In control dogs, there was basal FAK tyrosine phosphorylation as determined by blotting with anti-phosphotyrosine antibodies (Figure 2A). MR induction for 4-weeks led to a significant decrease in tyrosine phosphorylation of FAK without a decrease in FAK expression, as the same blot reblotted with anti-FAK antibodies showed equal FAK expression levels between control and MR dogs (Figure 2A). Similar decrease in FAK tyrosine phosphorylation was observed at 2-weeks after MR (Supplemental Figure S2). The lack of quantitative changes in total FAK expression between control and MR hearts was also associated with a lack of detectable changes in FAK distribution as evaluated by double immunostaining using anti-FAK polyclonal antibodies (Supplemental Figure S3). FAK immunolabeling was localized throughout the myocardium of control hearts, including the cardiomyocytes that also stained positively for sarcomeric α-actin, and some staining of the interstitial space. There was also FAK staining of endothelial cells and the medial layer of blood vessels (data not shown). In 4W-MR hearts, there was no qualitative change in FAK immunolabeling compared to normal hearts. Thus, FAK expression and distribution were not significantly affected by 4W-MR. Treatment with β1-RB significantly reduced the decrease in FAK tyrosine phosphorylation induced by MR at 4-weeks, but did not reach statistical significance in 2W-MR dogs compared to controls (Figure 2A and Supplemental Figure S2). As FAK can be tyrosine phosphorylated on a number of tyrosine residues including Tyr-397, -578/577, -861, and -925 in response to various stimuli, we next mapped the phosphorylation site on FAK using series of well-characterized phospho-specific antibodies.24 FAK-Tyr-397, -578/577, -861, and -925 phosphorylation decreased significantly in 4W-MR dogs compared to controls and treatment with β1-RB improved the decrease in FAK-Tyr-397, -578/577, -861 tyrosine phosphorylation sites without any detectable differences between these different sites. However, β1-RB was without significant effect on FAK-Tyr-925 phosphorylation suggesting a different mechanism of regulation of this FAK tyrosine phosphorylation site (Figure 2B).
Figure 2. β1-RB prevents FAK tyrosine downregulation induced by MR.
(A) Left ventricular extracts from control, 4W-MR, and 4W-MR+β1-RB dogs were immunoprecipitated (IP) with anti-FAK antibodies and immunoblotted with anti-phosphotyrosine antibodies. Top, representative autoradiogram (with each lane from a single gel exposed for the same duration). Bottom, fold induction, n=6 each group, *P<0.05 4W-MR vs. control, #P<0.05 4W-MR+β1-RB vs. 4W-MR. (B) representative immunoblots showing accumulation of phospho-FAK Tyr-397, -576/577, -861, and -925 in LV extracts from control, 4W-MR, and 4W-MR+β1-RB. Blots were stripped and blotted with anti-FAK antibodies.
Consistent with a reduction in FAK tyrosine phosphorylation, there was a decreased association between FAK and p130Cas, and FAK and paxillin, two important components of FA complex that associate with and are phosphorylated by FAK,15,16 in 4W-MR dogs compared to controls (Figure 3A). Treatment with β1-RB prevented both FAK-paxillin and FAK-p130Cas dissociation. To investigate whether p130Cas and paxillin are also affected by 4W-MR, analysis of p130Cas and paxillin tyrosine phosphorylation was performed. p130Cas and paxillin tyrosine phosphorylation was also decreased in 4W-MR dogs and correlated with those of FAK (Figures 3B and 3C). Treatment with β1-RB prevented the decrease in paxillin tyrosine phosphorylation but did not reach statistical significance for p130Cas phosphorylation. These data indicate that the loss of collagen following 4W-MR is associated with a decrease in FAK phosphorylation and its association with downstream signaling molecules. However, this downregulation seems to be selective for FAK and FAK associated molecules as tyrosine phosphorylation of Pyk2, a FA related kinase with strong homology to FAK, was not significantly affected in 2W-(Supplemental Figure S4) and 4W-MR hearts (Figure 3D).
Figure 3. Alteration of FAK downstream signaling induced by MR is prevented by β1-RB.
(A) FAK immunoprecipitates from LV lysates of control, 4W-MR, and 4W-MR+β1-RB dogs were resolved on SDS-PAGE and blotted with anti-phosphotyrosine, p130Cas, paxillin, or FAK antibodies. (B–C) p130Cas or paxillin immunoprecipitates from control, 4W-MR, or 4W-MR+β1-RB LV extracts were resolved on SDS-PAGE and blotted with anti-phosphotyrosine, anti-p130Cas (B), or anti-paxillin (C) antibodies. Top, representative autoradiograms (with each lane from a single gel exposed for the same duration). Bottom, fold induction, n=6 each group, *P<0.05 4W-MR vs. control, #P<0.05 4W-MR+β1-RB vs. 4W-MR. (D) Representative immunoblot showing accumulation of phospho-Pyk2 Tyr-402 in LV extracts from control, 4W-MR, and 4W-MR+β1-RB. Blot was stripped and blotted with anti-Pyk2 antibodies.
β1-RB prevents alteration of FAK downstream signaling induced by MR
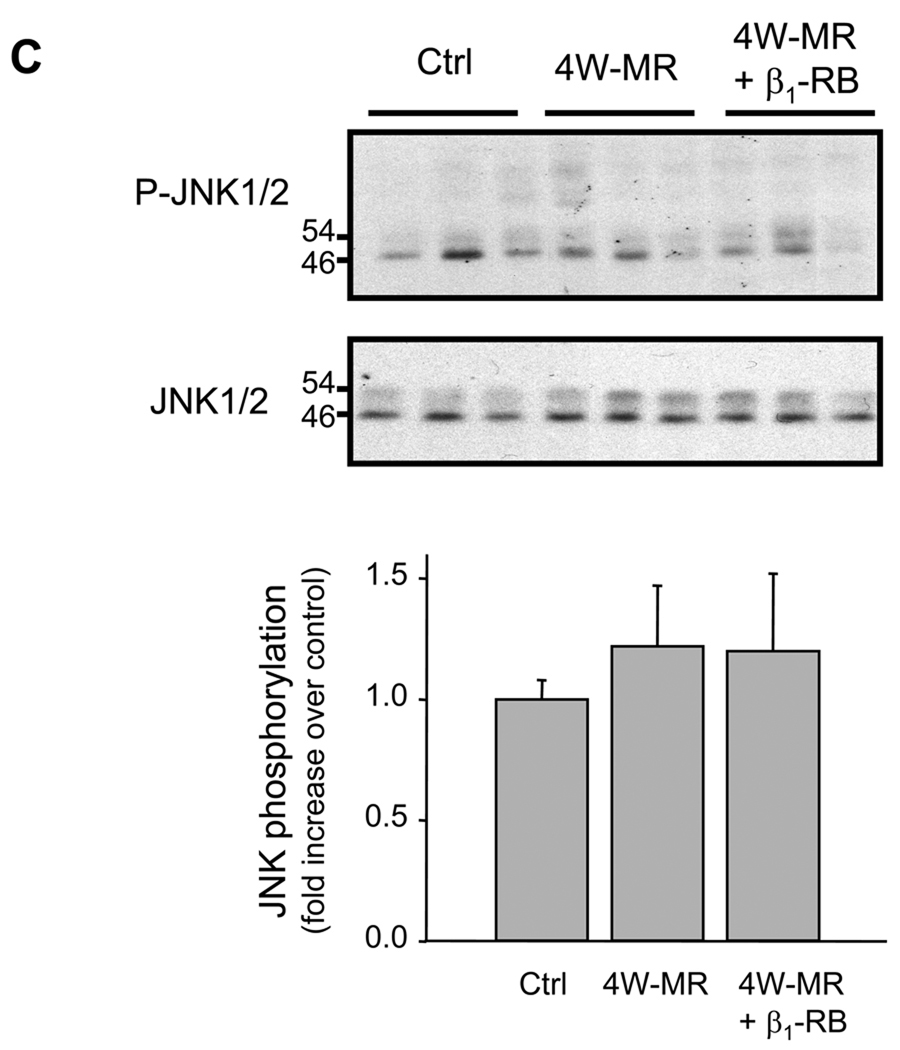
Putative signaling pathways downstream from FAK/p130Cas/paxillin could involve activation of the ERK1/2, JNK, and p38-MAPK pathways, each of which has been implicated in hypertrophic signal transduction.25–27 Therefore, we examined whether the loss of FA signaling observed in MR dogs was associated with changes in p38-MAPK, ERK1/2, and JNK phosphorylation by immunoblot analysis. Control animals presented basal p38-MAPK and ERK1/2 phosphorylation and induction of MR for 4-weeks led to an increase in p38-MAPK and ERK1/2 phosphorylation (Figures 4A, 4B). However, 4-weeks of MR had no detectable effect on JNK phosphorylation state (Figure 4C). Similar increase in p38-MAPK and ERK1/2 phosphorylation was also observed in 2W-MR group (Supplemental Figure S5 and data not shown). Interestingly, β1-RB completely abolished p38-MAPK phophorylation induced after 4W-MR whereas activation of ERK1/2 was not significantly affected at 2W and 4W after MR (Figures 4A, 4B, and Supplemental Figure S5). This suggests that the VO of isolated MR differentially regulates the activation of these 3 MAP-kinases with p38-MAPK and ERK1/2 being activated in response to MR and that p38-MAPK is dependent on β1-AR stimulation.
Figure 4. Differential activation of ERK1/2, p38 MAPK, and JNK after MR.
Top, representative immunoblots showing accumulation of phospho-p38-MAPK, -ERK1/2, and -JNK in LV extracts from control, 4W-MR, and 4W-MR+β1-RB. Blots were stripped and blotted with anti-p38-MAPK, -ERK1/2, or -JNK antibodies. Bottom, fold induction, n=6 each group, *P<0.05 4W-MR vs. control, #P<0.05 4W-MR+β1-RB vs. 4W-MR.
MR-induced FA signaling alteration is not associated with cell death by apoptosis
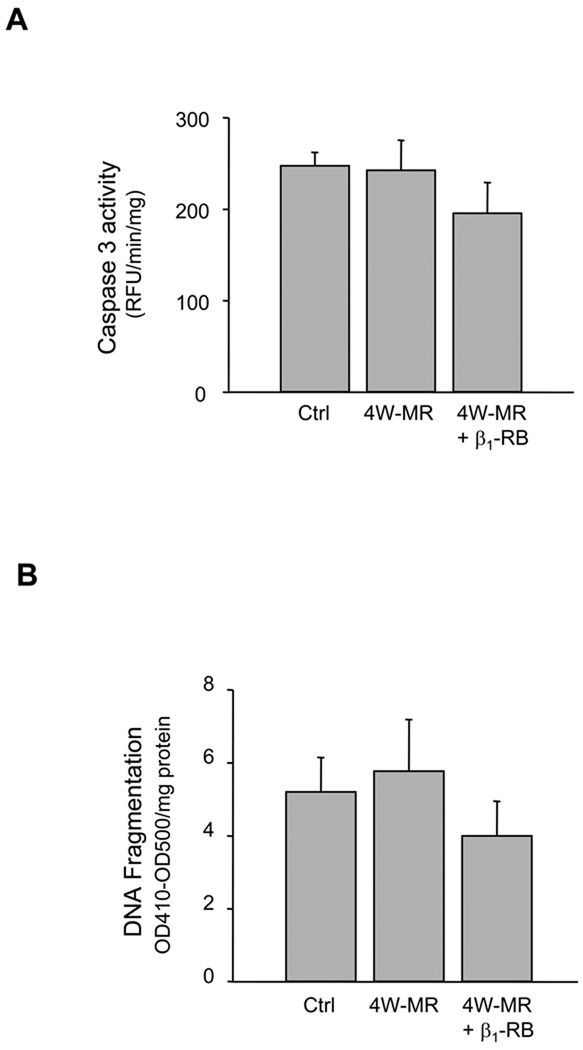
Both loss of ECM and/or loss of FAK signaling have been shown to lead to cell death termed anoikis.28–30 To examine whether apoptosis occurs after ECM loss and subsequent FA alteration during induction of MR, we measured caspase-3 activity and DNA fragmentation by ELISA and TUNEL assay in control, 4W-MR, and 4W-MR+β1-RB groups (Figure 5). We did not detect any difference between control, 4W-MR group, and 4W-MR+β1-RB in either caspase-3 activity (NL, 249 ±13 RFU/min/mg; 4W-MR, 242 ±27 RFU/min/mg protein, 4W-MR+β1-RB, 198 ±26 RFU/min/mg), DNA fragmentation (NL, 5.2 ±0.9 OD410–OD500/mg protein; 4W-MR, 5.8 ±1.4 OD410–OD500/mg protein; 4W-MR+β1–RB, 4.00 ±0.9 OD410–OD500/mg protein), or the percent of TUNEL positive cardiomyocytes (NL, 0.026% ±0.011%; 4W-MR, 0.021% ±0.002%; 4W-MR+β1-RB, 0.03% ±0.015%). These data showed that MR at this early compensatory hypertrophy stage is not associated with myocyte apoptosis despite a loss of ECM and FA signaling.
Figure 5. Induction of MR is not associated with myocardial cell apoptosis.
LV homogenates from control, 4W-MR, and 4W-MR+β1-RB groups were assessed for apoptosis. (A) Caspase-3 activity was measured using fluorogenic substrate. Results are expressed as relative fluorescence unit (RFU)/min/mg protein. (B) DNA fragmentation as measured by anti-histone antibody ELISA. Results are expressed as relative OD410-OD500/mg protein. Values are mean ±SE. (C) Morphometry of TUNEL staining of LV sections from control, 4W-MR, and 4W-MR+β1-RB.
MR-induced AKT and HSP27 phosphorylation is prevented by β1-RB
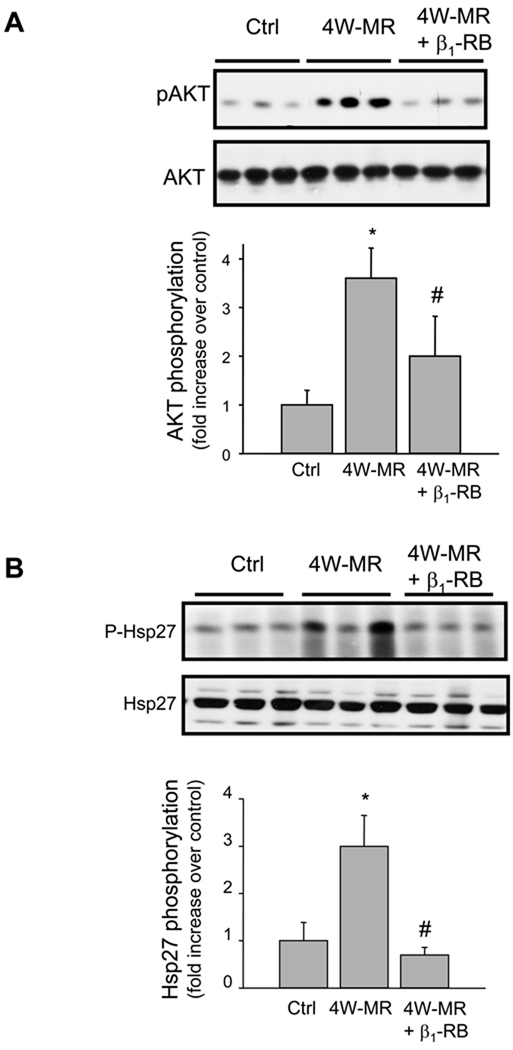
The absence of apoptotic markers in MR hearts despite the decrease in FA signaling led us to hypothesize that other compensatory signaling molecules may be activated to counteract the effect of ECM loss. One of the molecules that has been shown to play a role in survival and protection of myocytes against apoptosis is the PI3-kinase/AKT pathway.31 As FAK has been identified as the major site for binding of PI3-kinase, whose inositol lipid products are key mediators of Akt activation,32 we next examined whether induction of MR is associated with an increase in AKT phosphorylation and whether β1-RB prevents this activation. Immunoblotting with anti-phospho-AKT at Ser-473 residue, which has been shown to be required for maximal activation of AKT,33 showed an increase in AKT phosphorylation following 4W-MR (Figure 6A). The amount of total-Akt expression was not different between the two groups of animals. The increase in AKT phosphorylation was abrogated in 4W-MR+β1-RB dogs suggesting that β1-AR stimulation mediates AKT activation. Interestingly, these data also showed that FAK and AKT phosphorylation are differentially regulated by β1-ARs in the MR model with AKT phosphorylation being independent from FAK activation.
Figure 6. MR-induced AKT and Hsp27 phosphorylation is prevented by β1-RB.
Top, representative immunoblots showing accumulation of phospho-AKT and -Hsp27 in LV extracts from control, 4W-MR, and 4W-MR+β1-RB. Blots were stripped and reblotted with Anti-AKT or -Hsp27 antibodies. Bottom, fold induction, n=6 each group, *P<0.05 4W-MR vs. control, #P<0.05 4W-MR+β1-RB vs. 4W-MR.
Another molecule that has been shown to be downstream of p38-MAPK and confer protection and anti-apoptotic properties for the heart is Hsp27.34 MR induction for 4-weeks increased Hsp27 phosphorylation as assessed by immunoblot (Figure 6B). As for p38-MAPK activation, β1-RB abolished Hsp27 activation induced by 4W-MR (Figure 6B). These data together suggest that stimulation of β1-AR is involved in the activation of anti-apoptotic pathways, AKT and Hsp27, that may compensate for the loss of ECM and FA signaling in early MR.
β-AR signaling is not altered in MR isolated cardiomyocytes
Since β1-RB reduced FA signaling loss and prevented p38-MAPK, Hsp27 and AKT activation, we thought that alteration in β-AR signaling may occur in cardiomyocytes at this early stage of MR. To test this hypothesis, myocytes were isolated from LV of control and 4W-MR dogs and plated on laminin coated dishes for 6-hrs in 5% FBS DMEM. Myocytes were then switched to serum free medium for 1-hr prior to their treatment with isoproterenol (a non specific β1- and β2-AR agonist) in the presence or absence of β1-AR antagonist CGP20712A, or with a selective β2-AR agonist zinterol. In control-derived myocytes, isoproterenol induced FAK, p38-MAPK, Hsp27, and AKT phosphorylation that was prevented when cells were pretreated with CGP20712A. The β2-AR agonist zinterol also induced an increase in FAK, p38-MAPK, Hsp27, and AKT phosphorylation suggesting that the activation of these kinases is induced by both β1- and β2-AR stimulation (Figure 7A). Similarly, exposure of myocytes-derived from 4W-MR dogs with β1- or β2-AR agonists led to FAK, p38-MAPK, Hsp27, and AKT phosphorylation and there were no significant quantitative or qualitative differences observed between these myocytes and myocytes-derived from control animals (Figure 7B and 7C). Taken together, the effect of β1-RB in preventing FA signaling loss and increasing p38-MAPK/Hsp27 and AKT activation in LV tissue extracts cannot be explained by an intrinsic alteration in β1- or β2-AR-induced downstream signaling based on these in-vitro studies of isolated cardiomyocytes.
Figure 7. Preservation of β-AR signaling in MR isolated cardiomyocytes.
Cardiac myocytes were isolated from control (A) or 4W-MR (B) LV as described in materials and methods. (A, B) representative immunoblots showing accumulation of phospho-FAK, -p38-MAPK, -Hsp27, and -AKT after treatment with 10µmol/L isoproterenol (Iso) or 1µmol/L Zinterol (Zint) for 5-minutes compared with control (Ctrl): 1µmol/L of CGP20712A was added 30-minutes before isoproterenol stimulation. (C) Summary graphs represent quantitative data from 4 independent experiments from cells derived from 4 different animals. *P<0.05 vs. control.
DISCUSSION
We have previously shown that induction of MR for 4-weeks in the dog was associated with a marked increase in catecholamine release into the LV interstitial fluid space that is significantly attenuated by β1-RB started 24-hours after MR induction.1,2,7 The current study extends this work using samples from these same animals and demonstrates that decreased ECM accumulation in 4W-MR dogs is associated with alteration in FA signaling. FAK tyrosine phosphorylation and FAK association with p130Cas and paxillin is decreased in MR dogs. Interestingly, β1-RB prevented these signaling alterations without improving LV remodeling or completely restoring ECM accumulation. These results suggest that mechanical factors per se are not the trigger for FA signaling changes. Rather increased adrenergic drive and subsequent stimulation of β1-AR is the main trigger leading to impaired FA signaling early in the course of isolated MR.
In this early stage of isolated MR, loss of FAK tyrosine phosphorylation was associated with the loss of FAK interaction with p130Cas and paxillin, which are important docking sites for other signaling molecules that play an additional role in survival signaling.15,16 This is consistent with FAK involvement in the tyrosine phosphorylation of p130Cas and paxillin and indicates that MR induces downregulation of FA signaling as well as destruction of the FA complex. This is in stark contrast to the activation of FA signaling reported in the early compensated stages of experimentally-induced pressure overload in-vivo or in isolated cardiomyocytes subjected to pulsatile mechanical stretch.22,23,35 Interestingly, the decrease in FAK and paxillin tyrosine phosphorylation was not associated with their cleavage or caspase-3 activation in 2W (Supplemental Figure S2 and data not shown) and 4W-MR LVs (Figures 2A and 5A), suggesting that dephosphorylation and disruption of the FA complex occurs prior to caspase-3 activation and FAK/paxillin degradation. In the failing and dilated hearts where myocyte apoptosis was identified, FA proteins (FAK and paxillin) cleavage has been detected and may involve an increase in caspase-3 activity.36
The role of the constitutive phosphorylation of FA proteins in normal cellular function is not fully understood, but may be important for maintaining cell survival and FA integrity in the resting state.16,37 In mice with a selective inactivation of FAK in cardiomyocytes, an eccentric cardiac hypertrophy develops with age and even in the face of angiotensin II infusion or induction of pressure overload with transaortic constriction.19 In another study, persistent challenge of similar transgenic mice with transaortic constriction leads to enhanced cardiac fibrosis and cardiac dysfunction in comparison with challenged genetic controls.20 Despite these cardiac structural changes, both studies failed to detect an increase in myocyte apoptosis. This is in contrast to findings in myocyte-restricted deletion of the β1-integrin in adult mouse hearts where dilated cardiomyopathy and concomitant HF were observed.38 These findings in mice, in addition to our present study, showed that impaired FA signaling is associated with the development of eccentric cardiac hypertrophy. Recent studies in cardiomyocytes in-vitro have demonstrated that disruption of FAK signaling prevented hypertrophic responses induced by GPCRs and promoted cardiomyocyte apoptosis by anoikis.29,30,39
Protection against apoptosis in this early adaptive phase of isolated MR could be explained by an increase in survival signaling pathways Erk1/2, AKT, and Hsp27 activation that counter pro-apoptotic events resulting from the loss of FA signaling. In addition, there was no increase in JNK phosphorylation, which has been shown to mediate β1-AR-induced cardiomyocyte apoptosis.40 Surprisingly, β1-RB prevented both AKT and Hsp27 stimulation, implicating β1-AR stimulation in the activation of these survival signaling pathways that are independent of FAK activation. Nevertheless, there is emerging evidence that prolonged activation of some survival signaling pathways may have deleterious effects on cardiomyocyte survival. Herein, induction of an activated Akt1 gene in the mouse heart induced adaptive cardiac hypertrophy in the acute phase and dilated cardiomyopathy in the chronic phase, suggesting that Akt and Akt-dependent signaling pathways are involved in both physiological and pathological cardiac growth.41 These data emphasize that cardiac apoptosis control is multifactorial and that the balance between pro- and anti-apoptotic pathways over time may dictate the transition form heart hypertrophy to failure.
In this study, we showed that β1-RB prevented FA signaling loss and p38-MAPK, Hsp27, and AKT activation induced by 4W-MR. However, we couldn’t detect a significant difference in β1- or β2-AR expression by immunoblot of plasma membrane prepared from control- or MR-derived cardiomyocytes (data not shown). A defect in β-AR downstream signaling is also unlikely since myocytes derived from control and MR hearts showed similar β1- and β2-AR responses in FAK, AKT, p38-MAPK, and Hsp27 phosphorylation. Although these data indicate an intact β-AR signaling mechanism of cardiomyocytes in-vitro, it is noteworthy that cardiomyocytes were isolated from the whole LV and were stimulated with submaximal concentrations of agonists which may not allow detecting differences in kinase phosphorylation between control- and MR-derived cardiomyocytes. Furthermore, cardiomyocytes were grown on laminin substrate, which may not reproduce the marked loss of ECM in MR hearts in-vivo. This is especially important since plating myocytes on laminin substrate as opposed to glass selectively reduced β1-AR and enhanced β2-AR regulation of ICa.13 Thus, our short term in-vitro studies in MR-derived cardiomyocytes plated on laminin may negate the loss of ECM in MR hearts with increased adrenergic drive in-vivo.
The selective downregulation of FA signaling in the compensated phase of MR was associated by a small amount of LV dilatation and increased LV fractional shortening. This occurs in spite of the renin-angiotensin-system and adrenergic drive activation and is in stark contrast to the activation of FA signaling in experimentally-induced pressure overload.22,23 In these dogs, β1-RB decreased catecholamine release into the interstitial fluid in response to electrical and angiotensin-II stimulation7 and restored FA signaling without affecting LVEDD/wt and wall stress indexes. Our data suggest that prolonged and excessive adrenergic drive is a potential mediator of FA signaling alteration early in the adaptive phase of VO. Consistent with these findings, prolonged stimulation of β-ARs has been shown to promote disruption of β1-integrin signaling in cultured cardiomyocytes and stimulation of β1-integrin signaling was efficient to protect cardiomyocytes against β-AR-induced apoptosis.14 However, β1-RB did not prevent a marked loss of endocardial collagen, which could explain the failure to improve short-term diastolic remodeling and function. Long-term therapy of VO with β1-AR does improve isolated cardiomyocyte function but has no effect on interstitial collagen loss and LV dilatation and remodeling (preliminary data and ref.3). The primary loss of myocyte/fibroblast-ECM scaffolding and their interaction offers a new target, in addition to β1-AR, to attenuate excessive adrenergic drive in the VO of MR.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health HL76799 (AS), SCCOR in Cardiac Dysfunction P50HL077100 (LJD), American Heart Association 0430301N (AS), and Dept. of Veteran Affairs (LJD).
Footnotes
Disclosures
None
REFERENCES
- 1.Tallaj J, Wei C-C, Hankes GH, Holland M, Rynders P, Dillon AR, Ardell JL, Armour JA, Lucchesi PA, Dell'Italia LJ. {beta}1-adrenergic receptor blockade attenuates angiotensin II-mediated catecholamine release into the cardiac interstitium in mitral regurgitation. Circulation. 2003;108:225–230. doi: 10.1161/01.CIR.0000079226.48637.5A. [DOI] [PubMed] [Google Scholar]
- 2.Stewart J, James A, Wei C-C, Brower GL, Rynders PE, Hankes GH, Dillon AR, Lucchesi PA, Janicki JS, Dell'Italia LJ. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2003;35:311–319. doi: 10.1016/s0022-2828(03)00013-0. [DOI] [PubMed] [Google Scholar]
- 3.Tsutsui H, Spinale FG, Nagatsu M, Schmid PG, Ishihara K, DeFreyte G, Cooper G, IV, Carabello BA. Effects of chronic [beta]-adrenergic blockade on the left ventricular and cardiocyte abnormalities of chronic canine mitral regurgitation. J Clin Invest. 1994;93:2639–2648. doi: 10.1172/JCI117277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dell'italia LJ, Balcells E, Meng QC, Su X, Schultz D, Bishop SP, Machida N, Straeter-Knowlen IM, Hankes GH, Dillon R, Cartee RE, Oparil S. Volume-overload cardiac hypertrophy is unaffected by ACE inhibitor treatment in dogs. Am J Physiol Heart Circ Physiol. 1997;273:H961–H970. doi: 10.1152/ajpheart.1997.273.2.H961. [DOI] [PubMed] [Google Scholar]
- 5.Perry GJ, Wei C-C, Hankes GH, Dillon SR, Rynders P, Mukherjee R, Spinale FG, Dell'Italia LJ. Angiotensin II receptor blockade does not improve left ventricular function and remodeling in subacute mitral regurgitation in the dog. J Am Coll Cardiol. 2002;39:1374–1379. doi: 10.1016/s0735-1097(02)01763-1. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RH, Supiano MA, Oral H, Grossman PM, Montgomery DS, Smith MJ, Starling MR. Compared with control subjects, the systemic sympathetic nervous system is activated in patients with mitral regurgitation. Am Heart J. 2003;145:1078–1085. doi: 10.1016/S0002-8703(03)00111-X. [DOI] [PubMed] [Google Scholar]
- 7.Hankes GH, Ardell JL, Tallaj J, Wei CC, Aban I, Holland M, Rynders P, Dillon R, Cardinal R, Hoover DB, Armour JA, Husain A, Dell'Italia LJ. beta1-Adrenoceptor blockade mitigates excessive norepinephrine release into cardiac interstitium in mitral regurgitation in dog. Am J Physiol Heart Circ Physiol. 2006;291:H147–H151. doi: 10.1152/ajpheart.00951.2005. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg SF. The Molecular Basis for Distinct {beta}-adrenergic receptor subtype actions in cardiomyocytes. Circ Res. 1999;85:1101–1111. doi: 10.1161/01.res.85.11.1101. [DOI] [PubMed] [Google Scholar]
- 9.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, Stinson E. Beta1- and beta2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 10.Feldman AM, Cates AE, Veazey WB, Hershberger RE, Bristow MR, Baughman KL, Baumgartner WA, Van Dop C. Increase of the 40,000-mol wt pertussis toxin substrate (G protein) in the failing human heart. J Clin Invest. 1988;82:189–197. doi: 10.1172/JCI113569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth DA, Urasawa K, Helmer GA, Hammond HK. Down-regulation of cardiac guanosine 5'-triphosphate-binding proteins in right atrium and left ventricle in pacing-induced congestive heart failure. J Clin Invest. 1993;91:939–949. doi: 10.1172/JCI116315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ungerer M, Kessebohm K, Kronsbein K, Lohse MJ, Richardt G. Activation of β-adrenergic receptor kinase during myocardial ischemia. Circ Res. 1996;79:455–460. doi: 10.1161/01.res.79.3.455. [DOI] [PubMed] [Google Scholar]
- 13.Wang YG, Samarel AM, Lipsius SL. Laminin binding to beta1-integrins selectively alters beta1- and beta2-adrenoceptor signalling in cat atrial myocytes. J Physiol (Lond) 2000;527:3–9. doi: 10.1111/j.1469-7793.2000.t01-2-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Communal C, Singh M, Menon B, Xie Z, Colucci WS, Singh K. beta1 integrins expression in adult rat ventricular myocytes and its role in the regulation of beta-adrenergic receptor-stimulated apoptosis. J Cell Bioch. 2003;89:381–388. doi: 10.1002/jcb.10520. [DOI] [PubMed] [Google Scholar]
- 15.Ross RS. The extracellular connections: The role of integrins in myocardial remodeling. J Card Fail. 2002;8:S326–S331. doi: 10.1054/jcaf.2002.129263. [DOI] [PubMed] [Google Scholar]
- 16.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 17.Bloch W, Forsberg E, Lentini S, Brakebusch C, Martin K, Krell HW, Weidle UH, Addicks K, Fassler R. beta1 integrin is essential for teratoma growth and angiogenesis. J. Cell Biol. 1997;139:265–278. doi: 10.1083/jcb.139.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.llic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 19.Peng X, Kraus MS, Wei H, Shen TL, Pariaut R, Alcaraz A, Ji G, Cheng L, Yang Q, Kotlikoff MI, Chen J, Chien K, Gu H, Guan JL. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J. Clin. Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiMichele LA, Doherty JT, Rojas M, Beggs HE, Reichardt LF, Mack CP, Taylor JM. Myocyte-restricted focal adhesion kinase deletion attenuates pressure overload-induced hypertrophy. Circ Res. 2006;99:636–645. doi: 10.1161/01.RES.0000240498.44752.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg T. Expression of collagen binding integrins during cardiac development and hypertrophy. Circ Res. 1991;68:734–744. doi: 10.1161/01.res.68.3.734. [DOI] [PubMed] [Google Scholar]
- 22.Franchini KG, Torsoni AS, Soares PH, Saad MJ. Early Activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ Res. 2000;87:558–565. doi: 10.1161/01.res.87.7.558. [DOI] [PubMed] [Google Scholar]
- 23.Bayer AL, Heidkamp MC, Patel N, Porter MJ, Engman SJ, Samarel AM. PYK2 expression and phosphorylation increases in pressure overload-induced left ventricular hypertrophy. Am J Physiol Heart Circ Physiol. 2002;283:H695–H706. doi: 10.1152/ajpheart.00021.2002. [DOI] [PubMed] [Google Scholar]
- 24.Ruest PJ, Roy S, Shi E, Mernaugh RL, Hanks SK. Phosphospecific antibodies reveal focal adhesion kinase activation loop phosphorylation in nascent and mature focal adhesions and requirement for the autophosphorylation site. Cell Growth Differ. 2000;11:41–48. [PubMed] [Google Scholar]
- 25.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng C-F, Kitsis RN, Molkentin JD. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez MT, Sah VP, Zhao X-L, Hunter JJ, Chien KR, Brown JH. The MEKK-JNK pathway is stimulated by alpha1-adrenergic receptor and Ras activation and is associated with in-vitro and in-vivo cardiac hypertrophy. J. Biol. Chem. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 28.Frisch S, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res. 2002;90:1282–1289. doi: 10.1161/01.res.0000023201.41774.ea. [DOI] [PubMed] [Google Scholar]
- 30.Rafiq K, Kolpakov MA, Abdelfettah M, Streblow DN, Hassid A, Dell'Italia LJ, Sabri A. Role of protein-tyrosine phosphatase SHP2 in focal adhesion kinase down-regulation during neutrophil cathepsin G-induced cardiomyocytes Anoikis. J Biol Chem. 2006;281:19781–19792. doi: 10.1074/jbc.M513040200. [DOI] [PubMed] [Google Scholar]
- 31.O'Neill BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J. Clin. Invest. 2005;115:2059–2064. doi: 10.1172/JCI25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinisitide-regulated kinases: Kinase Activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 33.Bayascas JR, Alessi DR. Regulation of Akt/PKB Ser473 Phosphorylation. Mol Cell. 2005;18:143–145. doi: 10.1016/j.molcel.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH. Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation. 2004;110:3544–3552. doi: 10.1161/01.CIR.0000148825.99184.50. [DOI] [PubMed] [Google Scholar]
- 35.Torsoni AS, Constancio SS, Nadruz W, Jr, Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res. 2003;93:140–147. doi: 10.1161/01.RES.0000081595.25297.1B. [DOI] [PubMed] [Google Scholar]
- 36.Melendez J, Welch S, Schaefer E, Moravec CS, Avraham S, Avraham H, Sussman MA. Activation of pyk2/related focal adhesion tyrosine kinase and focal adhesion kinase in cardiac remodeling. J Biol Chem. 2002;277:45203–45210. doi: 10.1074/jbc.M204886200. [DOI] [PubMed] [Google Scholar]
- 37.Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 38.Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the {beta}1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 39.Taylor JM, Rovin JD, Parsons JT. A role for focal adhesion kinase in phenylephrine-induced hypertrophy of rat ventricular cardiomyocytes. J Biol Chem. 2000;275:19250–19257. doi: 10.1074/jbc.M909099199. [DOI] [PubMed] [Google Scholar]
- 40.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. {beta}-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 41.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.