Abstract
The relationship between cytochrome P4503A4 (CYP3A4) activity and docetaxel clearance in patients with varying degrees of liver function (LF) was evaluated. Docetaxel 40, 50, or 75 mg/m2 was administered to 85 patients with advanced cancer; 23 of 77 evaluable patients had abnormalities in liver function tests. Baseline CYP3A activity was assessed using the erythromycin breath test (ERMBT). Pharmacokinetic studies and toxicity assessments were performed during cycle 1 of therapy and population modeling was performed using NONMEM. Docetaxel unbound clearance was lower (317 vs. 470 L/h) and more variable in patients with liver function abnormalities compared to patients with normal LF. Covariates evaluated accounted for 83% of variability on clearance in patients with liver dysfunction, with CYP3A4 activity accounting for 47% of variation; covariates accounted for only 23% of variability in patients with normal LF. The clinical utility of the ERMBT may be in identifying safe docetaxel doses for patients with LF abnormalities.
Keywords: docetaxel, population pharmacokinetics, liver dysfunction
INTRODUCTION
Docetaxel, a member of the taxane class of chemotherapeutic agents, is approved for the treatment of breast, non-small cell lung, androgen-independent prostate, and stomach cancers. A warning in the Prescribing Information (http://products.sanofi-aventis.us/Taxotere/taxotere.html) states that docetaxel should generally not be given to patients with bilirubin > than the upper limit of normal (ULN) or elevated liver transaminases concurrent with alkaline phosphatase, due to an increased risk of severe toxicities, although no published data is available from completed clinical trials in patients with liver impairment. The higher rate of severe toxicities observed in patients with liver function abnormalities is likely associated with reduced drug clearance, as higher plasma exposure is associated with an increase in the odds of developing febrile neutropenia and other severe (grade 3/4) toxicities such as mucositis, skin toxicity, and toxic death.1–3
Docetaxel undergoes extensive metabolism by cytochrome P450 (CYP) 3A4 and 3A5, and the predominant route of elimination of parent drug and metabolites is via biliary and intestinal excretion.4, 5 There has been great interest to predict docetaxel clearance and derive alternative dosing strategies based on CYP3A4 phenotypic probes including the assessment of urinary cortisol excretion, midazolam plasma clearance, dexamethasone plasma clearance, and exhalation of 14CO2 after administration of the erythromycin breath test.6–10 Most of these studies did not evaluate the use of a phenotypic probe for prediction of docetaxel clearance in patients with significant liver function abnormalities. Patients with elevated liver function tests comprise a population of patients that are rarely treated with docetaxel due to safety concerns and identification of covariates that account for a considerable degree of pharmacokinetic variability may allow the development of safe dosing strategies for this patient population.
The objective of the present study was to prospectively evaluate the relationship between CYP3A activity, using the erythromycin breath test, and docetaxel clearance in patients with varying degrees of liver function. A population pharmacokinetic model was developed to assess the influence of CYP3A activity and other patient covariates on docetaxel pharmacokinetic variability.
RESULTS
Patient Demographics and CYP3A Activity
Eighty-five patients were enrolled to the study. Of these, 77 were evaluable for both pharmacokinetic and CYP3A phenotyping studies. Patients were not assessable for pharmacokinetic studies for the following reasons: 1) plasma samples became thawed during shipment for analytic analysis (seven patients), and 2) severe hypersensitivity reaction with discontinuation of drug treatment (1 patient). Patient characteristics for the 77 patients are listed in Table 1. Fifty-four patients had normal liver function (group 1), 11 patients had mild liver function abnormalities (group 2), 7 patients had moderate liver function abnormalities (group 3A), and 5 patients had severe liver function abnormalities (group 3B). Liver function abnormalities that defined each liver function group are illustrated in Table 2. Values for liver function tests, the erythromycin breath test (ERMBT), and unbound docetaxel clearance are summarized in Table 3. Patients in liver function groups 1 and 2 had, on average, similar ERMBT values (mean, 0.050 and 0.061 %dose/min) and docetaxel clearance values (median, 444 and 437 L/h). Patients in groups 3A and 3B had reduced ERMBT values by approximately 45% and 75%, respectively, and lower docetaxel clearance values by 49% and 81%, respectively.
Table 1.
Patient Characteristics
| No. of Patients |
Mean | Range | |
|---|---|---|---|
| Number of Patients | 77 | ||
| Age, years | 57 | 24 – 79 | |
| BSA, m2 | 1.88 | 1.40 – 2.50 | |
| Race | |||
| Black | 10 | ||
| White | 67 | ||
| Sex | |||
| Female | 36 | ||
| Male | 41 | ||
| AAG, mg/dL | 142 | 60 – 271 | |
| Liver Function Group | |||
| 1 (normal) | 54 | ||
| 2 (mild impairment) | 11 | ||
| 3A (moderate impairment) | 7 | ||
| 3B (severe impairment) | 5 | ||
| Primary tumor type | |||
| Breast | 20 | ||
| Prostate | 14 | ||
| Lung | 11 | ||
| Head and neck | 10 | ||
| Melanoma | 8 | ||
| Unknown primary | 5 | ||
| Other | 9 |
Table 2.
Definition of liver function groups based on elevations in liver function tests.a
| Alkaline Phosphatase | AST or ALT |
|||
|---|---|---|---|---|
| ≤ ULN | > 1 to < 1.5 × ULN | ≥ 1.5 × ULN | > 5 × ULN | |
| ≤ ULN | Group 1 | Group 1 | Group 1 | Group 2 |
| > 1 to < 2.5 × ULN | Group 1 | Group 1 | Group 2 | Group 2 |
| ≥ 2.5 × ULN | Group 1 | Group 2 | Group 3A | Group 3A |
| > 5 × ULN | Group 2 | Group 2 | Group 3A | Group 3A |
Patients in liver function groups 1, 2, and 3A had total bilirubin levels < 1.5 × ULN. Patients in liver function group 3 (not shown in table) had total bilirubin levels ≥ 1.5 × ULN with normal or any elevations in AST/ALT or alkaline phosphatase.
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; ULN, upper limit of institutional normal.
Table 3.
Liver function tests, ERMBT, and docetaxel clearance.
| Liver Function Group | ||||
|---|---|---|---|---|
| Parameter | Group 1 n = 55 |
Group 2 n = 10 |
Group 3A n = 7 |
Group 3B n = 5 |
| Liver function testsa | ||||
| AST (x ULN) | 0.9 (0.3 – 3.2) | 2.3 (0.6 – 4.7) | 5.6 (1.7 – 11) | 13 (0.8 – 27) |
| ALT (x ULN) | 0.6 (0.1 – 2.2) | 2.1 (0.4 – 6.6) | 2.0 (0.6 – 3.1) | 3.3 (0.9 – 4.5) |
| AP ( × ULN) | 0.9 (0.4 – 5.0) | 2.7 (1.1 – 8.7) | 6.2 (2.5 – 15) | 5.2 (0.9 – 8.5) |
| Total bilirubin (x ULN) |
0.5 (0.2 – 1.1) | 0.4 (0.2 – 0.7) | 0.7 (0.4 – 1.0) | 2.5 (1.5 – 5.2) |
| ERMBTa | ||||
| C20min (% dose/min) | 0.050 (0.013 – 0.12) |
0.061 (0.041 – 0.11) |
0.031 (0.0060 – 0.050) |
0.019 (0.0055 – 0.062) |
| Docetaxel unbound clearance (L/h)b,c |
524 (444) [198 – 1321] |
450 (437) [213 – 752] |
264 (261) [153 – 378] |
139 (96) [64 – 327] |
Data are mean (range).
Data are mean (median) [range].
Individual clearance values are from the empirical Bayesian estimates
Abbreviations: n, number of patients; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AP, alkaline phosphatase; ERMBT, erythromycin breath test; C20min, flux of 14CO2 at 20 min after administration of the erythromycin breath test; and ULN, upper limit of institutional normal.
Population Pharmacokinetic Model
Individual observed unbound and total plasma concentration versus time profiles in patients with normal liver function and liver function abnormalities are shown in Figure 1. Unbound concentrations were best fit using a four-compartment model. The half lives for the four phases of the model were: 7.14 minutes, 55 minutes, 14.9 hours and 4.8 days. To incorporate total plasma measurements into the model, a binding model was developed to relate unbound to total docetaxel plasma concentrations. Parameters associated with the binding model are shown in Table 4.
Figure 1.
Total (Ctot) and unbound (Cu) docetaxel plasma concentration versus time profiles for patients with liver function abnormalities (left column) and normal liver function (right column).
Table 4.
Population pharmacokinetic parameters, expressed as the estimate and % RSE, obtained from the final covariate model.
| Parameter | Model Estimate |
RSE (%) | IIV (%CV) | RSE (%) | |
|---|---|---|---|---|---|
| CLnormal LF (L/h) | 470 | 5.26 | 41 | 16.2 | |
| CLimpaired LF (L/h) | 317 | 7.29 | |||
| V1 (L) | 80.7 | 6.98 | 48.2 | 26.4 | |
| V2 (L) | 101 | 8.57 | |||
| V2 (L) | 2140 | 20.7 | |||
| V4 (L) | 16,100 | 8.45 | |||
| Q2 (L/h) | 76.4 | 4.33 | 33.9 | 42.8 | |
| Q3 (L/h) | 99.4 | 10.3 | |||
| Q4 (L/h) | 96.9 | 6.78 | |||
| Blin | 19.7 | 2.08 | 14.5 | 19.9 | |
| Bmax | 1.28 × 10−3 | 21.5 | |||
| B50 (mg/L) | 2.45 × 10−4 | 25.6 | |||
| Bp80,1 | 0.183 | 26.8 | |||
| Bp80,2 (h−1) | 1.30 | 32.7 | |||
| θaag-bin (dL/mg) | 3.66 × 10−3 | 13.8 | |||
| θaag-bmax (dL/mg) | 8.70 × 10−3 | 21.0 | |||
| θERMBT,normal LF (dL/mg) |
8.64 | 15.6 | |||
| θERMBT,impaired LF (dL/mg) |
16.4 | 9.57 | |||
| θaag-CL (dL/mg) | 2.21 × 10−3 | 8.01 | |||
| σcorr | 0.259 | 6.72 | 34.9 | 31.9 | |
| σprop | 0.177 | 6.38 | |||
| σadd (mg/dL) | 2.00 × 10−4 | 60.5 | |||
Abbreviations: IIV, inter-individual variability; Clnormal LF, unbound plasma clearance in patients with normal liver function; CLimpaired LF, unbound plasma clearance in patients with impairmed liver function; V, volume of distribution for the four compartments of the structural model; Q, intercompartmental clearances for the four compartments of the structural model; Blin, parameter in linear term of binding model; Bmax and B50, parameters in nonlinear term of binding model; Bp80,1 and Bp80, 2, parameters for effect of polysorbate 80 on fraction unbound in binding model; θAAG-blin and θAAG-bmax, parameters for the covariate AAG in the binding model; θERMBT,normal LF and θERMBT,impaired LF parameters for the covariate ERMBT on clearance; θaag-CL, parameter for the covariate AAG on clearance; σcor, σprop, and σadd, parameters for the residual error model in standard deviation units.
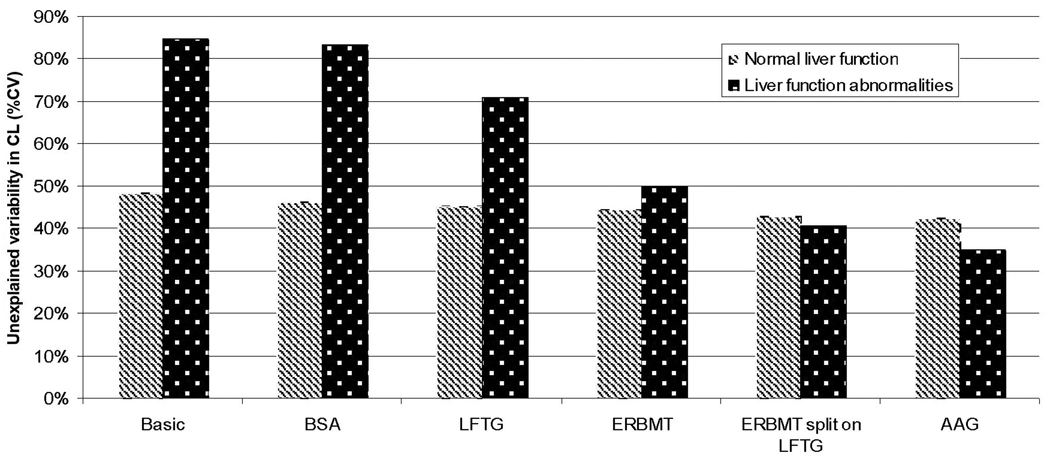
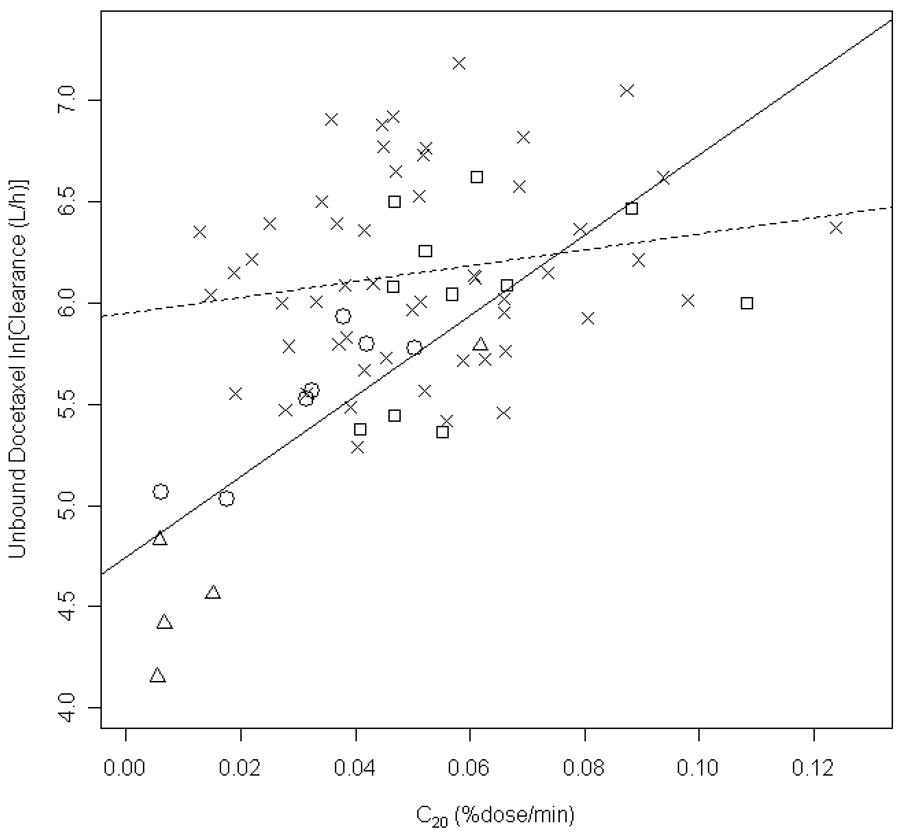
The ERMBT, liver function group (LFG), and AAG were found to be significant covariates on unbound clearance. The final population pharmacokinetic model parameters for unbound docetaxel are listed in Table 4. The parameters were, in general, well estimated and the observed data was well described by the model (Figure 2). Unbound docetaxel clearance was reduced by 33% in patients with liver function abnormalities (groups 2, 3A, and 3B) compared to patients with normal liver function with population mean values (fixed effects) of 317 L/h and 470 L/h, respectively. Figure 3 shows the associated unexplained variability on unbound clearance as each covariate was added to the model for patients with impaired hepatic function (groups 2, 3A and 3B) and those with normal liver function (group 1). In the base model, unexplained variability was significantly (p<0.001; ΔOFV=13.9) larger in those with liver function abnormalities compared to those with normal liver function when the two groups were allowed to be described by separate parameters (Figure 3). With the incorporation of all included covariates in the final model, including the ERMBT, liver function group, AAG, and BSA, unexplained variability on unbound clearance was reduced from 84% to 35% in patients with liver function abnormalities, indicating that these covariates accounted for 83% of variability on clearance. Figure 3 also shows that the ERMBT accounted for the most unexplained variability on unbound clearance in patients with liver impairment and AAG to a lesser extent. In patients with normal liver function, incorporation of covariates in the model reduced unexplained variability on unbound clearance from 48% to 42%, thus accounting for 23% of the variability. The ERMBT was significantly correlated with model-predicted unbound docetaxel clearance in patients with liver function abnormalities (R2 = 0.603, P<0.0001), but poorly so in patients with normal liver function (R2 = 0.03547; P = 0.177) (Figure 4). Figure 4 also illustrates the wide variability in docetaxel unbound clearance among all liver function groups. Once covariates were incorporated, there was no significant difference in unexplained variability in CL between patients with normal or abnormal liver function tests and in the final model a joint term for this variability was estimated (41%).
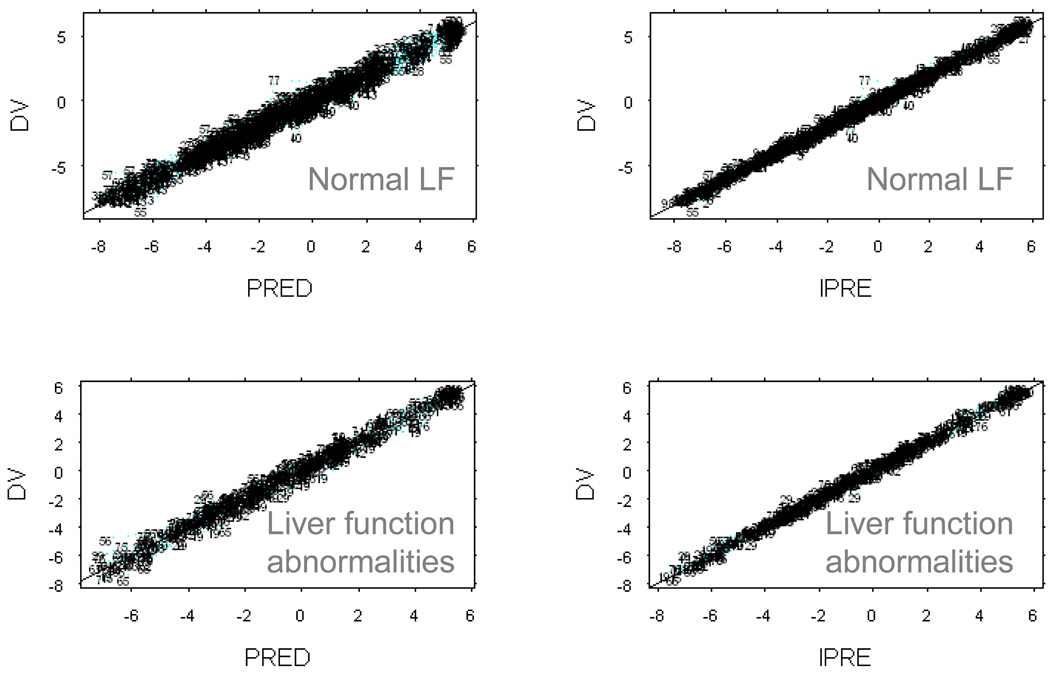
Figure 2.
Goodness of fit plots for patients with liver function abnormalities (lower row) and normal liver function (LF) (upper row). The left column shows the observed concentrations (DV) of both bound and unbound docetaxel plasma concentrations versus concentrations for the typical individual in the population (PRED). The right column shows the observed concentrations versus the individual predictions of concentration (IPRE).
Figure 3.
Unexplained variability in unbound docetaxel CL as each covariate was added to the model. Unexplained variability is estimated separately for patients with normal and abnormal liver function tests. Abbreviations: LFG, liver function group; body surface area, BSA, alpha-1 acid glycoprotein, AAG.
Figure 4.
Association between the erythromycin breath test variable C20,min, and log-transformed unbound docetaxel, estimated from the final population pharmacokinetic model. The symbol (x), represents patients with normal liver function, and the symbols (□), (○), and (Δ) represent patients with liver function abnormalities in liver function groups 2, 3A, and 3B, respectively. The dashed line is a linear-regression analysis of data in patients with normal liver function (R2 = 0.03547; P = 0.177), and the solid line is from data in patients with liver function abnormalities (R2 = 0.603, P<0.0001).
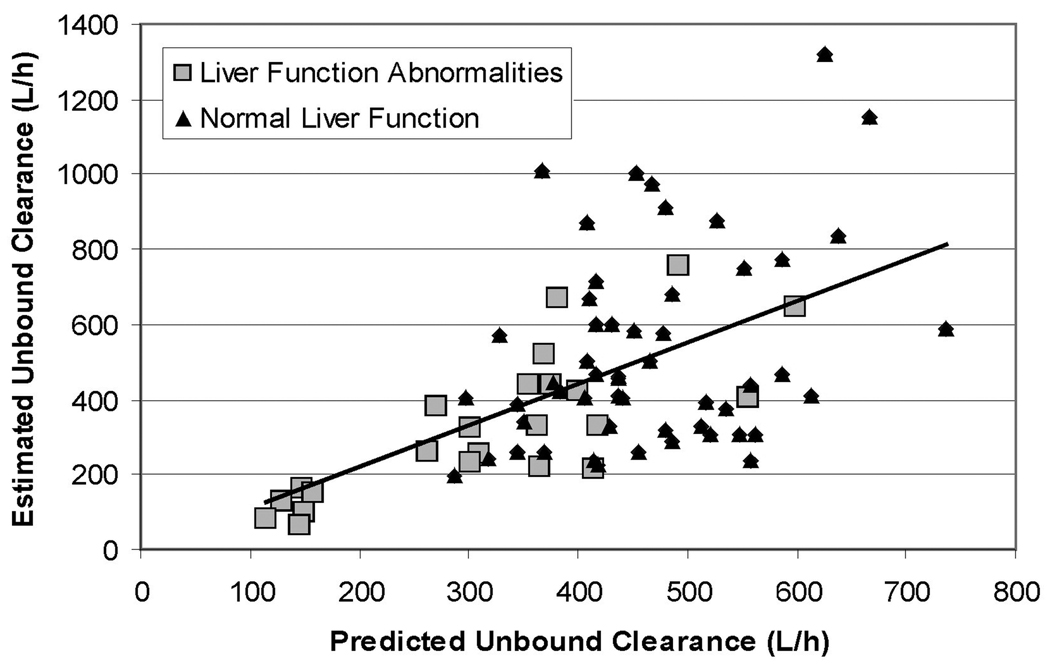
Results from the cross validation show that the model’s predictions of unbound clearance are not significantly different if any one individual is removed from the dataset used to estimate the model parameters (data not shown). Figure 5 shows the comparison of the (cross-validated) model predictions of unbound clearance (TVCLXV,i) to the estimated values of unbound clearance (CLi). Computing the mean absolute error of the prediction compared to the estimated clearance yields a value of 25.7% for the group with liver function abnormalities and 41.2% for the group with normal liver function. This indicates the type of error clinicians could expect when using the model to predict clearance in each group.
Figure 5.
Cross-valudated model predictions of the individual clearance values (TVCLXV,i) compared to individual clearance values estimated from the full data set (CLi). The model predicts the unbound concentrations well, with a mean absolute error of 36%.
Toxicity
Fourty-nine of 54 patients in liver function group 1 and all patients in groups 2, 3A, and 3B were evaluable for toxicity. Grade 3 and 4 toxicities that occurred during cycle 1 in group are listed in Table 5. Grade 3/4 neutropenia occurred in 71% of patients in group 1 receiving docetaxel 75 mg/m2, which is similar to that reported previously at this dose level (65%)11; the incidence of febrile neutropenia of grade 3 was 12%, which is twice as high as that reported previously (6%). Seven patients in group 1 had isolated elevations of a transaminase > 1.5 × ULN, which has been reported to be associated with a higher rate of febrile neutropenia grade 4 (http://products.sanofi-aventis.us/Taxotere/taxotere.html); however, only 1 of 7 patients with isolated elevations in a transaminases experienced febrile neutropenia grade 3. The higher incidence of febrile neutropenia may be due to the number of elderly patients in group 1 (36%), as grade 4 and febrile neutropenia were shown to be higher in elderly patients compared to younger cohorts, despite no pharmacokinetic differences.12, 13 Although the sample size was not calculated to detect statistical differences in docetaxel-mediated neutropenia between the different liver function groups, the incidence of grade 3 and 4 neutropenia was not greater in groups 2 and 3A compared to group 1, indicating that doses administered were tolerated similarly among the 3 groups. All patients in liver function group 3B receiving 40 mg/m2 (N = 4) experienced grade 4 neutropenia, and two of these experienced febrile neutropenia (grade 3 and 4 in one each) concurrent with grade 3 or 4 mucositis, indicating that 40 mg/m2 is not tolerable in patients with this degree of liver impairment. However, it is important to note that one patient in group 3B with a total bilirubin of 1.5 × ULN with normal AST/ALT and alkaline phosphatase was inadvertently administered 75 mg/m2. This patient experienced transient grade 4 neutropenia that resolved by day 15 and experienced no other toxicities during cycle 1, except grade 1 mucositis that resolved by day 22. This patient did not have reduced ERMBT value (0.062 %dose/min) (Table 3).
Table 5.
Worst grade toxicity during cycle 1.a
| Liver Function Group |
Number of Patients |
Neutropenia | Thrombocytopenia | Mucositis | |||
|---|---|---|---|---|---|---|---|
| Gr 3 | Gr 4 | Febrile | Gr 3 | Gr 3 | Gr 4 | ||
| 1 | 49 | 7 (14) | 28 (57) | 6 (12) | 0 | 0 | 0 |
| 2 | 11 | 4 (36) | 2 (18) | 0 | 0 | 0 | 0 |
| 3A | 7 | 0 | 2 (29) | 0 | 0 | 0 | 0 |
| 3B | 4 | 0 | 4 (100) | 2 (50) | 2 (50) | 1 (25) | 1 (25) |
| 1b | 0 | 1 (100) | 0 | 0 | 0 | 0 | |
Data are number of patients (%).
one patient with a total bilirubin of 1.5 × ULN with normal aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase was inadvertently administered 75 mg/m2.
Abbreviations: Gr, grade of toxicity.
DISCUSSION
The present population pharmacokinetic analysis demonstrated that patients with liver function abnormalities have reduced docetaxel clearance compared to those with normal liver function, but docetaxel clearance was more variable in the former (68% versus 47%). Examination of the separate liver function groups showed that patients with mild liver abnormalities (group 2) did not have reduced clearance and could have tolerated a full dose of docetaxel. Patients with moderate liver impairment, (group 3A), had reduced docetaxel clearance by 49% and tolerated a lower dose of 50 mg/m2. The greatest variability in docetaxel clearance and toxicity was observed in patients with severe liver impairment (group 3B). Severe toxicities were observed in 50% of the patients in this group indicating that docetaxel doses lower than 40 mg/m2 should be evaluated. However, a dosing algorithm based on the ERMBT in these patients is more likely to yield a safe and effective dose for the individual, rather than administering the same dose in all patients with severe liver dysfunction. The population pharmacokinetic model utilizing the ERMBT provides the framework for identifying patients with liver function abnormalities that should receive a reduced dose compared to those that could receive the clinical benefit of full doses of docetaxel.
In the present model, where 29% of the population had liver function abnormalities ranging from mild to severe, a significant amount (83%) of unexplained variability on docetaxel clearance was accounted for by the covariates ERMBT, liver function group and AAG. Several investigations have evaluated the effect of mild-to-moderate liver impairment on docetaxel pharmacokinetics, where a minor percentage of the population (approximately 5%) had elevated liver function tests. 9, 14 One study evaluated body surface area, liver function category (normal versus elevated liver function tests), alpha-1 acid glycoprotein, albumin, and elderly age as covariates and accounted for 34% of variability on docetaxel clearance.14 Another study evaluated CYP3A activity using the ERMBT and the liver function test, ALT, and accounted for 30% of variability on clearance.9 Neither population model examined variability separately for patients with elevated liver function tests.
The current model indicates that the ERMBT is unlikely to have clinical utility in predicting docetaxel clearance in patients with normal liver function, where a negligible amount of interindividual variability in docetaxel clearance (9%) was accounted for. Previous studies have noted relationships between CYP3A activity, using a variety of CYP3A phenotypic probes, and docetaxel clearance in patients with normal liver function. However, the potential clinical applicability of the studies may be limited due to small sample sizes,6–8, 10 and the study of populations with interindividual pharmacokinetic variability that is lower (e.g., 3- to 4-fold in range of clearance values)6, 7 than what would be expected for a representative population where docetaxel exhibits wide PK variability (e.g., 7- to 10-fold).
In conclusion, a population pharmacokinetic model was developed that adequately described unbound and total plasma concentration versus time profiles for docetaxel in patients with varying degrees of liver function abnormalities. The ERMBT alone accounted for the majority of interindividual variability in docetaxel clearance in patients with liver dysfunction. For anticancer agents that are cleared principally through the CYP3A metabolic pathway, the use of phenotypic measurements should be considered in future studies in patients with liver function abnormalities. The present population pharmacokinetic model provides the framework for developing a dosing strategy for docetaxel based on the ERMBT in patients with liver function abnormalities.
METHODS
Study Design and Patient Eligibility
Patients were enrolled to a clinical trial designed to prospectively address several objectives including investigating the effects of race/ethnicity (African American versus European Caucasian), age (< 65 years versus ≥ 65 years), and varying degrees of liver function on docetaxel pharmacokinetics. The study was opened to accrual in June, 2002 and closed in August, 2005 due to the lack of sufficient patient enrollment to the race/ethnicity group and moderate and severe liver impairment groups, with only 22%, 35%, and 50%, respectively, of the target enrollments achieved. Enrollment to the elderly group was completed and the results were described previously.13
A secondary objective of the study was to assess the relationship between liver function parameters, CYP3A activity, and docetaxel clearance in patients with varying degrees of liver function. For this analysis, patients were grouped according to their baseline liver function tests. Patients in liver function groups 1 (normal), 2 (mild impairment), and 3A (moderate impairment) had total bilirubin < 1.5 × the upper limit of institutional normal (ULN), but were classified according to the degree of elevations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase as illustrated in Table 2. Patients with total bilirubin ≥ 1.5 × ULN with normal or any elevations in AST/ALT or alkaline phosphatase were placed in liver function group 3 (severe impairment), which was consistent with the classification of severe liver impairment that was being evaluated in several ongoing phase I studies of docetaxel in patients with varying degrees of liver function.15, 16 Liver function test abnormalities defining liver function group 3A (AST/ALT > 1.5 × ULN concurrent with alkaline phosphatase > 2.5 × ULN) were based on those described originally in a population pharmacokinetic-pharmacodynamic analysis by Bruno et al, where patients with this level of liver function abnormalities were shown to have a 27% reduction in docetaxel clearance with a 1.5-fold increase in the odds of febrile neutropenia.1 Based on unpublished data from the study sponsor, patients assigned to group 1 were defined by the following: AST/ALT ≤ 1.5 × ULN concurrent with alkaline phosphatase ≤ 2.5 × ULN, or AST/ALT > 1.5 to < 5 × ULN with normal alkaline phosphatase, or normal AST/ALT with alkaline phosphatase > 2.5 to < 5 × ULN. Patients with isolated elevations in AST/ALT or alkaline phosphatase that did not fit the definition of liver function groups 1 or 3A, were enrolled into liver function group 2.
Patients were eligible when they had histologically or cytologically confirmed solid tumor malignancies, for which docetaxel was considered a viable treatment option. Other criteria for patient enrollment were described previously.13 The clinical protocols were approved by the local institutional review boards, and all patients provided written informed consent before enrollment. Before treatment, a complete registration form was received by the coordinating center (Baltimore, MD), and a study number and treatment group was assigned. Patients who were not evaluable for both CYP3A phenotyping and pharmacokinetic studies were replaced.
Drug Treatment
Docetaxel (Taxotere; Aventis Pharmaceuticals, Bridgewater, NJ) was obtained commercially. Individual drug doses were normalized to body-surface area and administered intravenously over 1 hour. The dose administered varied according to the degree of liver function abnormalities and assigned liver function group: group 1 and group 2, 75mg/m2; group 3A, 50mg/m2; and group 3B, 40mg/m2. The dose of 75 mg/m2 was administered to patients in group 2 (mild impairment), since no data were available to indicate these patients would have reduced docetaxel clearance. Docetaxel 50 mg/m2 was administered to patients with moderate liver impairment (Group 3A). This dose has been tolerated in patients with moderate liver impairment,15, 16 and represents a 33% dose reduction, which is in line with the 27% reduction in docetaxel clearance observed previously in this patient population. Docetaxel 40 mg/m2 was administered to patients with severe liver impairment (Group 3B). This dose represents a 47% reduction, and in several preliminary reports, was shown to be tolerated in patients with severe liver function abnormalities.15, 16 Dexamethasone, 8 mg orally every 12 hours for 5 doses (3 days), was administered starting 24 h before drug treatment. Patients did not routinely receive anti-emetic prophylaxis. After 1 cycle of therapy, treatment continued at the discretion of the treating physician until tumor progression, development of unacceptable toxicity, or patient withdrawal.
Erythromycin Breath Test (ERMBT)
Within 48 hours prior to docetaxel administration, CYP3A activity was determined using the ERMBT as described previously.17 Breath samples were shipped to Metabolic Solutions (Nashua, NH) for measurement of breath carbon dioxide. The data were reported as the flux of 14CO2. The parameter of interest was the percentage of dose exhaled per minute at 20 minutes after administration of the radiolabeled erythromycin (C20min [% dose/min]), which was the observed value.
Pharmacokinetic Sampling and Assay
Serial blood sampling was performed during the first cycle of treatment at the following time points: pre-treatment, 30 min during the infusion, 59 min (immediately before the end of the infusion), and post-infusion at 10 and 30 min, 1, 3, 7, 24, and 48 h, and days 8, 15, and 22. Samples were collected, processed and frozen until the time of analysis, as previously described.13
Total concentrations of docetaxel and polysorbate 80 were measured in plasma using a validated method based on HPLC with tandem mass spectrometric detection with lower limits of quantification of 400 pg/mL (0.5 nM) and 1.0 µg/mL, respectively.18 Unbound concentrations of docetaxel in plasma were measured using a validated method based on micro-equilibrium dialysis.19
Population Covariate-Free (Base) Model Development
A population (non-linear mixed effects) pharmacokinetic model was built to simultaneously describe unbound and total docetaxel plasma concentrations. Because concentrations varied over 5 orders of magnitude between the beginning and end of sampling, the data was log transformed before analysis. Unbound concentrations were missing for two individuals and total concentrations were available for all patients. Two-, three- and four-compartment linear models were tested for the disposition model. The analysis was performed using the first-order conditional estimation (FOCE) method with interaction in NONMEM version VIβ (S.L. Beal and L.B. Sheiner, University of California, San Francisco, CA). The final models used for the results in this paper were also run in NONMEM version VI with identical results.
The first modeling attempts were focused on analyzing data from patients with liver function abnormalities separately from those with normal liver function. However, in the group with liver function abnormalities (liver function groups 2, 3A and 3B combined), some parameters had poor precision due, in part, to the relatively low number of individuals with liver function abnormalities (N = 23) and the complexity of the model. As a consequence, data from the two groups (normal liver function and liver function abnormalities) were combined with the intention that more information could be obtained on the parameters that were similar between the two groups.
To combine the models, a ‘bottom-up’ strategy was used, which started with a single model for both liver function groups (normal and impaired) that incorporated the same model parameters used to describe the variance of the random measurement error (σ2’s), the variance of the interindividual variability (ω2’s) and the population mean effects (θ’s). The model was then expanded around liver function groups. This ‘bottom-up’ strategy assumes that, physiologically, many parameters should be the same between the various groups of individuals. This approach to model building is more conservative than a “top-down” strategy which assumes the two groups are described by separate models and then combining terms that are the same between groups.
Unbound concentrations were best fit using a four-compartment model compared to two- and three-compartment models based on visual analysis of the goodness of fit plots and the objective function values (e.g., use of a three- versus four-compartment model increased the OFV by 333 points).
To incorporate total plasma measurements into the model, a binding model was developed to relate unbound to drug bound to elements of the plasma. The binding model had three terms: a liner term, a non-linear term (E-max function) and a component meant to mimic the time-dependent effect of the formulation vehicle, polysorbate 80, on docetaxel plasma binding. Both the linear binding term and the non-linear term were found to have AAG as a linear covariate. All three terms were statistically significant and if omitted they increased the OFV of the model by 835, 111 and 21 points respectively. The equations for the binding model were:
Where Cu is the unbound concentration, Ctot is the total concentration, DURA is the duration of the infusion, is the mean of the measured values of AAG, Blin is the linear binding parameter, Bmax and B50 are the nonlinear binding terms, Bp80, 1 and Bp80, 2 are the parameters in the polysorbate 80 binding term and θAAG-blin and θAAG-bmax are the parameters for the covariate AAG.
The data supported five interindividual variability parameters in the model, one for each group of parameters. The model was parameterized using clearance, volumes of distribution and intercompartmental clearances; interindividual variability terms for these parameters were included in the model so that they also had correlation between each other (using the BLOCK notation in NONMEM). The data also supported the inclusion of an interindividual variability term on the linear binding parameter B as well on the residual error. Excluding these terms resulted in an OFV increase of 172 and 278 points, respectively.
The population model’s residual error contained three different terms. The first is a proportional error term (whose random parameter component εcor is assumed to be normally distributed with mean zero and variance σ2 cor) that includes the correlation between total and unbound concentrations taken at the same time, which was obtained using an L2 data item in NONMEM. The model also included a proportional error term for unbound concentrations alone (εprop and σ2prop), and an additive error term for total concentrations in patients with liver function abnormalities (εadd and σ2add). The additive error term was estimated with relatively poor precision (60%). Removing the term did not significantly increase the objective function (4.16 points), however, the term was needed for the model to minimize successfully and to calculate the covariance matrix of the estimates.
Population Covariate Model Development
The covariate model was built using the generalized additive model (GAM) search.20 Inclusion of highly correlated covariates such as height, weight and body surface area (BSA) has been shown to be poor practice21 so BSA was used as a size measurement. Other covariates tested on all parameters included sex, age, race (Black or White), alpha-1 acid glycoprotein (AAG), albumin, the erythromycin breath test parameter C20min, and liver function group (LFG). One individual had a missing AAG value in the dataset.
In model development, separate models were compared using graphical diagnostic plots (Xpose)20, the standard errors calculated from the model’s covariance matrices, and the models objective function values used in the likelihood ratio test. For hierarchical models, an objective function value (OFV) difference corresponding to a significance level of p<.01 was used for model discrimination (6.63 points for models that differ by one parameter).
Patient variables that were found to be significant covariates on unbound clearance included The ERMBT, liver function group (LFG), and AAG. The final covariate model for an individual’s unbound plasma clearance CLi was found to be:
The ERMBT was included as a linear covariate and was found to be more predictive of clearance if the values were separated based on liver function (one parameter for LFG 1 and one parameter for LFGs 2, 3A, and 3B combined). AAG was also found to be a linear covariate on clearance but was not split into two separate covariates based on liver function. BSA was included as a covariate on clearance (CL) as a proportional term. The addition of BSA increased the OFV slightly, 3.68 points, but was included for clinical relevance (a visual inspection of the fit showed no differences).
To evaluate the final model, a cross validation was performed. One individual at a time was removed from the data set and the model parameters were re-estimated. Unbound clearance for the individual excluded was predicted based on that individuals covariate measurements (TVCLXV,i). Comparisons were made between this value and the value of unbound clearance estimated for this individual using the model with parameter estimates taken from the full data set (TVCLi). This comparison should allow for identification of overly-influential individuals in the data set. Additionally, comparisons were made between TVCLXV,i and the individual clearance values estimated from the full data set (CLi). This comparison should give an indication of the type of results clinicians can expect when using this model to predict patients’ docetaxel clearance.
Patient Evaluation
Pretreatment evaluations included assessment of PS, height, weight, toxicity assessment, a complete blood count with differential (CBC), and the following serum chemistries: creatinine, alkaline phosphatase, AST, ALT, total bilirubin, α1-acid glycoprotein (AAG), and albumin.
Toxicity assessment and a CBC with differential were performed weekly for a total of 3 weeks (1 cycle). Toxicity assessments were performed according the National Cancer Institute Common Toxicity Criteria version 2.0. Management of toxicity was at the discretion of the treating physician per institutional guidelines.
ACKNOWLEDGMENTS
The funding source of this project was by Aventis Pharmaceuticals (Bridgewater, New Jersey) GIA# 19075 (S.D. Baker) and the Swedish Cancer Society (M.O. Karlsson).
Footnotes
CONFLICT OF INTEREST
Dr. Carducci is a consultant to and on the speaker’s bureau for Sanofi-Aventis.
REFERENCES
- 1.Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. Journal of Clinical Oncology. 1998;16:187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 2.Bruno R, Vivier N, Veyrat-Follet C, Montay G, Rhodes GR. Population pharmacokinetics and pharmacokinetic-pharmacodynamic relationships for docetaxel. Invest New Drugs. 2001;19:163–169. doi: 10.1023/a:1010687017717. [DOI] [PubMed] [Google Scholar]
- 3.Bruno R, Olivares R, Berille J, Chaikin P, Vivier N, Hammershaimb L, et al. Alpha-1-acid glycoprotein as an independent predictor for treatment effects and a prognostic factor of survival in patients with non-small cell lung cancer treated with docetaxel. Clin Cancer Res. 2003;9:1077–1082. [PubMed] [Google Scholar]
- 4.Shou M, Martinet M, Korzekwa KR, Krausz KW, Gonzalez FJ, Gelboin HV. Role of human cytochrome P450 3A4 and 3A5 in the metabolism of taxotere and its derivatives: enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics. 1998;8:391–401. doi: 10.1097/00008571-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Baker SD, Sparreboom A, Verweij J. Clinical pharmacokinetics of docetaxel : recent developments. Clin Pharmacokinet. 2006;45:235–252. doi: 10.2165/00003088-200645030-00002. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto N, Tamura T, Kamiya Y, Sekine I, Kunitoh H, Saijo N. Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. Journal of Clinical Oncology. 2000;18:2301–2308. doi: 10.1200/JCO.2000.18.11.2301. [DOI] [PubMed] [Google Scholar]
- 7.Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. Journal of Clinical Oncology. 2002;20:3683–3690. doi: 10.1200/JCO.2002.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Hirth J, Watkins PB, Strawderman M, Schott A, Bruno R, Baker LH. The effect of an individual's cytochrome CYP3A4 activity on docetaxel clearance. Clinical Cancer Research. 2000;6:1255–1258. [PubMed] [Google Scholar]
- 9.Slaviero KA, Clarke SJ, McLachlan AJ, Blair EY, Rivory LP. Population pharmacokinetics of weekly docetaxel in patients with advanced cancer. Br J Clin Pharmacol. 2004;57:44–53. doi: 10.1046/j.1365-2125.2003.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puisset F, Chatelut E, Dalenc F, Busi F, Cresteil T, Azema J, et al. Dexamethasone as a probe for docetaxel clearance. Cancer Chemother Pharmacol. 2004;54:265–272. doi: 10.1007/s00280-004-0823-0. [DOI] [PubMed] [Google Scholar]
- 11.Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. Journal of Clinical Oncology. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 12.Minami H, Ohe Y, Niho S, Goto K, Ohmatsu H, Kubota K, et al. Comparison of pharmacokinetics and pharmacodynamics of docetaxel and Cisplatin in elderly and non-elderly patients: why is toxicity increased in elderly patients? Journal of Clinical Oncology. 2004;22:2901–2908. doi: 10.1200/JCO.2004.10.163. [DOI] [PubMed] [Google Scholar]
- 13.ten Tije AJ, Verweij J, Carducci MA, Graveland W, Rogers T, Pronk T, et al. Prospective evaluation of the pharmacokinetics and toxicity profile of docetaxel in the elderly. Journal of Clinical Oncology. 2005;23:1070–1077. doi: 10.1200/JCO.2005.03.082. [DOI] [PubMed] [Google Scholar]
- 14.Bruno R, Vivler N, Vergniol JC, De Phillips SL, Montay G, Sheiner LB. A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm. 1996;24:153–172. doi: 10.1007/BF02353487. [DOI] [PubMed] [Google Scholar]
- 15.Baker SD, Ravdin P, Aylesworth C, Smetzer L, Bruno R, Vernillet L, et al. A phase I and pharmacokinetic study of docetaxel in cancer patients with liver dysfunction due to malignancies. Proc Amer Soc Clin Oncol. 1998;17:192a. [Google Scholar]
- 16.Synold TW, Newman E, Lenz H-J, Gandara D, Chow W, Leong L, et al. Prospective evaluation of docetaxel (D) pharmacokinetics (PK) and toxicity in patients with tumnor-related hepatic dysfunctin (HD) Proc Amer Soc Clin Oncol. 1999;18:168a. [Google Scholar]
- 17.Baker SD, van Schaik RH, Rivory LP, Ten Tije AJ, Dinh K, Graveland WJ, et al. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin Cancer Res. 2004;10:8341–8350. doi: 10.1158/1078-0432.CCR-04-1371. [DOI] [PubMed] [Google Scholar]
- 18.Baker SD, Zhao M, He P, Carducci MA, Verweij J, Sparreboom A. Simultaneous analysis of docetaxel and the formulation vehicle polysorbate 80 in human plasma by liquid chromatography/tandem mass spectrometry. Anal Biochem. 2004;324:276–284. doi: 10.1016/j.ab.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 19.Acharya MR, Baker SD, Verweij J, Figg WD, Sparreboom A. Determination of fraction unbound docetaxel using micro-equilibrium dialysis. Anal Biochem. 2004;331:192–194. doi: 10.1016/j.ab.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 21.Derksen S, Keselman J. Backward, forward and stepwise automated subset selection algorithms: frequency of obtaining authentic and noise variables. Br J Math Stat Psych. 1992;45:265–282. [Google Scholar]