This study presents Humana's experience with a multigene breast cancer assay and provides an analysis of the clinical utility and economics of this technology.
Abstract
Purpose:
National guidelines recommend a 21-gene recurrence score (RS) to aid in adjuvant treatment decision in patients with estrogen receptor (ER) –positive, lymph node (LN) –negative early-stage breast cancer (ESBC). This study was performed to assess the economic implication of the assay in community practices from the perspective of a US payer.
Methods:
The study analyzed 952 women with ESBC enrolled with Humana (Louisville, KY) who were tested with the 21-gene RS between June 2006 and June 2010. The proportion of women classified by the assay according to RS risk category, use, and costs of chemotherapy regimens and supportive care, and costs of adverse events were obtained from Humana. We adopted a validated Markov model to compute the cost implications of RS for a representative patient. The probability of risk of recurrence, the chemotherapy benefit, and the decision impact of RS were derived from published studies.
Results:
Two hundred fifty-five patients within the tested population received adjuvant chemotherapy. Adjuvant chemotherapy was administered to 10% of women at low risk, 36% of women at intermediate risk, and 72% of women at high risk of recurrence. On the basis of a meta-analysis in the reduction of chemotherapy after RS, the model estimated an average test saving of $1,160 per patient. The immediate direct savings for chemotherapy drugs, supportive care, and management of adverse events were $1,885, $2,578, and $472, respectively. Prevention of recurrence through appropriate treatment of patients at high risk resulted in additional savings of $199.
Conclusion:
The adoption of the 21-gene RS led to targeted management of women with ER-positive, LN-negative ESBC and consequently directed savings to the payer.
Introduction
Early-stage breast cancer (ESBC) accounts for more than half of newly diagnosed cases of breast cancer per year.1,2 Surgery plus appropriate postsurgical care—such as endocrine therapy and adjuvant chemotherapy—customized to each individual's cancer biology is now the standard of care. Prescription of endocrine treatment is largely dependent on the presence or absence of hormone receptors, which are identified via widely available laboratory tests such as immunohistochemistry staining and fluorescent in situ hybridization. Comparatively, the recommendation for adjuvant chemotherapy is less clear, as it is guided only by tumor size, grade, and the presence of unfavorable features.3
In a 2005 meta-analysis, the Early Breast Cancer Trialists Collaborative Group4 found that systematic adjuvant chemotherapy provided a 13% absolute reduction in recurrence at 15 years and a 10% absolute reduction in mortality for women younger than age 50 years. For women older than age 50 years, adjuvant chemotherapy provided a 5% reduction in recurrence and a 3% reduction in mortality. To contextualize the effect of adjuvant chemotherapy in this cohort, 20 women must be treated to prevent one recurrence, and 33 women must be treated to prevent one death.
Molecular assays to predict risk and treatment response for ESBC have become commercially available in the past 5 years. In 2007 and 2008, the American Society of Clinical Oncology and the National Comprehensive Cancer Network (NCCN) recommended a 21-gene recurrence score (RS) as a clinically valid tool for improving individual risk assessment for systematic chemotherapy. In 2010, a meta-analysis of published decision impact studies of 21-gene RS showed that the RS reduced overall use of chemotherapy by 27%.1
Previous health economic studies of the 21-gene RS found that the test would improve women's lives and save costs for society and payers.5,6 A limitation of these analyses, acknowledged by the investigators, was that they were based on hypothesized use of adjuvant chemotherapy according to guideline recommendations. Although such analyses are useful in the early stages of technology diffusion, policy makers are keenly interested in learning how the assay affected outcomes and costs compared with actual practice and after some period of experience with the assay. Here we incorporate the utility reported in the meta-analysis with real-world costs associated with adjuvant chemotherapy treatment as obtained from Humana (Louisville, KY) to evaluate the cost-effectiveness of the 21-gene RS.
Methods
Model Framework
The model used in the study was a previously validated Markov model that simulated the distant recurrence–free survival, the overall survival, and the costs of breast cancer management with and without the use of 21-gene RS.5,6 A cycle length of 1 year until recurrence or death was used.5–7 The duration of the cycle was selected, as recommended on cost-effective guidelines,8,9 to reflect the natural history of breast cancer and the development of breast cancer–related events. This analysis was conducted from a US payer perspective. Sensitivity analyses were performed to evaluate the overall cost-effectiveness of the assay. Table 1 lists the model parameters and data sources.
Table 1.
Model Inputs
| Model | Base Case Value | Source |
|---|---|---|
| Distribution of patients according to risk group | ||
| Low risk | 52.7% | Humana |
| Intermediate risk | 34.6% | Humana |
| High risk | 12.7% | Humana |
| Relative reduction in risk with chemotherapy | ||
| Patients at low risk | 131.0% | Paik et al10 |
| Patients at intermediate risk | 61.0% | Paik et al10 |
| Patients at high risk | 26.0% | Paik et al10 |
| Resource costs | ||
| Chemotherapy | $7,026 | Humana |
| Supportive care | $9,606 | Humana |
| Adverse events | $1,761 | Humana |
| Recurrence | $104,000 | Oratz et al11 |
| Utility of health states | ||
| QALY loss associated with chemotherapy | 0.5 | Muss et al,12 Simes et al13 |
| QALY loss associated with recurrence | 9.1 | Muss et al,12 Piccart et al,14 Ravdin et al,15 Simes et al13 |
| QALY loss associated with second primary cancer caused by chemotherapy | 9.0 | Muss et al,12 Piccart et al,14 Ravdin et al,15 Simes et al13 |
| Chemotherapy recommendation before RS | ||
| Before RS low risk | 50% | Meta-analysis |
| Before RS intermediate risk | 55% | Meta-analysis |
| Before RS high risk | 60% | Meta-analysis |
| Other parameters | ||
| Mean age, years | 59 | Humana |
| Discount rate | 3% | Weinstein et al16 |
| Time horizon | 40 | Lifetime horizon |
NOTE. See Appendix Table A2 for distributions used for sensitivity analyses.
Abbreviations: QALY, quality-adjusted life-year; RS, recurrence score.
Test Characteristics
The 21-gene RS breast cancer assay (Oncotype DX; Genomic Health, Redwood City, CA) uses a reverse transcriptase polymerase chain reaction process to quantify the presence of specific mRNA for 16 cancer genes and 5 reference genes. The test was performed on paraffin-embedded tumor samples that were obtained from surgery. The test results were reported as a single RS quantified on a scale of 0 (lower risk) to 100 (higher risk). For example, an RS of 6 predicts a 5% (95% CI, 4% to 8%) 10-year risk of recurrence, whereas a RS of 30 predicts a 20% (95% CI, 15% to 25%) 10-year risk of recurrence. Women may also be classified into three recurrence risk groups: low risk (RS < 18), intermediate risk (RS 18 to 30), and high risk (RS > 30).17
The assay was validated in two prospective-retrospective clinical studies of two randomized clinical trials (National Surgical Adjuvant Breast and Bowel Project studies [NSABP] B-14 and B-20). These studies showed that the assay independently estimated individual patients' risk of distant recurrence of breast cancer at 10 years and predicts the likelihood to adjuvant chemotherapy using cyclophosphamide, methotrexate, and fluorouracil regimens.10,17
The effect of adjuvant chemotherapy as a function of recurrence risk was based on analysis of the NSABP B-20 study. Women who were reported as having low recurrence risk were predicted to derive no benefit from adjuvant chemotherapy (relative risk [RR], 1.31; 95% CI, 0.46 to 3.78). Women with intermediate recurrence risk received minimal benefit from adjuvant chemotherapy (RR, 0.61; 95% CI, 0.24 to 1.59), and women with high recurrence risk benefitted significantly from adjuvant chemotherapy (RR, 0.26; 95% CI, 0.13 to 0.53).10
Take-Away Points.
Breast cancer is a significant burden of illness in the United States, affecting one in eight women. The total cost of breast cancer management is significant. Early economic analyses of novel molecular classifiers for management of early-stage breast cancer suggest that technology adoption could aid in appropriate allocation of health care resources.
This study summarizes the direct cost of care for more than 900 patients with early-stage breast cancer in a US insurance program.
We provide a real-life analysis on the economic impact of a multigene breast cancer assay and find it saves on costs for the health plan.
Decision Impact
A meta-analysis of six published studies on the decision impact of the 21-gene RS show that the adoption of 21-gene RS consistently reduced the use of chemotherapy.1,7,11,18–21 The weighted mean reduction in chemotherapy was approximately 27% (95% CI, 23% to 31%). Additional sensitivity analyses were performed to assess the robustness of the base case result.
Study Population
Humana affirmed payment coverage in July 2006. Its database included 925 women with LN-negative, ER-positive ESBC who did not restrict the use of their claims information and were tested with the 21-gene assay between June 2006 and June 2010.
The primary estimates obtained from the Humana database were the proportion of women predicted by the assay to have low, intermediate, and high risk of 10-year distant recurrence of breast cancer; the proportion of women receiving adjuvant chemotherapy on the basis of the assay results; the type of chemotherapy and supportive care received; the incidence of adverse events; and the costs of chemotherapy, supportive care, and adverse events.
Adjuvant chemotherapy regimens preferred by the NCCN 2009 breast cancer guidelines were identified by individual therapeutic components through Healthcare Common Procedure Coding System (J9090, J9170, J9000, J9001, J9180, J9190, J9250, J9260, and J9265). Chemotherapy-related supportive care, such as growth factors and antiemetics, were identified by codes J2505, J1440/1441, J2820, J0885, J2088, J2405, J1260, J2469, J8501, J2765, and J0780. Cost associated with adverse events cited by established adjuvant chemotherapy trials was matched between the Current Procedural Terminology (CPT-9) and International Classification of Disease (ICD-9) diagnosis codes.12,14,22–24 All data were de-identified to protect patient's confidentiality in accordance with Health Insurance Portability and Accountability Act and compiled by Humana.
Quality of Life
Health utility scores were obtained from published literature and used to calculate the quality-adjusted life-year (QALY). These scores represent the quantitative value of an individual's perspective of a specific health state; a value of 0 is equivalent to death, and a value of 1 equals perfect health. Utility scores were obtained for three states: breast cancer during chemotherapy, breast cancer recurrence, and second primary cancer caused by chemotherapy.13,15,25–27 Total utility scores were derived from the sum of disutility associated with chemotherapy and the disutility associated with recurrence. Analyses by Cole et al28 indicated a utility score of 0.5 to represent 6 months of chemotherapy treatment that yielded no QALY gain for patients at low risk. The utility score of 0.5 was used in the base case model to be consistent with the guideline on the use of adjuvant therapy.
Sensitivity Analysis
We conducted one-way sensitivity analysis and probabilistic sensitivity analysis (PSA). In the one-way sensitivity analysis, we varied the model driver individually and noted the impact of each parameter on key model outputs such as incremental cost, incremental QALY, and cost per QALY gained. The range of the one-way sensitivity analysis represents the 95% probable values computed through 1,000 model simulations (Appendix Table A1). These drivers were ordered according to decreasing level of importance and plotted in a tornado diagram with the order of importance on the vertical axis and the total cost to the plan on the horizontal axis.
PSA used a Monte Carlo simulation to evaluate the effect of uncertainty on key parameters on the total cost to the plan. These model drivers varied independently according to predetermined probability distributions, which were developed to capture the likely range for each parameter. In the PSA, each driver is modeled according to the distribution standard recommended for a specific type of parameter (Appendix Table A2).29
Results
Mean age of the study population was 59 years. Five hundred two patients (53%) were classified by the assay as being at low risk; 329 patients (35%) were classified as being at intermediate risk, and 121 patients (13%) were classified as being at high risk. Two hundred fifty-five (27%) women received adjuvant chemotherapy. Fifty (10%) of the women classified as being at low risk by the assay received adjuvant chemotherapy compared with 118 (36%) of the women at intermediate risk and 87 (72%) of the women at high risk.
Among the 255 women who received adjuvant chemotherapy, 105 (41%) were selected for docetaxel-based regimens, and 46 (18%) were treated with docetaxel and cyclophosphamide. Other adjuvant chemotherapy regimens identified were docetaxel and doxorubicin plus cyclophosphamide (4%); doxorubicin and cyclophosphamide plus paclitaxel (2%); cyclophosphamide, methotrexate, and fluorouracil (2%); fluorouracil, doxorubicin, and cyclophosphamide (1%); and fluorouracil, epirubicin, and cyclophosphamide (4%). Twenty-eight percent of the treated population received other chemotherapy regimens that did not match the NCCN recommendations.
At baseline, we projected that the 21-gene RS provided the women at low risk with an average of 0.134 QALYs gained from avoiding chemotherapy-related complications. For the women who were reclassified as being at high risk by the 21-gene assay, the adoption of 21-gene RS provided 0.027 QALYs gained in preventing distant recurrence. Humana spent $3,784,200 for 952 assays. The model projects that Humana had a net savings of $1,104,320 or an average of $1,160 per test. The immediate savings associated with reduction in chemotherapy costs, supportive care costs, and management of adverse events per woman tested was $1,885, $2,578 and $472, respectively. The model estimates an average of $199 saved per woman tested in the prevention of distant recurrence (Table 2).
Table 2.
Model Results
| End Point | 27% Decision Impact |
21% Decision Impact |
||
|---|---|---|---|---|
| Baseline | Second Primary Cancer* | Baseline | Second Primary Cancer* | |
| Cost per patient tested, $ | ||||
| Oncotype DX† | 3,975 | — | — | — |
| Reduced use of adjuvant chemotherapy | ||||
| Chemotherapy drugs | 1,885 | — | 1,482 | — |
| Supportive care | 2,578 | — | 2,025 | — |
| Adverse events | 472 | 897 | 371 | 701 |
| Recurrence (projected) | 199 | — | 122 | — |
| Total | 1,160 | 1,579 | 25 | 377 |
| Total cost, $ | ||||
| Per plan | 1,103,874 | 1,503,423 | 23,932 | 337,883 |
| Per member per month | 0.02 | 0.03 | 0.00 | 0.01 |
| QALYs gained | ||||
| Chemotherapy-related | 0.134 | — | 0.105 | — |
| Recurrence | 0.027 | — | 0.005 | — |
| Second primary cancer | Not applicable | 0.076 | Not applicable | 0.059 |
| Total | 0.162 | 0.237 | 0.110 | 0.170 |
| Cost saving per QALY gained | Cost saving | Cost saving | Cost saving | Cost saving |
NOTE. — indicates same value as column to the left.
Abbreviation: QALY, quality-adjusted life-year.
Chemotherapy-related second primary cancer.
Genomic Health, Redwood City, CA.
In a separate analysis, we accounted for chemotherapy-induced second primary cancer, a distant long-term adverse event not captured in the immediate adverse event costs. We applied the incidence rate of second primary cancer as reported by Schaapveld et al30 and adjusted it for the average of treated patients. We estimated that approximately 3.1% of the treated women might be at risk of a second primary cancer induced by chemotherapy. Including this potential long-term adverse effect, the model projected $1,503,423 in net savings. Savings from prevention of this rare but serious adverse event increased to $897 per woman. Women who were spared adjuvant chemotherapy and risk of developing a chemotherapy-induced second primary cancer gained 0.076 QALYs.
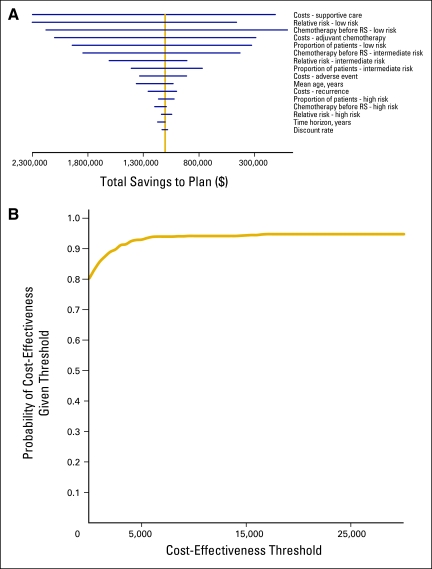
The one-way sensitivity analysis showed that the most influential cost driver is the RR of recurrence with chemotherapy for patients with RS low risk. Other highly influential parameters in ranked order are cost of supportive care and percent of patients with RS low risk who are recommended to chemotherapy before RS testing (Appendix Table A2). Figure 1A illustrates the impact of each parameter on the total cost to the health plan. In early 2010, Lo et al1 reported a prospective study on the decision impact of the 21-gene RS on adjuvant treatment. Applying the chemotherapy distribution reported by Lo et al to the Humana cohort, the model showed that the assay led to a modest net total saving of $23,932 to $337,883 for the plan.
Figure 1.
(A) Tornado diagram of one-way sensitivity analysis. (B) Sensitivity analysis cost-effectiveness acceptability curve.
We ran 1,000 probabilistic scenarios to predict the cost per QALY gained under a variety of conditions. Figure 1B presents the cost-effectiveness acceptability curve from the PSA. The curve shows an 81% probability that the 21-gene RS would be cost-saving to a health plan.
Discussion
Breast cancer is a significant burden of illness in the United States; it ranks as the second most common cancer in the nation. Although the exact economic cost of breast cancer management is unknown, a Medicare–Surveillance, Epidemiology, and End Results claims study by Warren et al31 shows an increasing trend in the costs of breast cancer care from 1991 to 2002. This study also reported a significant increase in the use of chemotherapy and an increase in the mean Medicare payment per therapy. Given the growing cost of the health care system, the economic implications of new health technologies should be carefully evaluated and validated.
The economics of 21-gene RS was first published in 20056 and then in 2007,5 in the context of the present standard of care, under the best practice scenario. In this study, we present a real practice economic validation of 21-gene RS in the US setting. In the base case, we estimated that the use of 21-gene RS led to more than one million dollars in net savings to the Humana plan during the study period. The model also projected approximately 2 to 3 months of QALY gained for the tested patients. For the patients at low risk, 21-gene RS adoption represented improved quality of life through avoidance of chemotherapy-related complications and potential chemotherapy-induced secondary cancers; for the patients at high risk, 21-gene RS demonstrates appropriate and justified used of adjuvant chemotherapy.
The Humana study shows the community distribution of RS risk groups and supports the validation trial by Paik et al that approximately 50% of the tested population could be classified as being at low risk.17 Moreover, this study presents the actual post-RS–directed treatment patterns in the community setting. We noted a variation in RS risk distribution and the subsequent treatment plan between the Humana cohort and the patient population (n = 89) from the study by Lo et al.1 This suggests that there is considerable heterogeneity in breast cancer practice.
The result of our analysis is sensitive to the current chemotherapy prescribing patterns and those of breast cancer management. Twenty-one–gene RS saves the most cost in a health care setting with significant use of chemotherapy. Without an extensive chart review on the treatment process for all 925 patients in the Humana cohort, we set our baseline decision impact of the assay on meta-analysis of the published studies and performed detailed sensitivity analyses on the basis of this parameter.
There are a number of potential limitations to this study. First, the cost-effectiveness of the 21-gene RS is highly influenced by the cost of adjuvant chemotherapy and supportive care. In our analysis, these costs were representative of the Humana network. As such, we recognize that the economic impact of 21-gene RS will vary according to health care systems. Second, the analysis is conducted from Humana's perspective and did not account for the patients' out-of-pocket costs or patients' indirect costs. In addition, the costs of monthly laboratory workups and outpatient office visits related to treatment were not included in this study—as such, the total societal saving of the 21-gene RS may have been underestimated in this study.
Within the base case parameters, analysis of Humana's claims data shows that the 21-gene RS testing of 925 women with LN-negative ESBC decreased their total plan cost by close to one million dollars. For patients at low risk of recurrence who decided against adjuvant chemotherapy, the benefit of 21-gene RS was realized in the avoidance of chemotherapy-related complications and long-term adjuvant chemotherapy–related adverse events, such as infertility and second primary tumors.
Patients at high risk of recurrence who elected adjuvant treatment on the basis of the 21-gene RS results were estimated to receive a 20% to 30% reduction in the risk of breast cancer recurrence and associated mortality. This study demonstrates the cost saving potential of the 21-gene RS and projects it as a cost-effective treatment decision tool with a 95% probability of cost-effectiveness ratio that exceeded $16,500 per QALY gained—a value much less than the acceptability threshold value of $50,000 per QALY.
Acknowledgment
We thank Julie Doberne, BA, for her contributions to the early stages of this article.
Appendix
Table A1.
Results of the One-Way Sensitivity Analysis
| Parameter | Range of Input | Range of Total Net Savings ($) | Difference ($) |
|---|---|---|---|
| Costs | |||
| Supportive care | $5,720-$14,756 | 111,308-2,419,597 | 2,308,289 |
| Adjuvant chemotherapy | $3,827-$10,924 | 286,701-2,099,697 | 1,812,996 |
| Adverse events | $1,005-$2,658 | 910,925-1,333,119 | 422,194 |
| Recurrence | $47,667-$187,611 | 1,001,267-1,256,166 | 254,899 |
| Relative risk if given chemotherapy | |||
| Low risk | 47%-364% | 460,861-2,724,608 | 2,263,747 |
| Intermediate risk | 26%-160% | 908,140-1,607,536 | 699,396 |
| High risk | 13%-51% | 1,136,871-1,044,620 | 92,251 |
| Chemotherapy before RS | |||
| Low | 39%-61% | 1,674.97-2,177,517.37 | 2,175,842 |
| Intermediate | 42%-70% | 428,929.10-1,844,429.42 | 1,415,500 |
| High | 59%-71% | 1,093,772.50-1,197,278.54 | 103,506 |
| Proportion of patients according to risk group | |||
| Low risk | 42%-64% | 325,001-1,940,500 | 1,615,499 |
| Intermediate risk | 24%-44% | 769,103-1,408,751 | 639,648 |
| High risk | 5%-23% | 1,163,515-1,023,183 | 140,332 |
| Mean age, years | 42.72-67 years | 1,362,982-1,032,230 | 330,753 |
| Time horizon | 30-50 years | 1,171,441-1,103,874 | 67,568 |
| Discount rate | 0.02%-0.05% | 1,081,634-1,131,727 | 50,093 |
Abbreviations: QALY, quality-adjusted life-year; RS, recurrence score.
Table A2.
Distribution for PSA
| Parameter | Base Case Value | PSA |
||||
|---|---|---|---|---|---|---|
| SE | Distribution | 95% CI | Shape (alpha) | Scale (beta) | ||
| Distribution of patients by risk group | ||||||
| Low risk | 52.7% | 0.006 | Dirichlet | 100.0 | 0.005 | |
| Intermediate risk | 34.6% | 0.008 | Dirichlet | 25.0 | 0.014 | |
| High risk | 12.7% | 0.006 | Dirichlet | 6.3 | 0.020 | |
| Relative reduction in risk with chemotherapy | ||||||
| Low risk | 131.0% | 0.533 | LogNormal | 0.46 to 3.72 | ||
| Intermediate risk | 61.0% | 0.476 | LogNormal | 0.24 to 1.55 | ||
| High risk | 26.0% | 0.354 | LogNormal | 0.13 to 0.52 | ||
| Resource cost | ||||||
| Chemotherapy | $7,026 | 1,757 | Gamma | 16.0 | 439.1 | |
| Supportive care | $9,606 | 2,401 | Gamma | 16.0 | 600.3 | |
| Adverse events | $1,761 | 440 | Gamma | 16.0 | 110.0 | |
| Recurrence | $104,000 | 36,400 | Gamma | 8.2 | 12,740.0 | |
| Utility of health states | ||||||
| QALY loss associated with chemotherapy | 0.5 years | 0.50 | LogNormal | 0.19 to 1.33 | ||
| QALY loss associated with recurrence | 9.1 years | 0.20 | LogNormal | 6.15 to 13.47 | ||
| QALY loss associated with second primary cancer caused by chemotherapy | 9.0 years | 0.20 | LogNormal | 6.08 to 13.32 | ||
| Chemotherapy recommendation before RS | ||||||
| Low risk | 50.0% | 0.060 | Normal | 0.382 to 0.618 | ||
| Intermediate risk | 55.0% | 0.070 | Normal | 0.442 to 0.658 | ||
| High risk | 60.0% | 0.030 | Normal | 0.502 to 0.698 | ||
| Other | ||||||
| Mean age | 59 years | 1 | Normal | 57.0 to 61.0 | ||
| Discount rate | 3.0% | Fixed | ||||
| Time horizon | 40 years | Fixed | ||||
Abbreviations: PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life-year; RS, recurrence score.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Katie Krebs, Humana (C); Louis Hochheiser, Humana (C) Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: John Hornberger, Genomic Health; Rebecca Chien, Genomic Health Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: John Hornberger, Rebecca Chien, Louis Hochheiser
Financial support: John Hornberger
Administrative support: John Hornberger, Rebecca Chien, Katie Krebs
Provision of study material or patients: Katie Krebs, Louis Hochheiser
Collection and assembly of data: John Hornberger, Rebecca Chien, Katie Krebs
Data analysis and interpretation: John Hornberger, Rebecca Chien, Katie Krebs, Louis Hochheiser
Manuscript writing: John Hornberger, Rebecca Chien, Louis Hochheiser
Final approval of manuscript: John Hornberger, Rebecca Chien, Katie Krebs, Louis Hochheiser
References
- 1.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER cancer statistics review, 1975-2006: Breast. http://seer.cancer.gov/csr/1975_2006/
- 3.National Comprehensive Cancer Network. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Breast Cancer, V. 1.2010. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. [DOI] [PubMed]
- 4.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 5.Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: An economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109:1011–1018. doi: 10.1002/cncr.22506. [DOI] [PubMed] [Google Scholar]
- 6.Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11:313–324. [PubMed] [Google Scholar]
- 7.Klang SH, Hammerman A, Liebermann N, et al. Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-managed health-care organization. Value Health. 2010;13:381–387. doi: 10.1111/j.1524-4733.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 8.Philips Z, Ginnelly L, Sculpher M, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess. 2004;8:iii–iv. ix–xi, 1–158. doi: 10.3310/hta8360. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: Report of the ISPOR Task Force on Good Research Practices–Modeling Studies. Value Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 10.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 11.Oratz R, Paul D, Cohn AL, et al. Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. J Oncol Pract. 2007;3:182–186. doi: 10.1200/JOP.0742001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360:2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simes RJ, Coates AS. Patient preferences for adjuvant chemotherapy of early breast cancer: How much benefit is needed? J Natl Cancer Inst Monogr. 2001;30:146–152. doi: 10.1093/oxfordjournals.jncimonographs.a003453. [DOI] [PubMed] [Google Scholar]
- 14.Piccart MJ, Di Leo A, Beauduin M, et al. Phase III trial comparing two dose levels of epirubicin combined with cyclophosphamide with cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer. J Clin Oncol. 2001;19:3103–3110. doi: 10.1200/JCO.2001.19.12.3103. [DOI] [PubMed] [Google Scholar]
- 15.Ravdin PM, Siminoff IA, Harvey JA. Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol. 1998;16:515–521. doi: 10.1200/JCO.1998.16.2.515. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 17.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 18.Hornberger J, Chien R. Meta-analysis of the decision impact of the 21-gene breast cancer recurrence score in clinical practice. Presented at the 33rd San Antonio Breast Cancer Symposium; December 8-12, 2010; San Antonio, TX. (abstr 20609) [Google Scholar]
- 19.Asad J, Jacobson AF, Estabrook A, et al. Does Oncotype DX recurrence score affect the management of patients with early stage breast cancer? Am J Surg. 2008;196:527–529. doi: 10.1016/j.amjsurg.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Henry LR, Stojadinovic A, Swain SM, et al. The influence of a gene expression profile on breast cancer decisions. J Surg Oncol. 2009;99:319–323. doi: 10.1002/jso.21244. [DOI] [PubMed] [Google Scholar]
- 21.Thanasoulis T, Brown A, Frazier T. The role of Oncotype DX assay on appropriate treatment for estrogen positive, lymph node negative invasive breast cancer. Presented at the 2008 American Society of Breast Surgeons Annual Meeting; New York, NY. 2008. [Google Scholar]
- 22.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of intergroup trial C9741/cancer and leukemia group B trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 23.Jones S, Holmes FA, O'Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 24.Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: Results from NSABP B-28. J Clin Oncol. 2005;23:3686–3696. doi: 10.1200/JCO.2005.10.517. [DOI] [PubMed] [Google Scholar]
- 25.Brennan V, Wolowacz S. A systematic review of breast cancer utility weights. Presented at the 13th Annual International Meeting of ISPOR; May 3-7, 2009; Toronto, Ontario, Canada. (abstr PCN77) [Google Scholar]
- 26.Lindley C, Vasa S, Sawyer WT, et al. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol. 1998;16:1380–1387. doi: 10.1200/JCO.1998.16.4.1380. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann C, Baldo C, Molino A. Framing of outcome and probability of recurrence: Breast cancer patients' choice of adjuvant chemotherapy (ACT) in hypothetical patient scenarios. Breast Cancer Res Treat. 2000;60:9–14. doi: 10.1023/a:1006342316373. [DOI] [PubMed] [Google Scholar]
- 28.Cole BF, Gelber RD, Gelber S, et al. Polychemotherapy for early breast cancer: An overview of the randomised clinical trials with quality-adjusted survival analysis. Lancet. 2001;358:277–286. doi: 10.1016/S0140-6736(01)05483-6. [DOI] [PubMed] [Google Scholar]
- 29.Briggs A, Claxton K, Sculpher M. New York, NY: Oxford University Press; 2006. Decision Modelling for Health Economic Evaluation. [Google Scholar]
- 30.Schaapveld M, Visser O, Louwman M, et al. Risk of new primary nonbreast cancers after breast cancer treatment: A Dutch population-based study. J Clin Oncol. 2008;26:1239–1246. doi: 10.1200/JCO.2007.11.9081. [DOI] [PubMed] [Google Scholar]
- 31.Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100:888–897. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]