We measured the financial consequences of new CRC treatment regimens. New regimens have increased cost directly through price and indirectly through nonstandard and second-line regimen use.
Abstract
Purpose:
To compare medical expenditures of patients receiving old and new colorectal cancer (CRC) regimens.
Study Design:
Using claims data, we identified two cohorts of privately insured patients diagnosed with CRC: first, those diagnosed before new treatment introduction (January 1, 2002, to December 31, 2002), and second, those diagnosed after new treatment introduction (June 1, 2004, to May 31, 2005). CRC diagnosis was identified using International Classification of Diseases–9 codes 153.xx, 154.xx, and 159.0. First- and second-line chemotherapy regimens were identified. Treatments and expenditures were then observed for up to 2 years after initial diagnosis.
Methods:
We estimated multivariate models to measure changes in cost with changes in treatment regimen. Approval dates of new regimens were used as natural experiments.
Results:
New regimens, such as fluorouracil, leucovorin, and oxaliplatin (FOLFOX), have rapidly replaced the most prevalent preperiod product (ie, fluorouracil/leucovorin). Changes in treatment have caused large increases in total expenditure, primarily through increases in chemotherapy prices. FOLFOX alone has increased total average cost by 14%. New treatments have not substituted other medical services; rather, they have indirectly raised costs through nonstandard regimen use and increases in second-line treatment use. We found no evidence that expenditure effects were driven by changes in follow-up duration.
Conclusion:
New CRC treatments have increased both regimen choice and expenditures. New regimens have primarily increased expenditures through direct treatment costs; we observed no offsetting expenditure reductions.
Introduction
Colorectal cancer (CRC) is the third most frequently diagnosed cancer and the second leading cause of death as a result of cancer in the United States.1 In 2009, an estimated 147,000 individuals were diagnosed with CRC, and approximately 50,000 died as a result of this condition. However, there have been significant advances in CRC treatment over the past decade. We measured the financial consequences of these innovations.
CRC is usually treated with multiagent regimens. The underlying principle of the combination chemotherapy regimen is that drugs can function through separate mechanisms and may have superior efficacy and effectiveness when administered jointly.2 Until recently, three regimens dominated the CRC first-line treatment market: fluorouracil (FU), available since the 1960s,3 which has been routinely administered with leucovorin (FU/LV) since the early 1990s4 or with irinotecan (IFL or FOLFIRI). The US Food and Drug Administration (FDA) approved five new CRC treatments between 1999 and 2004. The most popular regimen combines FU/LV with oxaliplatin (FOLFOX). Use of these drug combinations in metastatic CRC has been found to improve survival.5
The FDA also approved new biologic CRC treatments in 2004. These new biologic agents—bevacizumab, cetuximab, and panitumumab—are often referred to as monoclonal antibodies. In effect, these antibodies facilitate immune responses to rapidly proliferating cancer cells. The first agent to be introduced was bevacizumab, which works against the vascular endothelial growth factor and is often used in combination with FOLFOX or FOLFIRI. Cetuximab and panitumumab inhibit the epidermal growth factor receptor, and cetuximab has been tested and used in combination with FOLFIRI. More recent evidence suggests that cetuximab and panitumumab are only effective in patients who have tumors with a KRAS mutation.6,7 Table 1 lists common first-line CRC treatment regimens as well as the drugs that make up each regimen and their approval dates.
Table 1.
First-Line Regimens and Drug Approval Dates
| Regimen | FU | LV | Irinotecan (April 2000) | Capecitabine (May 2001) | Oxaliplatin (January 2004) | Bevacuzimab (February 2004) | Cetuximab (February 2004) |
|---|---|---|---|---|---|---|---|
| FU | ● | ||||||
| FU/LV | ● | ● | |||||
| IFL or FOLFIRI | ● | ● | |||||
| Capecitabine | ● | ||||||
| Capecitabine/irinotecan | ● | ● | |||||
| FOLFOX | ● | ● | |||||
| Capecitabine/oxaliplatin | ● | ● | |||||
| FU/LV + bevacizumab | ● | ● | ● | ||||
| IFL + bevacizumab | ● | ● | ● | ||||
| Other biologic | ● |
NOTE. Date of approval for first-line therapy of advanced/metastatic colorectal cancer.
Abbreviations: FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil/leucovorin with oxaliplatin; FU, fluorouracil; IFL, fluorouracil plus irinotecan; LV, leucovorin.
Although these advances have been welcomed by the oncology community, there has been significant concern about the cost of these treatments.8–10 An early editorial after the approval of new CRC treatments showed that the cost of an 8-week course using the Mayo regimen (ie, monthly administration of 5 consecutive days of FU/LV) was $63.11 This increased to $11,889 with FOLFOX, $21,033 with FOLFOX plus bevacizumab, and $30,675 with FOLFIRI plus cetuximab. Howard et al12 recently measured changes in CRC survival and cost during 2002 to 2005. The authors found that survival increased by 1.4 months, whereas total cost increased by $4,600 among patients with CRC in the Medicare population. Larger increases in survival and spending were observed during the period of 1995 to 2002.
We build on the existing literature by measuring the direct and indirect expenditure effects of new CRC treatment regimens for a privately insured population. Following Howard et al,12 we used approval dates as a natural experiment to measure the financial consequences of new CRC treatment regimens. These new regimens are associated with substantial increases in total treatment cost. We used a combination of empirical models to decompose this overall change. New regimens generate substantial increases in the direct cost of first-line treatments. We considered three potential indirect effects: changes in nonchemotherapy treatment costs, use of nonstandard regimens, and changes in the cost of incumbent products. Nonstandard regimens were defined as a mix of drugs that included typical drugs used in a regimen (eg, FU/LV) as well as other drugs that were not standard in the regimen (eg, FU/LV + capecitabine). Often, additional treatment for nonstandard regimens was initiated weeks to months after the start of the initial treatment regimen. We found no evidence that new regimens change nonchemotherapy medical costs. We did observe small decreases in the cost of older regimens. The rate of nonstandard regimen use increased after the introduction of new treatments; physicians supplemented existing regimens with new products, thus increasing costs. Finally, we considered issues of treatment duration and observed follow-up periods to test and correct for both selection bias and the potential for effective treatments to increase long-run costs through improved survival.
Methods
Data
We assembled an extensive data set of de-identified pharmacy and medical claims from the IMS Health LifeLink Health Plan Claims Database, provided by IMS Health. The database covers more than 55 million lives and 80 health plans. Data include all claims and encounters, including prescription drugs, inpatient services, outpatient office visits, ambulatory services (such as testing, imaging, and chemotherapy administration), emergency room visits, and home health services. Expenditures reflect total annual payments made by each enrollee (copayments, deductibles, excluded expenses) and by all third-party payers (primary and secondary coverage, net of negotiated discounts).
Study Sample
We created two distinct cohorts of patients with CRC age 18 to 64 years: one cohort of those diagnosed before new treatment availability (January 1, 2002, to December 31, 2002) and the other of those diagnosed after new treatment availability (June 1, 2004, to May 31, 2005). We identified CRC diagnoses on the basis of the existence of claims with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) for malignant neoplasm of colon, rectum, rectosigmoid junction, anus, or intestinal tract (codes 153.xx, 154.xx, and 159.0). All eligible patients had 1 year of no CRC-related claims preceding their diagnosis.
We identified 12,881 unique patients in the preperiod and 15,416 in the postperiod who had at least one claim with the above ICD-9 codes. To eliminate any false positives, we also required colorectal claims on five or more separate dates within 6 months of the index diagnosis, similar to methods used by Ramsey et al.13 The resulting sample included 5,597 patients in the preperiod and 6,309 in the postperiod. We also required each patient to have received CRC chemotherapy within 6 months of initial diagnosis. Our final analytic data set included 2,644 unique patients (10 months average observed follow-up) in the preperiod cohort and 3,213 (10.3 months average follow-up) in the postperiod cohort. We ascertained that no preperiod cohort patients were included in postperiod data.
All costs were inflated to 2007 US dollars. Costs were estimated by service type, including inpatient, outpatient, emergency room, laboratory, imaging, and chemotherapy. We also estimated cost across several time dimensions: first, observed cost with up to 1 year of follow-up; second, annualized cost, which is 365 times the ratio of observed cost divided by days of observation; and third, cost during first-line treatment period. Resections were identified using Current Procedural Terminology–4 procedure codes (44140 to 44160, 44204 to 44212, and 45110 to 45119). Resource utilization was based on claims and included use and costs incurred after initial CRC diagnosis.
Take-Away Points.
New oncology drugs have rapidly affected both the treatment and the cost of colorectal cancer (CRC). Although new regimens offer the hope of increased survival, they come at a high cost.
New regimens such as FOLFOX largely substituted for FU/LV and IFL/FOLFIRI.
New regimens have substantially increased the cost of CRC treatment.
Increases in FOLFOX-related costs alone account for approximately 14% of total average cost per patient.
New treatments have not been substitutes for other medical services; rather, they have increased the cost of existing regimens through nonstandard regimen use.
CRC Chemotherapy Regimens
We identified CRC chemotherapy drugs using J-codes in the claims. We used combinations of chemotherapy drugs to define chemotherapy regimen based on first chemotherapy treatment after diagnosis. This resulted in 11 exclusive first-line treatment regimens, listed in Table 1: FU, FU/LV, IFL or FOLFIRI, capecitabine (an oral FU alternative), capecitabine/irinotecan, FOLFOX, capecitabine/oxaliplatin, FU/LV plus bevacizumab, IFL plus bevacizumab, other biologic, and other.
Only the first five regimens were available during the preperiod. The other biologic and other categories included some regimens that could not be easily classified and that few individuals received. For example, patients receiving FU plus oxaliplatin plus irinotecan or FOLFOX plus irinotecan were classified in the other category. Rare regimens that included a biologic, such as capecitabine plus bevacizumab, were categorized in the other biologic category. As Table 1 suggests, some of the 11 exclusive categories of first-line treatments are not highly prevalent in our data. Accordingly, we constructed broader first-line therapy regimens by including capecitabine/oxaliplatin and capecitabine/irinotecan in the other category. Similarly, we included FU/LV plus bevacizumab and IFL plus bevacizumab in the other biologic category.
Second-line therapy was defined in two ways. Typically, chemotherapy regimens for CRC are expected to last 6 months. We observed this to a great extent in the data, but it was not consistent. We defined second-line treatment as the discontinuation of first-line treatment (ie, all agents in the initial regimen) and start of a new treatment regimen 6 to 12 weeks after the end of the initial regimen. We also tested other definitions of switching to second-line treatment, such as discontinuation of initial regimen and start of a new regimen at any time. However, this did not seem to affect the results. Our method of second-line therapy identification is similar to that used by Kutikova et al.14
Statistical Analyses
We measured expenditure differences across patients receiving different chemotherapeutic regimens. Naturally, patients receiving more expensive treatment regimens could be costlier for unobserved reasons. This potential selection effect could cause bias in naive models of treatment costs. Consequently, we used FDA approval dates as a natural experiment. In effect, we used patients diagnosed before approval as a control group. Previous studies have used similar identification strategies.12,15
The effects of regimens on expenditures were estimated by generalized linear models (with log link, gamma family). These models included extensive patient-level controls for clinical comorbidities and quarterly indicators for diagnosis dates. These quarterly indicators control for any factors unobserved to the researchers that were common across providers or patients. For example, these variables might capture the effect of unobserved price changes, pharmaceutical marketing efforts, or publication of medical research. Furthermore, we allowed preperiod regimen effects to differ during pre- and postperiods. We estimated the impact of changes in chemotherapy regimen on spending for first-line and non–first-line chemotherapy and on nonchemotherapy spending. We then investigated whether first-line treatments were associated with second-line therapy use (using a logistic model) and with observed length of follow-up time (using a Cox proportional hazard model). Both specifications included the same set of explanatory variables, as discussed.
We conducted a counterfactual exercise to facilitate parameter interpretation. For the postperiod cohort, we assumed that FOLFOX was not available, and patients received either FU/LV (67%) or IFL/FOLFIRI (33%) in proportion to their preperiod distributions. This allowed us to estimate the incremental effect of FOLFOX on cost.
Results
Descriptive Statistics
Table 2 presents descriptive statistics for the preperiod (2,644 unique patients) and postperiod (3,231 unique patients) cohorts. Average patient age at diagnosis was 56 years in the preperiod cohort and 55 years in the postperiod cohort. Patients in each cohort had approximately 10 months of follow-up. All cost and utilization measures were constructed for 1 year after diagnosis. Total observed cost was substantially higher for the average postcohort patient ($107,994) relative to the average precohort patient ($60,586). This increase was largely driven by increases in inpatient costs ($26,109 v $36,106), chemotherapy administration costs ($7,621 v $26,174), and other outpatient costs ($11,503 v $21,713). On average, each cohort had similar numbers of hospital stays (preperiod, 1.4; postperiod, 1.5), but preperiod cohort patients had slightly longer hospital stays (8.5 v 8.1 days). We did not observe any statistically significant differences in prevalence of comorbid conditions across the cohorts, with the exception of dementia and chronic pulmonary disease, which were more prevalent in the postperiod cohort.
Table 2.
Descriptive Statistics for Pre- v Postperiod Cohorts
| Statistic | Preperiod Cohort (diagnosed between January and December 2002) |
Postperiod Cohort (diagnosed between June 2004 and May 2005) |
P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| No. of patients | 2,644 | 3,231 | |||
| Male, % | 53.2 | 53.1 | .9783 | ||
| Age at diagnosis, years | 56.0 | 11.2 | 54.9 | 9.9 | < .001 |
| Follow-up time, months | 10.0 | 2.9 | 10.3 | 2.7 | < .001 |
| Costs up to 1 year of follow-up, $ | |||||
| Total | 60,586 | 52,385 | 107,994 | 85,162 | < .001 |
| Inpatient | 26,109 | 38,092 | 36,106 | 57,424 | < .001 |
| Inpatient/skilled nursing facility | 219 | 2,731 | 120 | 1,586 | .0834 |
| Pharmacy | 5,197 | 7,273 | 7,506 | 11,910 | < .001 |
| Emergency room | 199 | 578 | 754 | 4,388 | < .001 |
| Home health | 696 | 2,020 | 1,444 | 4,462 | < .001 |
| Office visits | 3,952 | 3,752 | 7,057 | 10,131 | < .001 |
| Laboratory | 892 | 1,080 | 1,185 | 1,353 | < .001 |
| X-ray | 4,199 | 6,269 | 5,933 | 7,436 | < .001 |
| Chemotherapy administration | 7,621 | 13,761 | 26,174 | 34,911 | < .001 |
| Other outpatient | 11,503 | 18,901 | 21,713 | 28,718 | < .001 |
| Utilization* | |||||
| No. of hospital stays | 1.4 | 1.4 | 1.5 | 1.4 | .0478 |
| Length of hospital stay, days | 8.5 | 7.5 | 8.1 | 6.9 | .0064 |
| No. of hospital days | 12.0 | 15.5 | 12.0 | 15.2 | .9305 |
| Comorbid conditions, % | |||||
| Myocardial infarction | 2.6 | 2.7 | .7723 | ||
| Congestive heart failure | 7.6 | 8.0 | .5765 | ||
| Peripheral vascular disease | 4.2 | 4.6 | .5212 | ||
| Cerebrovascular disease | 4.8 | 4.9 | .9188 | ||
| Dementia | 0.6 | 1.2 | .0225 | ||
| Chronic pulmonary disease | 10.0 | 12.6 | .0017 | ||
| Ulcer | 1.9 | 2.2 | .3526 | ||
| Mild liver disease | 1.0 | 1.2 | .4354 | ||
| Diabetes | 16.5 | 16.2 | .7635 | ||
| Diabetes with organ damage | 2.2 | 2.0 | .5799 | ||
| Hemiplegia | 1.1 | 0.7 | .1539 | ||
| Moderate/severe renal disease | 3.1 | 3.4 | .5569 | ||
| Moderate/severe liver disease | 1.2 | 1.5 | .2992 | ||
| Metastatic solid tumor | 65.3 | 65.8 | .7235 | ||
| AIDS | 0.2 | 0.3 | .4199 | ||
| Rheumatologic disease | 1.0 | 1.1 | .6822 | ||
| First-line chemotherapy regimen, % | < .001 | ||||
| FU | 18.2 | 23.1 | |||
| FU/LV | 54.4 | 24.7 | |||
| IFL or FOLFIRI | 20.7 | 7.0 | |||
| Capecitabine | 6.4 | 8.8 | |||
| Capecitabine/irinotecan | 0.1 | 0.0 | |||
| FOLFOX | 0.2 | 32.8 | |||
| Capecitabine/oxaliplatin | 0.0 | 0.8 | |||
| FU/LV + bevacizumab | 0.0 | 0.4 | |||
| IFL + bevacizumab | 0.0 | 0.8 | |||
| Other biologic | 0.0 | 1.4 | |||
| Other | 0.0 | 0.2 | |||
| Second-line therapy, %† | 39.0 | 42.0 | .0028 | ||
| Resection, % | 53.0 | 64.0 | < .001 | ||
NOTE. Data adapted from LifeLink Health Plans Claims Database.
Abbreviations: FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil/leucovorin with oxaliplatin; FU, fluorouracil; IFL, fluorouracil plus irinotecan; LV, leucovorin; SD, standard deviation.
All utilization measures represent use during the first year after diagnosis.
Second-line therapy is defined as chemotherapy 6 weeks to 3 months after initial treatment.
Table 2 also lists significant differences in the first-line regimens used by patients in the two cohorts. The most popular regimens in the preperiod cohort were FU/LV (54.4%), FU (18.2%), and IFL or FOLFIRI (20.7%). These regimens were still prevalent in the postcohort but not as popular, with only 7% of patients receiving IFL or FOLFIRI and 24.7% receiving FU/LV. Interestingly, the proportion of patients receiving FU increased slightly to 23.1%. The most popular first-line regimen in the postperiod was FOLFOX (32.8%).
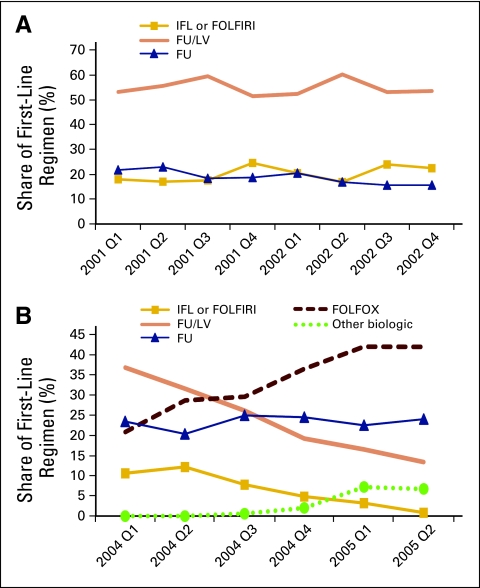
Figures 1A and 1B present quarterly trends in first-line regimens for the pre- and postperiod cohorts. respectively. Preperiod cohort regimen use was fairly stable over time, but there was a significant decline in FU/LV and IFL or FOLFIRI use during the postperiod. Proportion of patients receiving FU, another regimen available to the preperiod cohort, remained stable during the postperiod. Figure 1B also demonstrates significant increases in FOLFOX use. FOLFOX use rose from 20.8% in the first quarter of 2004 to 41.9% in the second quarter of 2005. Use of other biologic drugs as first-line regimens also increased from 2% to 6.7% during the same period.
Figure 1.
Share of each first-line regimen for (A) preperiod cohort (January 1, 2002, to December 31, 2002) and (B) postperiod cohort (June 1, 2004, to May 31, 2005). FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil/leucovorin with oxaliplatin; FU, fluorouracil; IFL, fluorouracil plus irinotecan; LV, leucovorin.
Multivariate Models
Table 3 lists results for a series of cost models. We report results from models of costs incurred only during first-line regimens; however, we found similar results for other cost measures, such as total observed cost up to 1 year of follow-up and total annualized cost (coefficient estimates on regimen indicators were similar across specifications that had these other cost measures as outcome variables). Model 1 examines total cost, model 2 examines cost of chemotherapy agents associated with the regimen only, model 3 examines cost of other chemotherapy agents not directly associated with the first-line regimen, and model 4 focuses on nonchemotherapy costs.
Table 3.
Treatment Regimens and Medical Expenditures: Multivariate Models
| Characteristic | Generalized Linear Model (log link, gamma family) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||
| Effect | SE | Effect | SE | Effect | SE | Effect | SE | |
| Age | −0.01* | 0.001 | −0.02* | 0.002 | −0.01† | 0.007 | −0.01* | 0.001 |
| Female | −0.00 | 0.020 | 0.13* | 0.045 | 0.31* | 0.118 | −0.00 | 0.023 |
| First-line chemotherapy regimen | ||||||||
| FU (reference) | ||||||||
| IFL/FOLFIRI | 0.20* | 0.045 | 4.55* | 0.102 | −2.13* | 0.274 | 0.18* | 0.053 |
| IFL/FOLFIRI × postperiod cohort | 0.03 | 0.071 | −0.28† | 0.160 | 2.20* | 0.418 | 0.12 | 0.083 |
| FU/LV | −0.28* | 0.037 | 2.62* | 0.085 | −1.24* | 0.224 | −0.33* | 0.044 |
| FU/LV × postperiod cohort | −0.06 | 0.050 | −0.74* | 0.114 | 0.75‡ | 0.303 | 0.05 | 0.059 |
| FOLFOX | 0.23* | 0.035 | 4.72* | 0.079 | 0.07 | 0.217 | 0.09‡ | 0.040 |
| Other§ | 0.26‡ | 0.127 | 4.93* | 0.292 | −0.69 | 0.723 | 0.11 | 0.147 |
| Other biologic‖ | 0.31* | 0.088 | 4.95* | 0.199 | 0.04 | 0.511 | 0.22‡ | 0.102 |
| Capecitabine | −0.15* | 0.042 | 2.92* | 0.095 | 0.04 | 0.240 | −0.06 | 0.048 |
| Comorbid conditions | ||||||||
| Myocardial infarction | 0.07 | 0.063 | 0.04 | 0.141 | −0.00 | 0.376 | 0.14† | 0.073 |
| Congestive heart failure | 0.25* | 0.039 | −0.16† | 0.087 | −0.34 | 0.227 | 0.23* | 0.045 |
| Peripheral vascular disease | 0.16* | 0.049 | −0.04 | 0.110 | −0.18 | 0.298 | 0.17* | 0.057 |
| Cerebrovascular disease | 0.15* | 0.047 | 0.07 | 0.108 | −0.14 | 0.292 | 0.21* | 0.055 |
| Dementia | 0.39* | 0.105 | −0.01 | 0.235 | −0.37 | 0.584 | 0.47* | 0.124 |
| Chronic pulmonary disease | 0.03 | 0.032 | −0.09 | 0.072 | −0.35† | 0.188 | 0.08‡ | 0.037 |
| Ulcer | 0.18‡ | 0.070 | −0.11 | 0.159 | −0.01 | 0.410 | 0.27* | 0.081 |
| Mild liver disease | 0.25* | 0.094 | −0.18 | 0.213 | −1.07‡ | 0.540 | 0.27‡ | 0.109 |
| Diabetes | 0.03 | 0.028 | 0.01 | 0.064 | 0.09 | 0.166 | 0.03 | 0.033 |
| Diabetes with organ damage | 0.14† | 0.074 | 0.01 | 0.165 | −0.14 | 0.433 | 0.02 | 0.086 |
| Hemiplegia | 0.41* | 0.108 | −0.13 | 0.246 | −0.73 | 0.630 | 0.55* | 0.125 |
| Moderate/severe renal disease | 0.42* | 0.057 | −0.16 | 0.129 | 0.21 | 0.322 | 0.48* | 0.066 |
| Moderate/severe liver disease | 0.16† | 0.087 | 0.06 | 0.199 | 0.63 | 0.489 | 0.42* | 0.100 |
| AIDS | −0.14 | 0.184 | −0.25 | 0.417 | 0.03 | 1.086 | −0.28 | 0.213 |
| Rheumatologic disease | 0.11 | 0.100 | −0.05 | 0.223 | 0.04 | 0.570 | 0.13 | 0.118 |
| Resection | 0.40* | 0.021 | 0.09† | 0.047 | −0.41* | 0.125 | 0.19* | 0.024 |
| Observations | 5,874 | 5,873 | 5,873 | 5,873 | ||||
NOTE. All specifications include indicators for quarter of diagnosis. Model 1, total cost during first-line treatment; model 2, first-line chemotherapy treatment agent costs during first-line treatment; model 3, non–first-line chemotherapy costs during first-line treatment; model 4, nonchemotherapy costs during first-line treatment. Data adapted from LifeLink Health Plans Claims Database.
Abbreviations: FU, fluorouracil; FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil/leucovorin with oxaliplatin; IFL, fluorouracil plus irinotecan; LV, leucovorin.
P < .01.
P < .1.
P < .05.
Capecitabine/oxaliplatin and capecitabine/irinotecan are included in Other category of first-line regimens.
FU/LV plus bevacizumab and IFL plus bevacuzimab are included in Other biologic category of first-line regimens.
We found that patients using new products incurred higher costs than patients treated with FU or IFL/FOLFIRI. In Table 3, model 1, the parameters for FOLFOX and other biologic were positive (0.23 and 0.31, respectively) and significant (P < .01). We used models 2 to 4 to identify mechanisms through which treatment regimen affects spending. In model 2, we found that FOLFOX and other biologics cost 400% more than FU, our reference regimen. Costs associated with our reference category—a generic regimen (ie, FU)—changed little over time, although costs associated with other incumbent products (IFL/FOLFIRI and FU/LV) fell when competing products were introduced.
First-line chemotherapy regimens are frequently supplemented with additional chemotherapy products. Model 3 shows that nonstandard regimen spending did increase for patients receiving IFL/FOLFIRI and FU/LV compared with that for those receiving FU. New products do not directly increase spending on nonstandard regimens. However, they may indirectly increase nonstandard regimen spending when they are used to supplement older regimens.
Finally, model 4 indicates that FOLFOX and other biologics are associated with relatively modest nonchemotherapy expenditure increases. Patients receiving older regimens such as IFL/FOLFIRI and FU/LV incur greater costs than patients receiving FU; however, this effect did not change during the postperiod. Across all specifications, younger patients had higher costs. The prevalence of other comorbidities and resection were also associated with higher total cost (model 1) and nonchemotherapy costs (model 4). As expected, none of the comorbidities had a statistically significant effect on chemotherapy costs (models 2 and 3).
We also examined the effect of different regimens on the probability of receiving second-line treatment and duration of observed claims data follow-up. Although we observed no differences in follow-up duration (Table 4; model 5), patients receiving newer regimens were more likely to receive second-line therapies (Table 4; model 6). These analyses are discussed in the Robustness section.
Table 4.
Models of Observed Follow-Up and Second-Line Treatments
| Characteristic | Model 5 |
Model 6 |
||
|---|---|---|---|---|
| Effect | SE | Effect | SE | |
| Age | −0.00* | 0.000 | −0.00 | 0.003 |
| Female | −0.01 | 0.009 | 0.09 | 0.055 |
| First-line chemotherapy regimen, % | ||||
| FU (reference) | ||||
| IFL/FOLFIRI | −0.04† | 0.021 | 0.44* | 0.125 |
| IFL/FOLFIRI × postperiod cohort | −0.01 | 0.033 | 0.34‡ | 0.195 |
| FU/LV | 0.01 | 0.017 | −0.20‡ | 0.105 |
| FU/LV × postperiod cohort | −0.04‡ | 0.023 | 0.41* | 0.141 |
| FOLFOX | −0.01 | 0.016 | 0.30* | 0.097 |
| Other§ | −0.12† | 0.058 | −0.04 | 0.358 |
| Other biologic‖ | −0.12* | 0.041 | 0.95* | 0.240 |
| Capecitabine | −0.01 | 0.019 | 0.08 | 0.117 |
| Comorbid conditions | ||||
| Myocardial infarction | −0.02 | 0.029 | −0.17 | 0.176 |
| Congestive heart failure | 0.03‡ | 0.018 | −0.06 | 0.107 |
| Peripheral vascular disease | 0.02 | 0.022 | −0.03 | 0.136 |
| Cerebrovascular disease | 0.01 | 0.022 | 0.02 | 0.132 |
| Dementia | −0.11† | 0.048 | −0.01 | 0.291 |
| Chronic pulmonary disease | −0.00 | 0.014 | −0.11 | 0.088 |
| Ulcer | 0.01 | 0.032 | 0.21 | 0.192 |
| Mild liver disease | 0.02 | 0.043 | −0.45‡ | 0.272 |
| Diabetes | 0.02 | 0.013 | 0.12 | 0.079 |
| Diabetes with organ damage | 0.03 | 0.034 | 0.27 | 0.200 |
| Hemiplegia | 0.06 | 0.049 | −0.04 | 0.304 |
| Moderate/severe renal disease | 0.01 | 0.026 | −0.08 | 0.159 |
| Moderate/severe liver disease | −0.02 | 0.040 | −0.09 | 0.243 |
| AIDS | 0.03 | 0.084 | −0.22 | 0.543 |
| Rheumatologic disease | −0.01 | 0.045 | −0.09 | 0.280 |
| Resection | 0.06* | 0.010 | 0.78* | 0.059 |
| Observations | 5,874 | 5,874 | ||
NOTE. Second-line therapy is defined as chemotherapy 6 weeks to 3 months after initial treatment. All specifications include indicators for quarter of diagnosis. Model 5, observed follow-up days after diagnosis (Cox proportional hazard model); model 6, probability of second line treatment (logit). Data adapted from LifeLink Health Plans Claims Database.
Abbreviations: FU, fluorouracil; FOLFIRI, fluorouracil plus irinotecan; FOLFOX, fluorouracil/leucovorin with oxaliplatin; IFL, fluorouracil plus irinotecan; LV, leucovorin.
P < .01.
P < .05.
P < .1.
Capecitabine/oxaliplatin and capecitabine/irinotecan are included in Other category of first-line regimens.
FU/LV plus bevacizumab and IFL plus bevacuzimab are included in Other biologic category of first-line regimens.
We conducted a counterfactual exercise to help interpret our findings. As Figure 1 suggests, FOLFOX was substituted for FU/LV and IFL/FOLFIRI in the postperiod. Although approximately 55% of patients received FU/LV and 20% received IFL/FOLFIRI in the preperiod, the shares of these two first-line regimens declined to approximately 15% and 0%, respectively, by the end of the postperiod. At the same time, FOLFOX gained approximately 40% of the market share. We predicted postperiod costs assuming FOLFOX had never been approved. In practice, we limited this analysis to the postperiod and randomly assigned FOLFOX users to either FU/LV or IFL/FOLFIRI proportionately based on their relative preperiod market shares. We used this random allocation because our multinomial logistic regression model of first-line treatment regimen choices did not identify any patient characteristics influencing treatment choice. We assigned two thirds of patients receiving FOLFOX in the postperiod to FU/LV and one third to FU. Using parameter estimates from model 1, we predicted the costs under these older treatment regimens. Mean predicted annual cost for first-line treatment was $133,617 (standard deviation, $44,951) for the postperiod. In the absence of FOLFOX, this cost would have been $115,204 (standard deviation, $38,254). FOLFOX directly increased average postperiod chemotherapeutic expenditures by more than $18,000. This corresponds approximately to a $15,000 increase in average cost per patient with up to 1 year of follow-up.
Robustness
Our primary empirical concern is that unobserved differences in patient severity (eg, stage at diagnosis) may drive treatment selection. Physicians and patients know more about CRC severity and the appropriateness of a given treatment. If, for example, relatively high-severity patients received FOLFOX, we might overestimate the effect of FOLFOX on expenditures. We performed a series of robustness tests to address potential problems such as unobserved severity.
The patterns of preperiod treatment choices described in Figure 1A suggest that the distribution of patient and physician treatment preferences was stable across time in the absence of treatment innovations. This stability provides support for our overall empirical strategy. Howard et al12 provide additional evidence for the stable distribution of CRC severity across time. We directly tested whether observed measures of patient severity affected treatment choice. We estimated multinomial logistic regression models to predict first-line chemotherapy regimen choices in the pre- and postperiod cohorts. Observable patient characteristics had almost no effect on first-line regimen choice. The exceptions were that older patients were less likely to receive FOLFOX or FOLFIRI, and patients with kidney or liver disease were less likely to receive biologic products. The choice model results were not particularly interesting and were excluded for brevity. These results are available on request from the authors. Although these results suggest that severity did not drive treatment selection, it is possible that selection is only dependent on unobserved patient severity measures.
Our empirical models contain implicit tests for selection bias based on unobserved severity. If the higher cost of FOLFOX were driven by unobserved severity, then the average severity among patients receiving FU/LV and IFL/FOLFIRI would fall during the postperiod. We found that neither observed duration nor nonchemotherapy costs changed during the postperiod. Although first-line chemotherapy costs fell, this was a price rather than quantity effect. The decrease in observed follow-up for patients receiving other biologic products could be the result of selection bias; however, if so, this effect must be small, because we observed no changes in IFL/FOLFIRI or FU/LV follow-up.
The reported empirical and robustness results are all based on expenditures during first-line regimen. Differences in regimen duration might bias these results. Consequently, we estimated models based on first-year expenditures and observed expenditures before second-line treatment initiation. In all cases, the results were consistent with those reported. Overall, these results strongly suggest that our findings are robust.
Discussion
This study documents increasing CRC treatment heterogeneity after the introduction of new chemotherapy agents. New regimens, such as FOLFOX, were largely substituted for FU/LV and IFL/FOLFIRI as first-line therapy for metastatic CRC. These treatment changes resulted in large expenditure increases in CRC care. The total average cost during follow-up after diagnosis rose from $60,586 in the preperiod to $107,994 in the postperiod. Cost increases from FOLFOX accounted for 14% of the total average postperiod cost. Although the new regimens are costly, there is substantial evidence that these treatments improve survival.16–19 Our analysis suggests that our price effects are independent of unobserved severity; however, our data are poorly suited to measuring any improved outcomes from new treatments.
We also examined indirect effects on medical costs. The new regimens had no effect on nonchemotherapy medical costs; they did not substitute for other forms of medical expenditure. However, they may have influenced the cost of existing treatment regimens. First, the prices of competing regimens fell during the postperiod, possibly because of competition. Second, the cost of nonstandard regimens rose for patients receiving older regimens as their primary first-line treatment. Third, the probability of receiving second-line therapy increased for nearly all treatments.
Notably, our results are in contrast to those of Howard et al,12 who performed an analysis of CRC treatment using Medicare data; however, they employed SEER data, which includes stage at diagnosis. Although our populations differed, our sample selection and empirical strategies were quite similar. Howard et al found that new regimens diffused more rapidly and achieved much higher market share.12 They also found smaller cost effects, with total average expenditure increasing by $4,600 during the analogous period. These differences may be a consequence of unobserved severity differences or federal policy. Differences between Medicare and private insurance reimbursement might also explain this variation in regimen cost and utilization. These differences, along with the 2003 Medicare Prescription Drug, Improvement, and Modernization Act,20 merit further study.
Our report has several limitations. First, our data are not linked to cancer registries. As such, we did not observe stage of cancer at diagnosis, severity of condition, or exact survival. However, we restricted each cohort to newly diagnosed patients to reduce the heterogeneity of disease severity across individuals. Thus, we are reluctant to assess the clinical effectiveness of existing and new therapies. Furthermore, adverse effects and quality-of-life factors may have influenced discontinuation of therapy. Finally, because this research is based on claims data, it is sensitive to potential diagnosis coding errors.
New oncology drugs have rapidly affected both the treatment and cost of CRC. Although new regimens offer the hope of increased survival, they come at a high cost. Our findings imply that new regimens have been largely substituted for older regimens as first-line therapy for metastatic CRC. These treatment changes have resulted in large expenditure increases for CRC care. New treatments have not been substituted for other medical services; rather, they have increased the cost of existing regimens through nonstandard regimen use. In general, the implications of costly new drugs on rising health care spending have been a concern with regard to various therapeutic classes such as antihypertensives, cardiovascular drugs, and specialty drugs for the treatment of rheumatoid arthritis and multiple sclerosis.21–26 As new clinical research becomes available, there are continuing changes in the treatment and management of cancer that warrant future studies to evaluate the costs and benefits of these new cancer treatments. Moreover, future work must study the impact of pharmacogenomic information (eg, the role of KRAS mutation on success with cetuximab) on the evolution of treatment patterns as oncologists' familiarity with new agents increases.
Acknowledgment
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health information service: LifeLink Health Plan Claims Database (data start year to data end year). All rights reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health or any of its affiliated or subsidiary entities.
Authors' Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Author Contributions
Conception and design: Pinar Karaca-Mandic, Jeffrey S. McCullough, Nilay D. Shah
Financial support: Nilay D. Shah
Administrative support: Nilay D. Shah
Collection and assembly of data: Nilay D. Shah
Data analysis and interpretation: Pinar Karaca-Mandic, Jeffrey S. McCullough, Holly Van Houten, Nilay D. Shah
Manuscript writing: Pinar Karaca-Mandic, Jeffrey S. McCullough, Mustaqeem A. Siddiqui, Holly Van Houten, Nilay D. Shah
Final approval of manuscript: Pinar Karaca-Mandic, Jeffrey S. McCullough, Mustaqeem A. Siddiqui, Holly Van Houten, Nilay D. Shah
References
- 1.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2009;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeVita VT, Schein PS. The use of drugs in combination for the treatment of cancer: Rationale and results. N Engl J Med. 1973;288:998. doi: 10.1056/NEJM197305102881905. [DOI] [PubMed] [Google Scholar]
- 3.Chu E. Clinical colorectal cancer: Ode to 5-fluorouracil. Clin Colorectal Cancer. 2007;6:609. [Google Scholar]
- 4.Erlichman C. Fluorouracil and leucovorin for metastatic colorectal cancer. J Chemother. 1990;2(suppl 1):38–40. doi: 10.1080/1120009x.1990.11739003. [DOI] [PubMed] [Google Scholar]
- 5.Wolpin BM, Mayer RJ. Systemic treatment of colorectal cancer. Gastroenterology. 2008;134:1296–1310. doi: 10.1053/j.gastro.2008.02.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amado RG, Wolf M, Peeters M, et al. Wild-Type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 7.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 8.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarlane J, Riggins J, Smith TJ. SPIKE$: A six-step protocol for delivering bad news about the cost of medical care. J Clin Oncol. 2008;26:4200–4204. doi: 10.1200/JCO.2007.15.6208. [DOI] [PubMed] [Google Scholar]
- 10.Meropol NJ, Schulman KA. Cost of cancer care: Issues and implications. J Clin Oncol. 2007;25:180–186. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- 11.Schrag D. The price tag on progress: Chemotherapy for colorectal cancer. N Engl J Med. 2004;351:317–319. doi: 10.1056/NEJMp048143. [DOI] [PubMed] [Google Scholar]
- 12.Howard DH, Kauh J, Lipscomb J. The value of new chemotherapeutic agents for metastatic colorectal cancer. Arch Intern Med. 2010;170:537–542. doi: 10.1001/archinternmed.2010.36. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey SD, Martins RG, Blough DK, et al. Second-line and third-line chemotherapy for lung cancer: Use and cost. Am J Manag Care. 2008;14:297–306. [PubMed] [Google Scholar]
- 14.Kutikova L, Bowman L, Chang S, et al. The economic burden of lung cancer and the associated costs of treatment failure in the United States. Lung Cancer. 2005;50:143–154. doi: 10.1016/j.lungcan.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Cutler D, McClellan M, Newhouse J, et al. Are medical prices declining? Evidence from heart attack treatments. Q J Econ. 1998;113:991–1024. [Google Scholar]
- 16.Lucarelli C, Nicholson S, Song M. Bundling among rivals: A case of pharmaceutical cocktails. http://www.nber.org/papers/w16321.
- 17.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 19.Kabbinavar F, Irl C, Zurlo A, et al. Bevacizumab improves the overall and progression-free survival of patients with metastatic colorectal cancer treated with 5-fluorouracil-based regimens irrespective of baseline risk. Oncology. 2008;75:215–223. doi: 10.1159/000163850. [DOI] [PubMed] [Google Scholar]
- 20.Medicare Prescription Drug, Improvement, and Modernization Act of 2003. 2003 Dec 8; Pub L 108-173. [Google Scholar]
- 21.Ganguli A, Hong SH. Impact of new antihypertensive drugs on the healthcare utilization of hypertensive patients. Am J Pharm Benefits. 2009;1:138–144. [Google Scholar]
- 22.Joyce GF, Goldman DP, Karaca-Mandic P, et al. Impact of specialty drugs on the use of other medical services. Am J Manag Care. 2008;14:821–828. [PMC free article] [PubMed] [Google Scholar]
- 23.Lichtenberg FR. The effect of using newer drugs on admissions of elderly Americans to hospitals and nursing homes: State-level evidence from 1997 to 2003. Pharmacoeconomics. 2006;24(suppl 3):5–25. doi: 10.2165/00019053-200624003-00002. [DOI] [PubMed] [Google Scholar]
- 24.Lichtenberg FR. The impact of new drugs on US longevity and medical expenditure, 1990-2003: Evidence from longitudinal, disease-level data. Am Econ Rev. 2007;97:438–443. [Google Scholar]
- 25.Miller GE, Moeller JF, Stafford RS. New cardiovascular drugs: Patterns of use and association with non-drug health expenditures. Inquiry. 2005;42:397–412. doi: 10.5034/inquiryjrnl_42.4.397. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Soumerai SB. Do newer prescription drugs pay for themselves? A reassessment of the evidence. Health Aff (Millwood) 2007;26:880–886. doi: 10.1377/hlthaff.26.3.880. [DOI] [PubMed] [Google Scholar]