Abstract
Background
Results of international clinical trials assessing when to initiate antiretroviral therapy (ART) will not be available for several years.
Objective
To inform HIV treatment decisions over the short- and long-term regarding the optimal CD4 threshold at which to initiate ART in South Africa, while awaiting “when to start” trial results.
Design
Cost-effectiveness analysis using a computer simulation model of HIV disease.
Data Sources
Published data from randomized trials and observational cohorts in South Africa.
Target Population
HIV-infected patients in South Africa.
Time Horizon
Five-year and lifetime.
Perspective
Modified societal.
Interventions
No treatment, initiate ART at CD4<250/μl, and initiate ART at CD4<350/μl.
Outcome Measures
Morbidity, mortality, life expectancy, medical costs, and cost-effectiveness.
Results of Base-Case Analysis
If 10-100% of HIV-infected patients are diagnosed and linked to care, initiating ART at CD4<350/μl would reduce severe opportunistic diseases by 22,000-221,000 and deaths by 25,000-253,000 during the next 5 years, compared to initiating ART at CD4<250/μl; cost increases would range from $142 million (10%) to $1.4 billion (100%). Either ART strategy increased long-term survival by at least 7.9 years, with a mean per person life expectancy of 3.8 years for no ART and 12.5 years for ART at <350/μl. Compared to initiating ART at <250/μl, initiating ART at <350/μl had an incremental cost-effectiveness ratio of $1,200/year of life saved.
Results of Sensitivity Analysis
Initiating ART at CD4<350/μl remained cost-effective over the next 5 years even if the probability that the trial would demonstrate superiority to earlier therapy is as low as 17%.
Limitations
This model does not consider the possible benefits of ART initiation at CD4>350/μl nor reduced HIV transmission.
Conclusions
Earlier ART initiation in South Africa will likely reduce morbidity and mortality, improve long-term survival, and be very cost-effective. While awaiting trial results, treatment guidelines should be liberalized to allow for earlier ART initiation (CD4<350/μl) than is currently recommended.
Keywords: Resource-limited setting, antiretroviral therapy, ART, cost-effectiveness, HIV
Introduction
Recent data from cohort studies and mathematical models in the developed world suggest that treatment outcomes of HIV-infected patients improve when antiretroviral therapy (ART) is initiated at CD4 thresholds of 350/μl, and perhaps even 500/μl (1-4). In resource-limited settings, the question of “when to start” antiretroviral therapy in HIV-infected patients is even more critical in the context of higher rates of mortality and opportunistic diseases — including tuberculosis and other severe bacterial infections — at CD4 counts >200/μl (5). At CD4 counts between 200-350/μl, rates of such opportunistic disease in South Africa may be 10-fold higher than those seen in the United States (5, 6). Several international clinical trials, including one in South Africa, are currently enrolling patients. These trials will explicitly address the clinical benefits of earlier antiretroviral therapy (ART) initiation (CD4 <350/μl or CD4 <500/μl) compared to the current World Health Organization (WHO) standard of care (stage 3 or 4 disease or when CD4 counts falls below 200/μl) (7-9).
While clinical trials may provide insight into the optimal timing of ART in resource-limited settings, trials can only address short-term outcomes and will not be available to inform practice for at least several years (8, 9). Our objective was to inform crucial decisions now, until these trials are reported, using a model-based analysis to examine treatment strategies with different timing of antiretroviral therapy initiation in South Africa.
Methods
Analytic Overview
Treatment Strategies
Using a computer-based model of HIV disease, we examined the policy decision regarding when to initiate antiretroviral therapy in South Africa. We considered three interim treatment strategies, while awaiting results of the “when to start” trials: 1) no treatment (for comparison purposes); 2) start ART at CD4 <250/μl (or severe opportunistic disease) (7); and 3) start ART at CD4 <350/μl (or severe opportunistic disease). Co-trimoxazole prophylaxis initiated at CD4 <500/μl was incorporated in all strategies, in accordance with WHO recommendations (10). We examined the impact of this decision over both short-term (5-year) and lifetime horizons and emphasize that all of these strategies would involve acting optimally on the results of the trial, once they are available in 5 years.
To report on cost-effectiveness, we adopted a modified societal perspective, only considering HIV-associated direct medical resource utilization. All costs were reported in 2006 US dollars using country-specific gross domestic product (GDP) deflators and the 2006 mean exchange rate between the South African Rand and the US dollar (6.8 Rand/1US$) (11, 12). All costs and life expectancies were discounted at 3% per year (13). WHO guidelines designate health interventions as “cost-effective” if the cost per quality adjusted life year (QALY) is less than three times the country's per capita GDP, and “very cost-effective” if the cost per QALY is less than the country's per capita GDP (14, 15). Although our analysis computes cost-effectiveness ratios in terms of years of life saved (rather than QALYs), these thresholds provide general guidance. As a reference point, we compared the results to South Africa's 2006 per capita GDP (US$5,400) (11).
Projections over the next five years
We first examined the “when to start” policy over the next five years to inform decisions regarding whether it would be best to consider therapy at CD4 <350/μl rather than CD4 <250/μl while waiting five years for clinical trial results. We did this in two steps. First, we projected the number of South African patients requiring ART over the five-year time horizon, and their anticipated short-term clinical outcomes (defined as deaths and opportunistic diseases) and costs under alternative ART initiation scenarios. To do so, we used model-based methods, similar to those previously described (16), to examine over the 5-year time horizon, how many HIV-infected people in South Africa would be eligible for an ART at <350/μl vs. <250/μl treatment decision. This estimate assumes steady HIV incidence over the next five years, and accounts for HIV- and non-HIV-related deaths prior to the <350/μl CD4 threshold.
Next, combining data from the WHO and the President's Emergency Plan for AIDS Relief (PEPFAR), we estimated the fraction of HIV-infected people diagnosed and linked to care in South Africa (Technical Appendix Table 2) (17, 18). We provided the estimated impact if: 10% of patients are linked to care (the fraction estimated to be on ART); 30% are linked to care (the estimate of those receiving either ART or other general PEPFAR services); and 100% are linked to care (as an upper bound).
Finally, we assumed that a clinical trial will provide perfect information in 5 years about whether ART initiation at <350/μl is more efficacious compared to the current standard of care and developed a decision criterion under which it would be cost-effective (<3 times the GDP) to invoke a CD4 <350/μl initiation policy now while awaiting clinical trial results. This criterion included a threshold value for the probability that the trials will demonstrate the superiority of starting ART at CD4<350/μl.
Developing a decision criterion for whether to start ART at <350/μl now
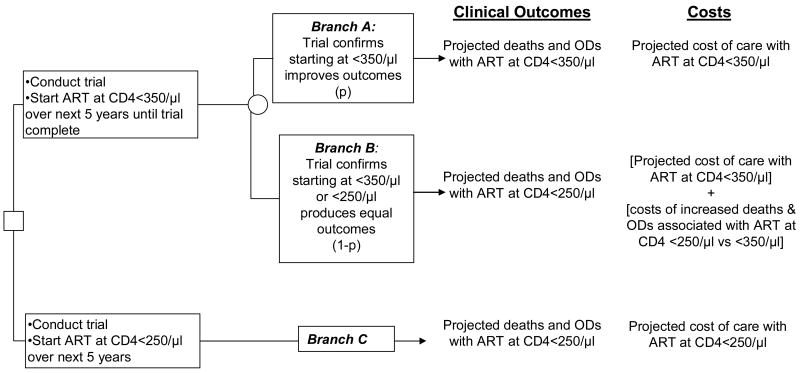
To develop a decision criterion for earlier ART initiation now, we examined two potential policy scenarios (ART at <350/μl vs. <250/μl) over the next five years and their associated clinical and economic outcomes (Figure 1). These outcomes excluded any long-term benefits, detriments, or costs potentially associated with either decision beyond the 5-year horizon. While they included ART-related toxicities, the calculated outcomes also excluded any excess toxicity that might be associated with earlier ART beyond the 5-year horizon. If the decision was to start at CD4 <350/μl, there is a probability, “p”, that the trial will demonstrate a benefit to initiation at CD4 <350/μl in 5 years (Branch A) and a probability, (1-p), that initiation at CD4 <350/μl will produce equivalent outcomes to starting at CD4 <250/μl (Branch B). In the latter case, the associated costs of ART at CD4 <350/μl not only include those of earlier initiation but also the HIV medical costs accrued due to the additional deaths ($536 each) and opportunistic diseases (ranging from $105-1,006 each) anticipated in the ART at <250/μl strategy compared to those anticipated under the strategy described in Branch A (19). If the decision was to initiate ART at CD4 <250/μl over the next five years (Branch C), clinical outcomes and costs would be those derived for the short-term ART at <250/μl strategy. Averaging out the simple tree in Figure 1, we created the decision rule under which it would be economically efficient to start at CD4 <350/μl now. We defined this decision rule examining alternative values for p and employing the cost-effectiveness willingness-to-pay threshold of 3 × GDP ($16,200/YLS).
Figure 1.
Decision tree outlining the ART strategy options over the next five years while awaiting “when to start” trial results. The payoffs in terms of both clinical outcomes and costs are delineated to the right of the tree. The probability, “p”, represents the chance that the trial will demonstrate a clinical benefit to ART at <350/μl. Using a cost-effectiveness willingness-to-pay threshold of 3 × the per capita GDP in South Africa ($16,200/YLS), the tree suggests an optimal policy of ART at <350/μl now for values of p such that:
As described in the Results section, values of p ≥ 0.17 satisfy this decision rule.
Lifetime projections
After projecting five-year outcomes, we then projected the per-person life expectancy and mean lifetime HIV treatment costs for patients starting ART at CD4 <350/μl compared to those starting at CD4 <250/μl. We used these outcomes to produce incremental cost-effectiveness ratios; sensitivity analyses were used to examine the impact of key input parameters on the cost-effectiveness results.
CEPAC-International Model
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC) International model is a state-transition model of HIV disease in resource-limited settings, with data derived for several country-specific analyses, including South Africa (16, 20, 21). Briefly, a cohort of hypothetical patients pass one at a time through “health states,” in monthly cycles, from entry into HIV care until death. Health states are defined to be both clinically and economically relevant and are stratified by current CD4 count, current HIV RNA level, and history of opportunistic disease. Opportunistic diseases are categorized into the following groups based on etiology, severity, and similarities in prophylaxis and treatment: mild or severe bacterial infections, mild or severe fungal infections, tuberculosis, toxoplasmosis, non-tuberculous mycobacteriosis, Pneumocystis jiroveci pneumonia, and other mild and severe diseases (5). Deaths in the model occur from acute opportunistic events (within 30 days of the event), chronic AIDS (not within 30 days of an opportunistic disease), or non-HIV-related causes (22).
Effective ART in the model functions to suppress HIV RNA and increase CD4 counts (23, 24). Above and beyond the beneficial effect of increased CD4 count on opportunistic diseases and chronic HIV-related death (5), antiretroviral therapy per se results in an additional reduction in opportunistic diseases and chronic HIV-related death, as recently reported in Côte d'Ivoire and in the US (25, 26). Clinical assessments are assumed to occur every 3 months, and CD4 and HIV RNA testing every 6 months while on therapy, consistent with South African recommendations (27). According to current standard of care, the model utilizes two sequential lines of antiretroviral therapy; the second-line is initiated when observed CD4 count decreases by 30% from its peak observed on-treatment level, or when a severe opportunistic disease is observed at least 6 months after initiating therapy (27). In accordance with current treatment guidelines, the second regimen for each patient is continued until death (7, 28).
Input Parameters
Description of trial-eligible patients for short-term projections
For the short-term projections, we developed a hypothetical cohort of HIV-infected patients with the appropriate clinical attributes (Table 1). We defined both a prevalent HIV-infected cohort -- to indicate those currently infected -- and an incident HIV-infected cohort – to indicate those who will become infected and trial-eligible during the 5-year horizon (see Technical Appendix). We used methods previously described to estimate the characteristics (CD4 count and viral load distribution) of the prevalent cohort in South Africa that might be eligible now if ART were offered to patients with a CD4 count <350/μl (16). For the prevalent cohort, the mean CD4 count is 321/μl (SD 146/μl); 21% of patients in this cohort are eligible for the treatment decision of ART initiation at <350/μl versus <250/μl. We also used projections from the Actuarial Society of South Africa (ASSA) to forecast the number of incident HIV infections anticipated over the 5-year trial horizon (29). The incident cohort has a mean CD4 count of 534/μl (SD 164/μl). In the first year after incident infection, 15% of patients in this cohort are eligible for the treatment decision of ART initiation at <350/μl versus <250/μl.
Table 1. Model input parameters.
| Variable | Base-Case Value | References |
|---|---|---|
| Baseline cohort characteristics | ||
| Mean Age (years) | 32.8, SD=9 | (5) |
| Gender distribution (% male) | 55 | (5) |
| Mean CD4 count (cells/μl) | ||
| 5-year projections (prevalent cohort) | 321, SD=146 | (16) |
| 5-year projections (incident cohort) | 534, SD=164 | (16) |
| Long-term projections | 375, SD=10 | Assumption |
| HIV RNA distribution (%) | (28) | |
| >100,000 copies/ml | 42.5 | |
| 30,001-100,000 copies/ml | 28.3 | |
| 10,001-30,000 copies/ml | 17.9 | |
| 3,001-10,000 copies/ml | 7.8 | |
| 501-3,000 copies/ml | 2.3 | |
| ≤ 500 copies/ml | 1.2 | |
| Natural history of disease | ||
| Monthly CD4 decline (cells/μl) | (30) | |
| >30,000 copies/ml | 6.4 | |
| 10,001-30,000 copies/ml | 5.4 | |
| 3,001-10,000 copies/ml | 4.6 | |
| 501-3,000 copies/ml | 3.7 | |
| ≤500 copies/ml | 3.0 | |
| Monthly risk of severe opportunistic diseases (%)* (Range by CD4) | (5) | |
| Bacterial | 0.08-0.71 | |
| Fungal | 0.02-2.22 | |
| Tuberculosis | 0.21-1.96 | |
| Toxoplasmosis | 0.00-0.06 | |
| Non-tuberculous mycobacteriosis | 0.00-0.30 | |
| Pneumocystis jiroveci pneumonia | 0.00-0.12 | |
| Other WHO stage 4 defining diseases | 0.25-2.57 | |
| Monthly risk of mild opportunistic diseases (%) (Range by CD4) | (5) | |
| Fungal | 0.59-3.51 | |
| Other | 2.51-3.11 | |
| Monthly risk of HIV-related death (%) (Range by CD4) | (5) | |
| With no OD history | 0.09-3.33 | |
| With OD history | 0.29-7.94 | |
| Efficacy of antiretroviral therapy (HIV RNA suppression rate at 48 weeks) | ||
| First-line therapy† | 84% | (23) |
| Second-line therapy† | 71% | (24) |
| Efficacy of cotrimoxazole (% reduction in probability of occurrence) | ||
| Bacterial, severe | 49.8 | (31, 32) |
| Toxoplasmosis | 83.3 | (31, 32) |
| Pneumocystis jiroveci pneumonia | 97.3 | (20) |
| Costs (2006 US$) | ||
| First-line ART, monthly | 24 | (36) |
| Second-line ART, monthly | 47 | (36) |
| Cotrimoxazole, monthly | 1 | (34) |
| Routine care (range by CD4 count), monthly | 10-129 | (5, 19, 33) |
| Inpatient hospital care, per day | 221 | (19) |
| Outpatient clinic care, per visit | 11 | (19) |
| CD4 count, per test | 10 | (35) |
| HIV RNA, per test | 49 | (35) |
SD: standard deviation, ART: antiretroviral therapy, OD: opportunistic disease
Risk of opportunistic disease varies by CD4 stratum, divided into <50/μl, 51-200/μl, 201-350/μl, 351-500/μl, >500/μl
First-line ART: non-nucleoside reverse transcriptase inhibitor-based regimen; Second-line ART: protease-inhibitor based regimen
Since the baseline characteristics (CD4 and HIV RNA distributions) of patients in the prevalent cohort and the incident cohorts differ, survival data were derived separately for each cohort. Using the CEPAC International model, we initialized the prevalent and incident cohorts to create a composite picture of the CD4 and HIV RNA distribution of each cohort, given their duration of infection (16). We projected the survival for HIV-infected individuals in the absence of ART (for the no ART comparison), and with ART starting at <350/μl or at <250/μl (16). Annual probabilities of survival (conditional on survival to the beginning of the year) were calculated by dividing the number of HIV-infected individuals alive at the end of a given calendar year by the number alive at the end of the previous year. This was done to reflect a patient's probability of surviving through the year, given that the patient was alive at the beginning of the year.
Cohort characteristics for the lifetime projections
For the long-term projections, the simulated cohort was designed to resemble the characteristics of HIV-infected people in South Africa. Clinical and demographic characteristics were based on data from the Cape Town AIDS Cohort (CTAC) (5). In the absence of specific data from South Africa, rates of CD4 count decline, stratified by baseline HIV RNA level, were from the Multicenter AIDS Cohort Study (MACS) in the US (Table 1) (30). Patients entering the model were assumed to be initiating HIV care with a mean age of 32.8 years. For the long term projections, we used a mean baseline CD4 count of 375/μl to simulate enrollment criteria for the “when to start” trials; 42.5% of patients had baseline HIV RNA >100,000 copies/ml (Table 1).
Opportunistic disease prophylaxis and efficacy of antiretroviral therapy
In the absence of reported data from South Africa, the efficacy of co-trimoxazole prophylaxis in the model was derived from clinical trials in Côte d'Ivoire (31, 32). In Côte d'Ivoire, cotrimoxazole confers protection against bacterial infections, Pneumocystis jiroveci pneumonia, isospora and malaria, and toxoplasmosis; isospora and malaria are not reported in the South African data (Table 1) (20). Two sequential antiretroviral regimens were assumed available. First-line was a non-nucleoside reverse transcriptase inhibitor-based regimen with a reported 84% of patients experiencing HIV RNA suppression at 48 weeks (mean CD4 count increase of 184/μl, IQR 108-271/μl) (23). Patients who failed the first-line regimen received a protease inhibitor-based second-line regimen. In this regimen, we incorporated the need for “recycled” nucleoside reverse transcriptase inhibitors, with a published estimate of 71% of patients experiencing HIV RNA suppression at 48 weeks (mean CD4 count increase of 151/μl, IQR 105-239/μl) (24).
Costs
The analysis considered HIV-associated direct medical resource utilization, including inpatient days, outpatient visits, laboratory tests and medication costs (19, 33-37). Direct non-medical costs and indirect costs (i.e. patient time and lost wages) were excluded. Healthcare utilization was derived from CTAC using a utilization analysis and unit costing approach (5, 19, 33). Costs were derived according to the number of inpatient hospital days and outpatient clinic visits associated with each month of routine HIV care in the absence of opportunistic disease, with each type of opportunistic disease, and during the month of death.
Role of the Funding Sources
The funding sources had no input in study design, analysis and interpretation of data, the writing of the report, or the decision to submit for publication. The corresponding author has full access to all the data in the study and holds final responsibility for the decision to submit the paper for publication.
Results
Outcomes projected over a five-year horizon
Over a five-year time horizon, we estimated that 4.7 million HIV-infected people in South Africa will become eligible to start ART in the CD4 250-350/μl treatment window. Among such individuals, 1.2 million are eligible now, 1.6 million will be eligible over the next year, and 1.9 million will become eligible over the ensuing three years.
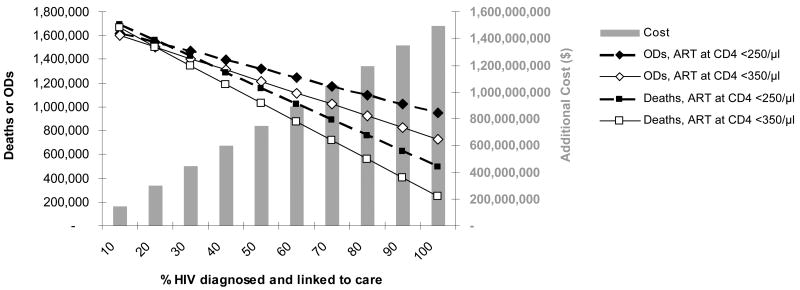
Assuming that 10%, 30% and 100% of the 4.7 million people are identified and linked to care, we projected the opportunistic diseases, deaths, and costs over the next five years of alternative ART strategies while awaiting results of the “when to start” trials (Table 2). At the conservative HIV diagnosis and linkage to care estimate of 10%, initiation of ART at <350/μl compared to starting at <250/μl would result in fewer total opportunistic diseases (1,599,900 vs. 1,622,000) and fewer total deaths (1,664,500 vs. 1,689,700). ART at CD4<350/μl would also lead to a discounted $142 million cost increase over the next five years, reflecting the additional treatment costs, which are offset in part by the reduced incidence of opportunistic diseases. At the maximum (100% diagnosis and linkage to care), 221,000 opportunistic diseases and 253,000 deaths could be averted. In this situation, additional costs of the ART at CD4<350/μl strategy would exceed $1.4 billion. Figure 2 provides results for the clinical and economic impact if between 10% and 100% of HIV-infected, eligible patients are identified and present for care. Clinical and cost results move together; the fewer patients identified, the fewer deaths and opportunistic diseases averted, and the lower the added total costs of an earlier ART strategy.
Table 2. Clinical and economic outcomes over the next five years from starting ART at <350/μl or at <250/μl in South Africa while awaiting trial results.
| Strategy | Total Opportunistic Diseases | Total Deaths | Discounted Total Costs ($) |
|---|---|---|---|
| At 10% HIV case identification and linkage to care | |||
|
| |||
| ART at CD4 <350/μl or OD | 1,599,859 | 1,664,458 | 9,974,640,200 |
| ART at CD4 <250/μl or OD | 1,621,969 | 1,689,739 | 9,832,663,100 |
|
| |||
| Difference (<350/μl minus <250/μl) | (22,110) | (25,281) | 141,977,100 |
|
| |||
| At 30% HIV case identification and linkage to care | |||
|
| |||
| ART at CD4 <350/μl or OD | 1,406,618 | 1,348,856 | 10,436,784,100 |
| ART at CD4 <250/μl or OD | 1,472,947 | 1,424,699 | 10,010,852,800 |
|
| |||
| Difference (<350/μl minus <250/μl) | (66,329) | (75,843) | 425,931,300 |
|
| |||
| At 100% HIV case identification and linkage to care | |||
|
| |||
| ART at CD4 <350/μl or OD | 730,272 | 244,249 | 12,054,287,800 |
| ART at CD4 <250/μl or OD | 951,370 | 497,059 | 10,634,516,900 |
|
| |||
| Difference (<350/μl minus <250/μl) | (221,097) | (252,810) | 1,419,770,900 |
OD: opportunistic disease
( ): denotes fewer ODs and deaths with ART at <350/μl
Figure 2.
Model-based projections over the next five years, under an ART at <350/μl (in white) and an ART at <250/μl (in black) initiation strategy. Total deaths are indicated by squares and total opportunistic diseases by diamonds for the two strategies (left vertical axis). The excess total costs of ART at <350/μl compared to ART at <250/μl over a 5-year horizon are indicated by bars (right vertical axis). The x-axis represents results at varying proportions of HIV cases identified and linked to care in the population. (OD: opportunistic disease)
The decision criterion for whether to start ART at CD4 <350/μl now
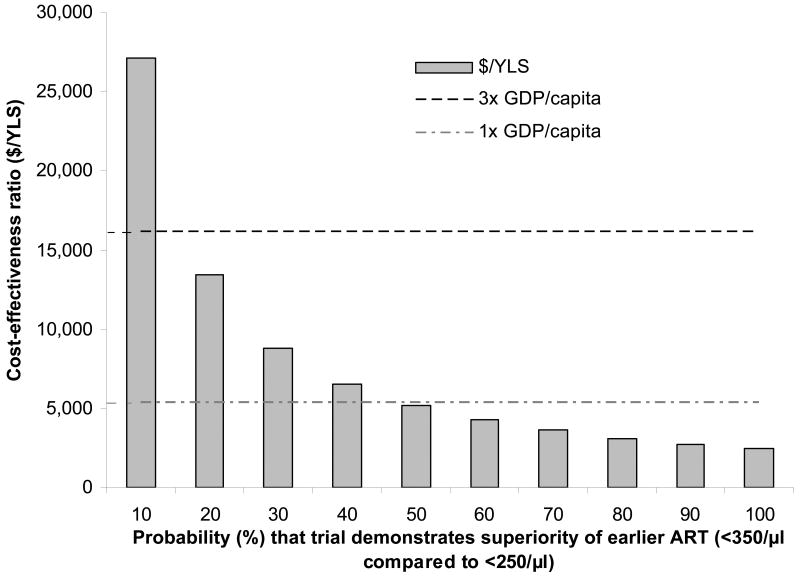
We then examined the probability that the data from the “when to start” trials would provide enough statistical evidence to state that ART at <350/μl is superior to ART at <250/μl – confirming the model-based results. If this was certain (100% probability), the incremental cost-effectiveness of ART at <350/μl compared to ART at CD4 <250/μl was $2,400/YLS, considering only the costs and benefits over the next five years. Should the probability that the trials show a benefit to ART at <350/μl decrease to 10%, the incremental cost-effectiveness over the next five years increased to $27,100/YLS. Using the established WHO cost-effectiveness guideline (that the cost per YLS is below the 3× GDP threshold), a policy option to initiate ART at CD4 <350/μl should be used over the next five years as long as the probability that the trial will confirm model-based results is greater than or equal to 17% (Figure 3). In sensitivity analyses, we varied the composite increase in deaths associated with ART at CD4 <250/μl compared to ART at CD4 <350/μl from 2-fold more deaths (base case) to 1.5 fold more deaths. The decreased benefits of ART at <350/μl can simulate situations either where earlier therapy is less effective on an individual level than the model projects or where there is lower linkage to care in the ART at <350/μl compared to the ART at <250/μl due to the absence of clinical trial data. Under such a scenario, a policy option to initiate ART at CD4 <350/μl should be used over the next five years, as long as the probability that the trial will confirm model-based results is greater than or equal to 28% (Technical Appendix, Figure 1).
Figure 3.
The incremental cost-effectiveness of ART at <350/μl vs. ART at <250/μl at alternative values of “p”, the probability that the trial will confirm model-based results indicating a benefit for earlier therapy (see Methods and Figure 1). The incremental cost-effectiveness is provided for the 5-year time horizon and reported in dollars per year of life saved. (YLS: years of life saved, GDP: per capita gross domestic product in South Africa (US$5,400)). The height of the bar provides the cost-effectiveness ratio of ART at <350/μl vs. ART at <250/μl for alternative values of p; bars that remain below the horizontal dashed line (<3× GDP) are considered to be “cost-effective” and those that remain below the horizontal dotted-dashed line (<1× GDP) are considred to be “very cost-effective.”
Lifetime projections
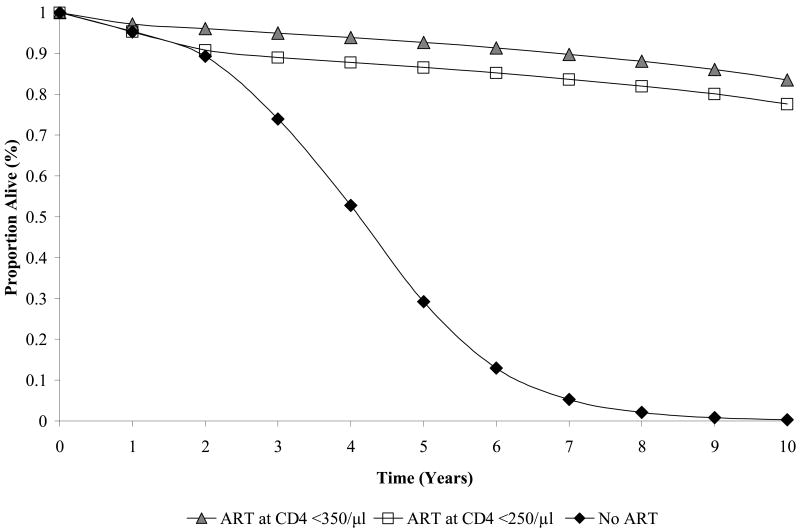
When long-term outcomes were projected for a cohort with a mean CD4 count of 375/μl, the no treatment strategy resulted in a mean survival of 3.83 years (4.11 undiscounted), compared to 11.71 years (15.23 undiscounted) with ART at CD4 <250/μl, and 12.48 years (16.27 undiscounted) with ART at CD4 <350/μl (Table 3). The survival curves corresponding to the ART at CD4 <350/μl and <250/μl strategies diverged within about one year, after which time they became essentially parallel, when nearly all patients in the <250/μl strategy have initiated ART; by year 3, therapy initiation at <350/μl maintained a consistent 6% absolute advantage in the proportion of the cohort alive through year 10 (Figure 4). Per-person lifetime direct costs were lowest with no ART ($3,930, Table 3). Lifetime costs increased to $12,730 per person with initiation of antiretroviral therapy at <250/μl and $13,620 with ART at <350/μl. The incremental cost-effectiveness ratio was $1,100 per year of life saved (YLS) for ART at <250/μl compared to no treatment and $1,200/YLS for ART at <350/μl compared to <250/μl (Table 3).
Table 3. Life expectancy, cost, and cost-effectiveness of strategies for HIV care in South Africa.
| Strategy | Discounted Per Person Lifetime Cost ($) | Discounted (undiscounted) Per Person Life Expectancy (Years) | Incremental Cost-Effectiveness Ratio ($/YLS)† |
|---|---|---|---|
| Base-Case | |||
| No Treatment | 3,930 | 3.83 (4.11) | — |
| ART at <250/μl or OD | 12,730 | 11.71 (15.23) | 1,100 |
| ART <350/μl or OD | 13,620 | 12.48 (16.27) | 1,200 |
ART: antiretroviral therapy; TB: tuberculosis; YLS: year of life saved; OD: opportunistic disease
Years and costs are discounted at 3% per year.
Incremental cost-effectiveness ratios are calculated compared to the next less costly strategy. Due to rounding, the incremental cost-effectiveness ratios may not match exactly the ratios of lifetime costs and projected survival reported in the table.
Figure 4.
Model-generated survival curves for ART starting at <350/μl, <250/μl, or no ART (co-trimoxazole alone). The annual mortality hazard two years after entry into care was 0.01 for ART at <350/μl, 0.05 for ART at CD4 <250/μl, and 0.06 with no ART. Two years after entry into care, the composite annual hazard of severe opportunistic disease, tuberculosis, or death was 0.06 for ART at <350/μl, 0.16 for ART at CD4 <250/μl, and 0.17 with no ART (data not shown).
Sensitivity analyses on lifetime projections
Because the long-term results consistently favored ART initiation at <350/μl, we designed the sensitivity analyses to bias against earlier initiation. Specifically, we examined large decrements in second-line antiretroviral efficacy in the earlier therapy strategies. Second-line efficacy would have to be <39% suppressed at 48 weeks – a 32% relative decrease from the base-case – to match the projected survival with ART at <250/μl. To examine the effect of pill fatigue and failed retention in care (38, 39), we also considered higher rates of discontinuation of care. We assumed that some patients who started ART at <350/μl discontinued antiretroviral therapy at the time they would have switched to second-line regimens, thereby realizing only the benefits of first-line therapy (though they still received prophylaxis and treatment for opportunistic diseases). Treatment discontinuation would need to occur in greater than 19% of patients receiving ART at <350/μl at the time of failure to decrease survival to that associated with ART at <250/μl. Finally, when we included a third-line antiretroviral regimen, which may become available in the future, survival and costs increased in both ART treatment strategies. The incremental cost-effectiveness of ART at <350/μl compared to at <250/μl was largely unchanged ($1,000/YLS). Detailed results of each of these analyses, and many others, are provided in the technical appendix (TA Table 4).
Discussion
While “when to start” trials in South Africa and other resource-limited settings will yield important information in the upcoming 5 to 10 years, this analysis suggests that, until trial data are available, starting ART at <350/μl would likely yield better clinical outcomes than starting later, and the magnitude of such benefits multiply with increased rates of HIV diagnosis and linkage to care. ART at <350/μl is also expected to be highly cost-effective in the interim. Many of the clinical benefits of starting earlier occur beyond the five-year time horizon of the trial (manifested in increased life expectancy). Even so, our results suggest it remains cost-effective over a five-year horizon to start ART at <350/μl, as long as the trial is anticipated to demonstrate, with a probability greater than or equal to 17%, improved clinical outcomes with starting ART earlier.
When initiated according to current treatment guidelines, we find that antiretroviral therapy for HIV infection in South Africa at <250/μl is very cost-effective in the long term with a ratio of $1,100/YLS (7); for therapy initiation at <350/μl, the cost-effectiveness ratio is $1,200/YLS. That these ratios are similar suggests that if HIV treatment is worth initiating, early initiation provides comparable value to later treatment. We specifically designed sensitivity analyses to see how these results might change and found that very high rates of drug resistance and pill fatigue would be required to make earlier therapy not cost-effective. Because the results depend heavily on the frequency of opportunistic diseases at higher CD4 counts, morbidity rates should be assessed carefully at high CD4 counts, because initiation of therapy even earlier than CD4 <350/μl in South Africa may be justified.
Conducting the current trials remains critically important in informing the “when to start” question. Evidence-based guidelines continue to maintain that randomized controlled trials are the “gold standard” for developing policy; modeling analyses are still considered lower levels of evidence (40, 41). As such, randomized trials will likely be used as the benchmark evidence for HIV treatment throughout the world. In the meantime, this model-based analysis suggests that opening up the option to start ART earlier in the disease course would very likely improve clinical outcomes, at least until trial results are available. Our results suggest that there may be 25,000 lives at stake; waiting 5 years to get trial results would likely be extremely costly in human terms.
Despite findings that ART initiation at CD4<350/μl may be beneficial, one study on patient characteristics at presentation to care in South Africa suggests that a discussion of earlier versus deferred ART initiation may not be germane at present; in that study, the mean CD4 count of patients starting antiretroviral therapy was only 96/μl (42). However, as the WHO's Guidelines for Using HIV Testing Technologies in Surveillance (43) become more widely implemented, and HIV screening and linkage to care improves, an increasing number of patients will likely be identified who are eligible for earlier therapy initiation (7). Decisions need to be made as to how to best optimize their care, and efforts to identify them must continue, if a policy of earlier therapy is to have a meaningful impact. This analysis demonstrates that treatment at <350/μl is highly effective and confers similar value to treatment at <250/μl.
There are instances, however, when a policy of therapy at <350/μl may not be optimal, specifically if treatment capacity is limited, as is currently the case in many places (16). In such settings, prioritization is already problematic–whether antiretroviral therapy should be provided on a first-come first-served basis or on a CD4-based policy (44). With inadequate treatment capacity, a change to a policy of treatment for all patients with CD4 <350/μl, without prioritization for the sickest patients, could result in more deaths in the near term, not fewer, even if earlier therapy is associated with long-term benefits. Thus, guidelines that move towards antiretroviral therapy initiation at higher CD4 counts should only be implemented if there is adequate capacity to treat all of those eligible and at highest risk.
The results of this analysis should be interpreted within the context of several limitations. First, this analysis does not represent an assessment of the estimated value of perfect information (EVPI), which would examine whether the trial is “worth doing.” Rather, with trials already enrolling, we address the question of the optimal clinical strategy while awaiting results of those trials. Second, input data are incorporated from multiple sources. While uniformly derived, not all data are from similar cohorts in South Africa. Sensitivity analyses demonstrate that within reasonable reported ranges, the major conclusions are robust to these data estimates. Third, in international settings where CD4 testing is not universally available, implementation of ART strategies by CD4 threshold may require investments in infrastructure. This model also does not account for the potential benefits of antiretroviral therapy, unrelated to opportunistic diseases, that might be attributable to treatment at CD4 thresholds higher than the current standard of care (45). We also do not capture any additional benefits in preventing HIV transmission due to viral load reduction that earlier ART may confer (46). To the extent that these benefits occur, earlier therapy would be even more advantageous. Finally, in many African countries, earlier therapy may have other additional benefits because of higher rates of malaria and bacterial diseases than those documented in South Africa (5, 31).
As ongoing “when to start” randomized trials in resource-limited settings continue to enroll and accrue follow-up toward the primary outcomes, decisions must be made now regarding the optimal ART initiation policy in these settings. While awaiting trial results in settings of adequate treatment capacity, this study demonstrates that it is likely both effective, and very cost-effective, to liberalize the opportunity for antiretroviral therapy to be initiated at CD4 counts <350/μl in South Africa.
Supplementary Material
Acknowledgments
We are indebted to the entire CEPAC-International team and investigators for their contributions, including Eugène Messou, Catherine Seyler, and Siaka Touré (Programme PACCI, Abidjan, Côte d'Ivoire); Yazdan Yazdanpanah (Service Universitaire des Maladies Infectieuses et du Voyageur, Centre Hospitalier de Tourcoing, EA 2694, Faculté de Médecine de Lille, and Laboratoire de Recherches Économiques et Sociales, Centre National de la Recherche Scientifique Unité de Recherche Associée 362, Lille, France); Nagalingeswaran Kumarasamy, J. and A. K. Ganesh (Y.R. Gaitonde Centre for AIDS Research & Education, Chennai, India); Glenda Gray, James McIntyre, and Lerato Mohapi (Perinatal HIV Research Unit, WITS Health Consortium, Johannesburg, South Africa); Kara Cotich, Sue Goldie, C. Robert Horsburgh, April Kimmel, Marc Lipsitch, Alethea McCormick, Chara Rydzak, George R. Seage III, and Hong Zhang (Harvard School of Public Health, Boston, MA, USA); Heather E. Hsu (University of Pittsburgh School of Medicine, Pittsburgh, PA, USA); Ingrid V. Bassett, Melissa A. Bender, Sarah Chung, Andrea Ciaranello, Benjamin P Linas, Zhigang Lu, Brandon Morris, Anjali Saxena, Caroline Sloan, Lauren Uhler, and Bingxia Wang (Massachusetts General Hospital, Boston, MA, USA).
We are also indebted to the CEPAC-International Scientific Advisory Board, including Richard Chaisson (Johns Hopkins University, Baltimore, MD, USA); Victor De Gruttola (Harvard School of Public Health, Boston, MA, USA); Joseph Eron (University of North Carolina, Chapel Hill, NC, USA); R.R. Gangakhedkar (National AIDS Research Institute, Pune, India); Jonathan Kaplan (Centers for Disease Control and Prevention, Atlanta, GA, USA); Salim Karim (University of KwaZulu Natal, Durban, South Africa); Thérèse N'Dri Yoman (University of Cocody-Abidjan, Abidjan, Côte d'Ivoire); Douglas Owens (Stanford University, Palo Alto, CA, USA); and John Wong (Tufts Medical Center, Boston, MA, USA).
We very much appreciate the helpful insights offered by Daniel R. Kuritzkes, MD and Paul E. Sax, MD. We would also like to thank Bethany Morris for technical assistance.
This research was funded by the National Institute of Allergy and Infectious Diseases (R01 AI058736, K24 AI062476, P30 AI060354 and U01 AI068634), and the Doris Duke Charitable Foundation (Clinical Scientist Development Award).
Literature Cited
- 1.Sterne Jonathan, et al. When should HIV-1-infected persons initiate ART? Collaborative analysis of HIV cohort studies [abstract 72LB]. 16th Conference on Retroviruses and Opportunistic Infections; Montréal, QC, CA. 2009. [Google Scholar]
- 2.Braithwaite RS, Roberts MS, Chang CC, et al. Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy: a decision model. Ann Intern Med. 2008;148(3):178–85. doi: 10.7326/0003-4819-148-3-200802050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197(8):1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 4.Kitahata M, Gange S, Moore R, et al. Initiating rather than deferring HAART at a CD4+ count >500 cells/mm3 is associated with improved survival [abstract 71]. 16th Conference on Retroviruses and Opportunistic Infections; Montréal, QC, Canada. 2009. [Google Scholar]
- 5.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42(4):464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 6.Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344(11):824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 2006 revision. [12 February 2008]; Accessed at http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf on. [PubMed]
- 8.Agence Nationale de Recherches sur le SIDA. (ANRS F. Early Antiretroviral Treatment and/or Early Isoniazid Prophylaxis Against Tuberculosis in HIV-Infected Adults (ANRS 12136 TEMPRANO) [15 October 2008]; Accessed at http://clinicaltrials.gov/ct2/show/NCT00495651 on.
- 9.National Institutes of Health; [15 October 2008]. Preventing Sexual Transmission of HIV With Anti-HIV Drugs. Accessed at http://clinicaltrials.gov/ct2/show/NCT00074581 on. [Google Scholar]
- 10.World Health Organization. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults: Recommendations for a public health approach. [17 April 2007];2006 Accessed at http://www.who.int/hiv/pub/guidelines/ctxguidelines.pdf on.
- 11.The World Bank Group. Quick Query Selected From World Development Indicators. [8 Janurary 2008]; Accessed at http://ddp-ext.worldbank.org/ext/DDPQQ/member.do?method=getMembers&userid=1&queryId=135 on.
- 12.Oanda Corporation. FXHistory: historical currency exchange rates. [14 January 2008]; Accessed at http://www.oanda.com/convert/fxhistory on.
- 13.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 14.World Health Organization. Report of the Commission on Macroeconomics and Health. 2001. Macroeconomics and health: investing in health for economic development. [Google Scholar]
- 15.World Health Organization - CHOosing Interventions that are Cost Effective. Prices for hospitals and health centres. [15 October 2008];2004 Accessed at http://www.who.int/choice/en/ on.
- 16.Walensky RP, Wood R, Weinstein MC, et al. Scaling up antiretroviral therapy in South Africa: the impact of speed on survival. J Infect Dis. 2008;197(9):1324–32. doi: 10.1086/587184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization; 2005. [17 March 2009]. Summary country profile for HIV/AIDS treatment scale-up: South Africa. Accessed at http://www.who.int/hiv/HIVCP_ZAF.pdf on. [Google Scholar]
- 18.PEPFAR. FY 2008 country profile: South Africa. [17 March 2009]; Accessed at http://www.pepfar.gov/documents/organization/116231.pdf on.
- 19.Cleary S, Boulle A, McIntyre D, Coetzee D. Cost-effectiveness of antiretroviral treatment for HIV-positive adults in a South African township. [19 April 2007];2004 Accessed at http://www.hst.org.za/uploads/files/arv_cost.pdf on.
- 20.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings--the case of Côte d'Ivoire. N Engl J Med. 2006;355(11):1141–53. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 21.Walensky RP, Weinstein MC, Yazdanpanah Y, et al. HIV drug resistance surveillance for prioritizing treatment in resource-limited settings. AIDS. 2007;21(8):973–82. doi: 10.1097/QAD.0b013e328011ec53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez A, Salomon J, Ahmed O, Murray C, Mafet D. (WHO). Life tables for 191 countries: data, methods and results. 2001 [Google Scholar]
- 23.Coetzee D, Hildrebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 24.Delfraissy JF, Flandre P, Delaugerre C, et al. Lopinavir/ritonavir monotherapy or plus zidovudine and lamivudine in antiretroviral-naive HIV-infected patients. AIDS. 2008;22(3):385–93. doi: 10.1097/QAD.0b013e3282f3f16d. [DOI] [PubMed] [Google Scholar]
- 25.Losina E, Yazdanpanah Y, Deuffic-Burban S, et al. The independent effect of highly active antiretroviral therapy on severe opportunistic disease incidence and mortality in HIV-infected adults in Côte d'Ivoire. Antivir Ther. 2007;12(4):543–51. [PMC free article] [PubMed] [Google Scholar]
- 26.Cole SR, Hernan MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158(7):687–94. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 27.South Africa National Department of Health. National antiretroviral treatment guidelines. [7 June 2008];2004 Accessed at http://hivinsite.ucsf.edu/doc/cr09-sf-01.doc on.
- 28.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19(18):2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 29.Actuarial Society of South Africa. ASSA2003 Summary Statistics. [27 February 2008]; Accessed at http://www.actuarialsociety.co.za/aids/content.asp?id=1000000449 on.
- 30.Mellors JW, Muñoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12):946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 31.Anglaret X, Chêne G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353(9163):1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 32.Yazdanpanah Y, Losina E, Anglaret X, et al. Clinical impact and cost-effectiveness of co-trimoxazole prophylaxis in patients with HIV/AIDS in Côte d'Ivoire: a trial-based analysis. AIDS. 2005;19(12):1299–308. doi: 10.1097/01.aids.0000180101.80888.c6. [DOI] [PubMed] [Google Scholar]
- 33.Badri M, Cleary S, Maartens G, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther. 2006;11(1):63–72. [PubMed] [Google Scholar]
- 34.Gauteng Department of Health. Gauteng Hospitals Numeric, Gauteng Province, South Africa. 2004 [Google Scholar]
- 35.Health Systems Trust. South African Health Review 2005. [15 January 2008]; Accessed at http://www.hst.org.za/publications/682 on.
- 36.Médecins Sans Frontières. Untangling the web of price reductions: A pricing guide for the purchase of ARV's in developing countries. [1 June 2007];2006 July; Accessed at http://www.doctorswithoutborders.org/news/hiv-aids/untangled.pdf on. Vol. 2007; July 2006.
- 37.South Africa National Department of Health. National Health Reference Price List 2008. [15 January 2008]; Accessed at http://www.doh.gov.za/docs/nhrpl-f.html on.
- 38.Myer L, el-Sadr W. Expanding access to antiretroviral therapy through the public sector--the challenge of retaining patients in long-term primary care. S Afr Med J. 2004;94(4):273–4. [PubMed] [Google Scholar]
- 39.Kress KD. HIV update: emerging clinical evidence and a review of recommendations for the use of highly active antiretroviral therapy. Am J Health Syst Pharm. 2004;61 3:S3–14. doi: 10.1093/ajhp/61.suppl_3.S3. quiz S15-6. [DOI] [PubMed] [Google Scholar]
- 40.Masur H, Kaplan JE, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons--2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Ann Intern Med. 2002;137(5 Pt 2):435–78. doi: 10.7326/0003-4819-137-5_part_2-200209031-00002. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Preventive Services Task Force. Guide to Clinical Preventive Services, recommendations of the U.S. Preventive Services Task Force. [15 October 2008]; Accessed at http://www.ahrq.gov/clinic/pocketgd08/pocketgd08.pdf on.
- 42.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20(12):1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. Guidelines for Using HIV Testing Technologies in Surveillance: Selection, Evaluation, and Implementation. 2001 [PubMed] [Google Scholar]
- 44.Bennett S, Chanfreau C. Approaches to rationing antiretroviral treatment: ethical and equity implications. Bull World Health Organ. 2005;83(7):541–7. [PMC free article] [PubMed] [Google Scholar]
- 45.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 46.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.