Abstract
Objectives
To characterize opiate effects on motor function of the oesophagus in patients presenting with dysphagia.
Methods
Retrospective review of 15 patients with dysphagia referred for oesophageal manometry while on chronic opiates. Manometry was completed during opiate use and in three cases, after opiates were discontinued.
Results
All patients demonstrated motility abnormalities. Incomplete lower oesophageal sphincter (LES) relaxation (11.5 ± 1.6mmHg) was seen in most cases. Ten patients demonstrated non-peristaltic contractions in ≥ 3 out of 10 swallows. Additional abnormalities included high amplitude contractions; triple peaked contractions; and increased velocity. The average resting lower oesophageal sphincter (LESP) met criteria for hypertensive LES in 3 patients. These features were suggestive of spasm or achalasia. Repeat manometry off opiates was performed in 3 cases. LES relaxation was noted to be complete upon repeat manometry in these cases. There was also improved peristalsis and normal velocity.
Conclusions
A range of manometric abnormalities were seen in patients with dysphagia in the setting of opiate use: impaired LES relaxation, high amplitude/velocity, and simultaneous oesophageal waves. These data suggest the oesophagus is susceptible to the effects of opiates and care must be taken before ascribing dysphagia to a primary oesophageal motility disorder in patients taking opiates.
Keywords: Dysphagia, oesophagus, opiates
INTRODUCTION
The deleterious side effects of opiates with respect to the gastrointestinal tract have long been recognized. Much research has investigated opiates and gut dysfunction. It is well established that opioid analgesics delay intestinal transit. This occurs by stimulation of non-propulsive contractions in the intestine and colon via both central and peripheral actions. Opioid receptors of all three classical types (μ, δ, and κ) are expressed in the central nervous system as well in the enteric nervous system. Gastrointestinal effects are most likely mediated through μ and κ receptors located on myenteric neurons but possibly through opioid receptors on the smooth muscle itself (1, 2). In the mid and distal gut, μ and κ receptors have been demonstrated within the intestinal wall. μ receptors are most dense in the submucosal plexus on nerve terminals, and κ receptors are found in greater density in the myenteric plexus. Oesophageal localization studies in man date back to the 1980s and mostly describe LES distribution (3). Opioid receptors have been demonstrated in the oesophagus, but are not as well-characterized as in the rest of the gut.
In contrast to the wealth of understanding of opioid receptor physiology regarding the stomach, small intestine and colon, far less is known in the oesophagus. Despite the paucity of knowledge of opioid receptor distribution and physiology in the human oesophagus, opiates have demonstrated clear motor effects on the human oesophagus. Physiologic studies on healthy volunteers have shown that morphine decreases lower oesophageal sphincter relaxation (4). To date, however, there is very scarce information published on effects of opiates on the oesophagus in clinical practice. Case reports have documented acute dysphagia following intrathecal fentanyl (5, 6). Retrospective series also note dysphagia with intrathecal fentanyl, suggesting a central effect (7). It is well known that agonists for peripheral opioid receptors are able to produce gastrointestinal effects without the central analgesic and respiratory depressant effects. It is also evident that central nervous system administration of opiates affects gastrointestinal motility as well, as intracranial administration has the same effects as peripheral administration in animals, and intrathecal injections in humans have similar effects to peripheral administration (8). These reports of dysphagia and the known effects of oral opiates on the intestines suggest that oral opiates may affect swallow function adversely. Our aim was to characterize the manometric profile of dysphagia in patients taking opiates.
MATERIALS AND METHODS
Retrospective analysis was performed on 15 patients presenting to the oesophageal motility lab at Mayo Clinic Rochester with indication of dysphagia and found to have a history of opiate use at the time of manometry. Chart review over a two year period found 15 patients had undergone oesophageal manometry while taking opiates. No patient had any pre-existing condition identified which would account for dysphagia. Oesophageal manometry was read by any one of a group of three gastroenterologists experienced in oesophageal motility testing without prior knowledge of patients’ medications. The routine in our oesophageal motility lab is that patients are asked to stop medications prior to testing, and this is documented in the medical record. However, patients often have not been instructed to stop chronic narcotics by the primary physician. None of the patients in this study had discontinued opiates prior to testing. Three patients did return for testing having discontinued use of opiates prior to the procedure. Standard oesophageal manometry was repeated in these three patients.
Oesophageal Motility Testing
All oesophageal motility testing was conducted in standard fashion with the solid-state multichannel manometry catheter (Konigsberg Instruments, Pasadena, CA, USA) with data collected using Bioview acquisition software (Sandhill Scientific Co., Denver, CO, USA). The catheter was placed transnasally into the stomach following intranasal topical anesthesia. The lower oesophageal sphincter was identified by standard pull-through technique. After the most distal transducer was placed in the LES, baseline LES pressure was recorded in the absence of swallowing. Then, ten wet swallows were completed with the patient in a semi-recumbent position. Each swallow was completed following oral administration of 5mL of water with at least 30 seconds between swallows.
Oesophageal manometry was interpreted by one of a group of gastroenterologists experienced in interpretation of oesophageal motility tests. As noted, gastroenterologists interpreting manometry were not aware of the opiate status of the patients at the time of interpretation of the study. Parameters measured included resting average LES pressure, average minimum LES pressure upon swallow, average per cent relaxation of LES upon swallow, peak oesophageal contraction amplitude, contractile front velocity, and number of non-peristaltic contractions per 10 wet swallows. Data are expressed as mean ± standard error of the mean.
RESULTS
Of the 15 patients reviewed in this study, three patients underwent manometry while on opiates and again once off opiates. The results of manometry in these three patients are included in the entire group and also considered separately for comparison to testing off opiates. As a group, the 15 patients undergoing manometry while on opiates were observed to have resting LESP of 38.2 ± 6.3 mmHg. Average LES relaxation was 65.6 ± 5.5% of baseline LESP, with relaxation below 8 mmHg (the standard at our institution for normal swallow-induced relaxation) occurring in only 4 patients (Figure 1). The average swallow-induced LESP nadir was 11.5 ± 1.6 mmHg. Relaxation to > 80% of baseline LES pressure occurred in only 3 patients. With ≥ 30% non-peristaltic contractions considered as criteria for abnormal oesophageal motility, 12 of 15 patients had oesophageal dysmotility. Three of 15 patients demonstrated absence of normal peristalsis of each of 10 swallows, most having the manometric appearance of spasm or achalasia. Individual and summary manometry data are found in tables 1 and 2.
Fig 1.
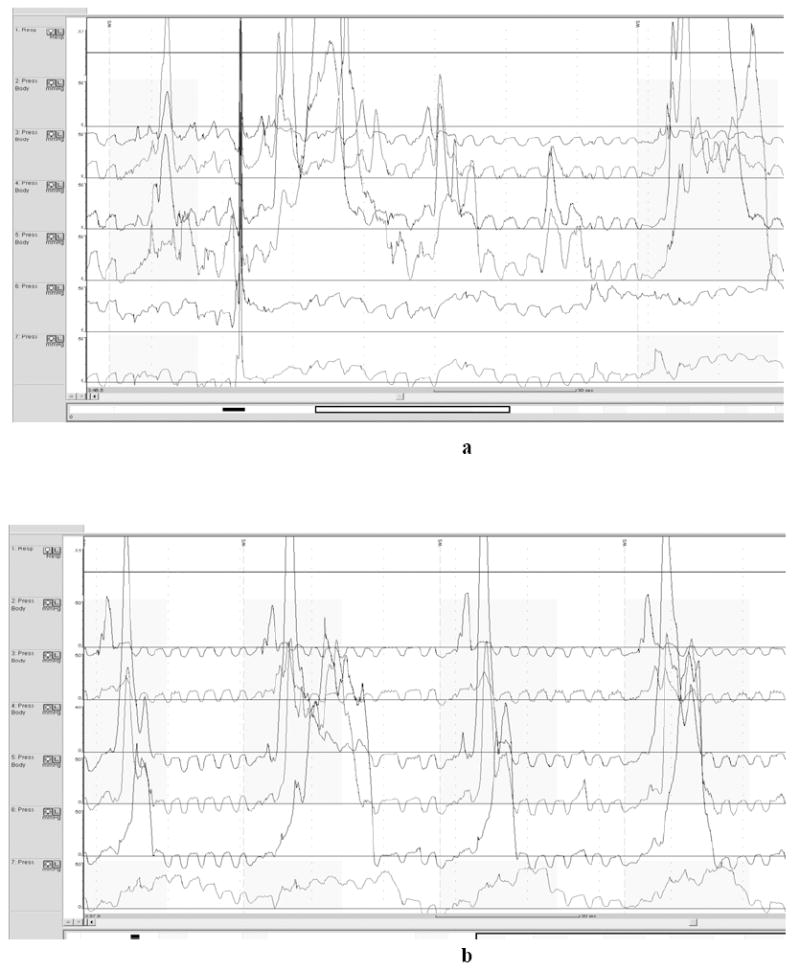
a demonstrates simultaneous contraction, high amplitude contractions, and incomplete LES relaxation on opiates. b shows continued high amplitude contractions, but improved peristalsis and LES relaxation off opiates
Table 1.
Individual manometric data of dysphagic patients on opiates
| Patient no. | Baseline LOSP (mmHg) | Percent relaxation | Residual LOSP (mmHg) | Oesophageal velocity (cm/s) |
|---|---|---|---|---|
| 1 | 13.8 | 93 | 1.0 | 10.9 |
| 2 | 27.7 | 38 | 17.2 | 13.6 |
| 3 | 117.5 | 87 | 15.3 | 9 |
| 4 | 26.8 | 55 | 12.1 | 7.5 |
| 5 | 38.1 | 50 | 19.1 | 19.2 |
| 6 | 34.6 | 64 | 12.5 | 10.6 |
| 7 | 36.4 | 76 | 8.7 | ∞ |
| 8 | 55.2 | 89 | 6.1 | ∞ |
| 9 | 30.2 | 11 | 26.9 | ∞ |
| 10 | 23.8 | 67 | 7.9 | 17.3 |
| 11 | 32.5 | 70 | 9.8 | 14.9 |
| 12 | 32.8 | 64 | 11.8 | 7.4 |
| 13 | 16.1 | 62 | 6.1 | 6.2 |
| 14 | 48.3 | 80 | 9.7 | 10.2 |
| 15 | 39.3 | 78 | 8.6 | 6.9 |
indicates all simultaneous contractions precluding velocity calculation
Table 2.
Summary manometric data of patients tested on and off opiates
| No. patients | Resting LOSP (mmHg) | Swallow induced LOSP nadir (mmHg) | No. patients with >3/10 non-peristaltic swallows | |
|---|---|---|---|---|
| All patients | 15 | 38.2 ± 6.3 | 11.5 ± 1.6 | 12 |
| On opiates before re-evaluation | 3 | 28.5 ± 6.7 | 13.9 ± 6.5 | 3 |
| Off opiates re-evaluated | 3 | 17.9 ± 5.2 | 2.7 ± 2.5 | 0 |
Propagation velocity of the primary oesophageal peristaltic wave was calculated in patients that had at least 5 of 10 swallows generating peristalsis. Accelerated velocities were found in 9 of 12 patients, suggesting diffuse or at least segmental oesophageal spasm. The mean oesophageal velocity was 11.1 ± 1.2 cm/sec. Normal is less than 8.0cm/sec. Oesophageal velocity could not be calculated in patients with all non-peristaltic contractions; simultaneous contractions could be considered to have an infinite velocity, but for calculation purposes were left out.
Three patients were re-evaluated having been off opiates for greater than 72 hours. Manometry in these patients while on opiates revealed distal oesophageal spasm in 2 of three patients and incomplete LES relaxation in 2 of 3 patients. LES relaxation was complete in all 3 patients upon repeat manometry off opiates, all less than 8 mmHg residual LES pressure. The oesophageal velocity decreased in all three patients off opiates with all in the normal range at retesting. None of these three patients met manometric criteria for oesophageal spasm at repeat manometry off opiates. These data are summarized in Table 2. Representative manometric traces from these patients are displayed in figures 1 - 3.
Fig 3.
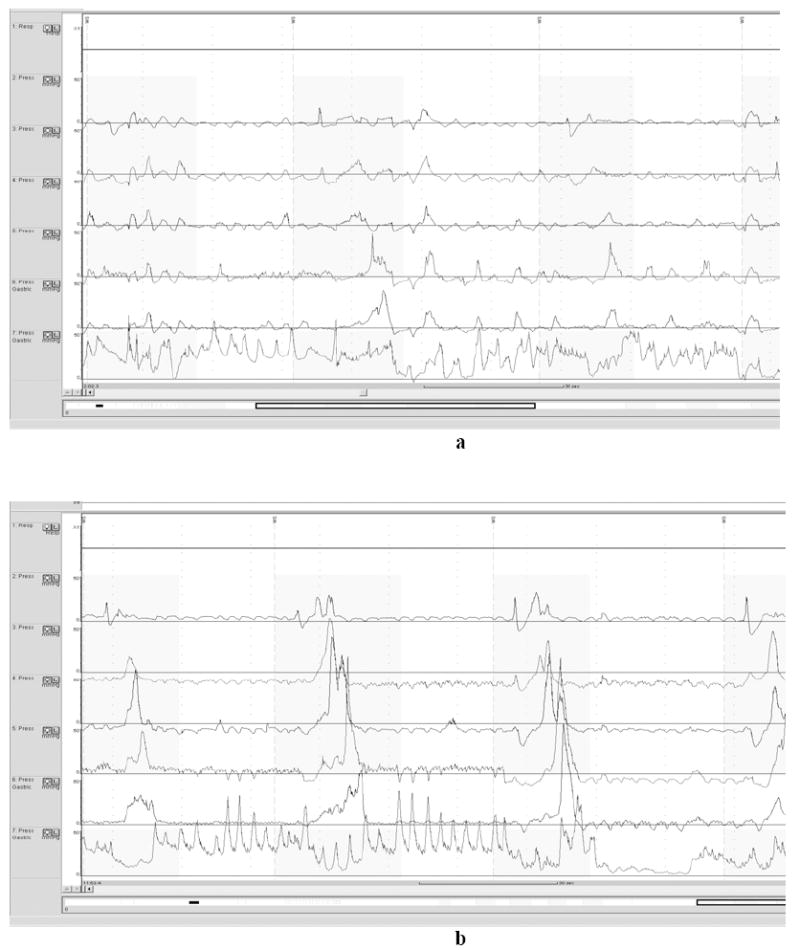
a demonstrates numerous simultaneous, non-peristaltic contractions (first and last swallow shown) on opiates. b demonstrates resolution off opiates.
DISCUSSION
This retrospective study examines oesophageal motility abnormalities in patients on opiate analgesics seen at Mayo Clinic Rochester. To date, there is a large base of information regarding opiate medications and their effects on the gut, but scarce information of the effects of these drugs in the oesophagus. We have characterized a number of manometric abnormalities on a cohort of patients with dysphagia and concomitant opiate use. This may have implications for interpretation of motility studies in patients on chronic opiate therapy.
Elevated resting LES pressure was observed in the group. The average resting LESP was 38 ± 6.2 mmHg. Three patients had resting LESP > 40mmHg, meeting our institutional criteria for hypertensive LES. This can manifest as dysphagia. Furthermore, these patients did not have adequate LES relaxation with swallow. This is typical of achalasia. However, it was unusual to observe aperistalsis in patients on opiates – only 3 of 15 patients demonstrated 10/10 wet swallows without peristalsis. The presence of peristalsis argues against vigorous achalasia, but incomplete relaxation of the LES does suggest a “spastic achalasia-like” oesophageal dysmotility that may manifest as dysphagia. The 3 patients retested off opiates displayed adequate LES relaxation with swallows – all LES relaxation pressures were < 8 mmHg.
Oesophageal body contraction was abnormal in most of the patients. Only three patients demonstrated normal peristalsis with ten wet swallows. The other 12 patients were found to have ≥ 30% non peristaltic contractions with wet swallows. These would meet criteria for either hypotensive peristalsis or oesophageal spasm and could account for dysphagia. Three patients were retested off opiates, and all had return of normal peristalsis, that is, > 80% of swallows demonstrating peristaltic contractions.
These findings corroborate finding from a previous physiologic study on healthy volunteers receiving morphine injection demonstrating increase in resting LESP and decreased swallow-induced relaxation within 15 minutes of administration of morphine (9). Penagini et al. performed a manometric study of healthy volunteers placed on morphine and found that morphine decreased both the magnitude and the duration of swallow-induced LES relaxation. Importantly, they demonstrated that this effect was reversed by naloxone, a μ-opioid receptor antagonist. While we did not administer opioid antagonists to the patients in our study, we did confirm incomplete swallow-induced LES relaxation in patients taking opiates (though not necessarily morphine). We have also shown return to normal swallow-induced LES relaxation in those patients taken off opioid medications and retested, suggesting the effect was mediated by the medication. The Penagini et al. study also featured the finding of increased velocity of primary peristalsis following administration of morphine. We not only observed increased velocity, but also we observed frequent simultaneous contractions. It is possible, though not established, that velocity of contraction lies along a continuum and that simultaneous contraction is at the extreme end of that continuum. Simultaneous contraction – not observed when retesting was performed off opiates – may be an effect of opiates.
The mechanism of these effects is unclear. Given the apparent effects on both LES relaxation and oesophageal peristalsis, a pathway through nitric oxide is possible. Interference with normal nitric oxide activity by administration of a nitric oxide scavenger has previously been shown to increase oesophageal contractile velocity, produce simultaneous contractions, inhibit normal swallow induced LES relaxation, and produce symptoms in healthy volunteers (10). There is no evidence for opiates to act as nitric oxide scavengers, but given the very similar manometric findings, it is conceivable that opiates may disrupt normal nitric oxide mediated neuromuscular function.
There is the possibility that our study selected patients with underlying oesophageal dysmotility who coincidentally were on opiates at the time of their presentation. This is certainly a possibility in a small cohort such as this. There are variable reports on the prevalence of dysphagia (11, 12) with the most recent suggesting a prevalence > 20%. However, we have identified a small but varied group with respect to age, sex, and medical history who shared the symptom of dysphagia and a history of opiate use. We further examined three of these patients after discontinuation of opiates and demonstrated manometric improvement. This suggests an effect secondary to opiates. This would corroborate the retrospective data from Albright in which a series of 71 of 6002 patients experienced acute dysphagia following sufentanil administration, treated with nalbuphine or naloxone with relief (7).
The manometric findings in this study were not consistent among patients, but as demonstrated most in multiple studies from Kahrilas and colleagues on patients with achalasia, there are multiple manometric variants that may present with dysphagia (13, 14). Findings previously observed in asymptomatic volunteers receiving morphine – impaired swallow-induced LES relaxation – were observed in the majority (12 of 15) of symptomatic dysphagic patients in our study. The oesophageal body dysmotility observed in our study included simultaneous and non-peristaltic contractions as well as increased oesophageal contraction velocity, sharing some features of oesophageal spasm, and in some cases, achalasia. The Penagini study did observe changes in oesophageal motility but this was only increased velocity and not disruption of primary peristalsis. It may be hypothesized that these changes represent a common physiologic process along a continuum, and that dysphagic patients have progressed from high velocity to disordered and in some cases, simultaneous contractions. This would require further study, but could provide insight into oesophageal physiology and could change our approach to interpretation of clinical oesophageal manometry of patients on opiates. Overall, our observations suggest that oesophageal manometric abnormalities in patients on opiates may not be due to a primary oesophageal motility disorder and may be secondary to a medication effect.
Fig 2.
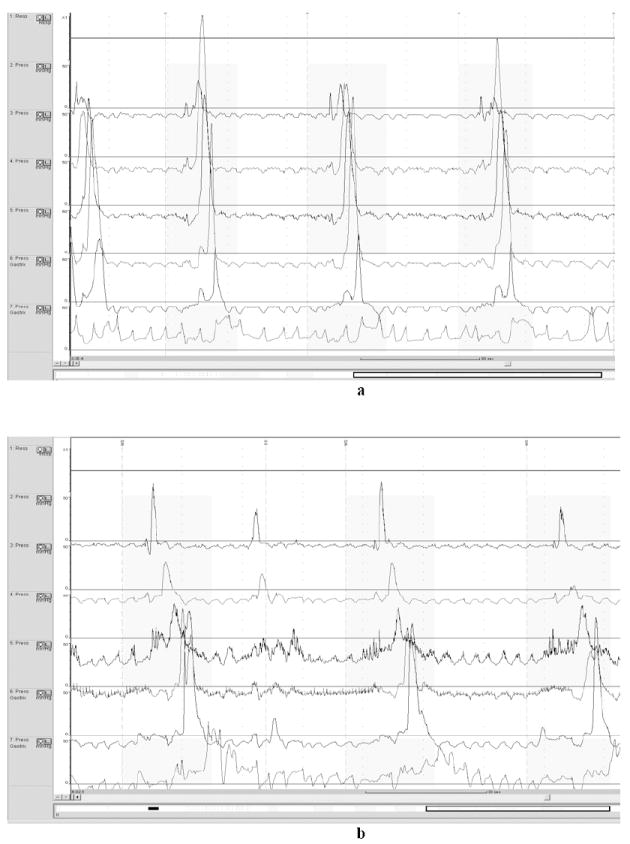
a demonstrates incomplete LES relaxation and high velocity contractions in the oesophageal body on opiates. b demonstrates improvement in LES relaxation and normal velocity off opiates.
Acknowledgments
Dr. Murray is supported in part by grants DK71003 and DK57892 from the National Institutes of Health.
Abbreviations
- LES
Lower oesophageal sphincter
- LESP
Lower oesophageal sphincter pressure
References
- 1.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 2.Sternini C, Patierno S, Selmer IS, Kirchgessner A. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 2):3–16. doi: 10.1111/j.1743-3150.2004.00553.x. [DOI] [PubMed] [Google Scholar]
- 3.Rattan S, Goyal RK. Identification and localization of opioid receptors in the opossum lower esophageal sphincter. The Journal of pharmacology and experimental therapeutics. 1983;224(2):391–7. [PubMed] [Google Scholar]
- 4.Penagini R, Picone A, Bianchi PA. Effect of morphine and naloxone on motor response of the human esophagus to swallowing and distension. The American journal of physiology. 1996;271(4 Pt 1):G675–80. doi: 10.1152/ajpgi.1996.271.4.G675. [DOI] [PubMed] [Google Scholar]
- 5.Currier DS, Levin KR, Campbell C. Dysphagia with intrathecal fentanyl. Anesthesiology. 1997;87(6):1570–1. doi: 10.1097/00000542-199712000-00037. [DOI] [PubMed] [Google Scholar]
- 6.Smiley RM, Moore RP. Loss of gag reflex and swallowing ability after administration of intrathecal fentanyl. Anesthesiology. 2007;106(6):1253. doi: 10.1097/01.anes.0000265423.95135.78. [DOI] [PubMed] [Google Scholar]
- 7.Albright GA, Forster RM. The safety and efficacy of combined spinal and epidural analgesia/anesthesia (6,002 blocks) in a community hospital. Regional anesthesia and pain medicine. 1999;24(2):117–25. doi: 10.1016/s1098-7339(99)90071-8. [DOI] [PubMed] [Google Scholar]
- 8.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63(7):649–71. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 9.Dowlatshahi K, Evander A, Walther B, Skinner DB. Influence of morphine on the distal oesophagus and the lower oesophageal sphincter--a manometric study. Gut. 1985;26(8):802–6. doi: 10.1136/gut.26.8.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray JA, Ledlow A, Launspach J, Evans D, Loveday M, Conklin JL. The effects of recombinant human hemoglobin on esophageal motor function in humans. Gastroenterology. 1995;109(4):1241–8. doi: 10.1016/0016-5085(95)90584-7. [DOI] [PubMed] [Google Scholar]
- 11.Wilkins T, Gillies RA, Thomas AM, Wagner PJ. The prevalence of dysphagia in primary care patients: a HamesNet Research Network study. J Am Board Fam Med. 2007;20(2):144–50. doi: 10.3122/jabfm.2007.02.060045. [DOI] [PubMed] [Google Scholar]
- 12.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112(5):1448–56. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 13.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135(5):1526–33. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirano I, Tatum RP, Shi G, Sang Q, Joehl RJ, Kahrilas PJ. Manometric heterogeneity in patients with idiopathic achalasia. Gastroenterology. 2001;120(4):789–98. doi: 10.1053/gast.2001.22539. [DOI] [PubMed] [Google Scholar]


