Abstract
Disproportionation of oxoiron(IV) porphyrin (Compound II) to oxoiron(IV) porphyrin radical cation (Compound I) was studied in three P450 model systems with different electronic structures. Direct conversion of Compound II to Compound I has been observed for 5,10,15,20-tetrakis(2,6-dichlorophenyl)porphyrin (TDCPP) in acid-catalyzed reactions in a mixed solvent of acetonitrile and water (1:1, v/v) containing excess m-CPBA oxidant, with a second-order rate constant of (1.3 ± 0.2) × 102 M−1 s−1. The acid-catalyzed disproportionation heavily depends on the electron demand of the substituted aryl groups on the porphyrin macrocycle. The disproportionation equilibrium constants show drastic change for the three porphyrin systems.
Keywords: oxoiron, disproportionation, Compound I, Compound II, P450 model
High-valent oxoiron porphyrin intermediates play important roles in the mechanisms of hemoproteins such as the peroxidases and cytochrome P450 enzymes [1, 2]. Much effort has been devoted to the enigmatic spectral and kinetic characteristics of highly oxidized states [3–7]. Since the first report in 1981 [8], oxoiron(IV) porphyrin radical cations, models for Compounds I in enzymes including cytochrome P450, have been generated and characterized for several different porphyrin systems [7, 9–11]. Another important oxoiron(IV) species, referred to as Compound II in heme enzymes, involves a single-electron oxidation from ferric porphyrin. In principle, Compound II species could be formed by the homolytic O−O bond cleavage in an acylperoxo-iron(III) complex [12]. Increasing interest has been directed at the study of Compound II analogues, especially regarding their reactivity towards organic substrates and the protonation of ferryl-oxo bonds [11, 13–17]. For example, oxoiron(IV) complexes were also able to effect two-electron oxidations [17, 18], which suggests that they might serve as one of the active intermediates in oxygen transfer process by cytochrome P450.
Electronic effects were found to be important factors in controlling the reactivity of high-valent oxoiron species. For example, it was demonstrated that the axial ligands on iron significantly affected the reactivity of Compound I analogues in oxygenation reactions [19–21]. For a series of oxoiron(IV) tetraarylporphyrin radical cations prepared by Fujii [22], the effects of the aryl ring meso substituents on electronic structures and reactivities revealed that the reactivity of the oxygen atom of Compound I depends on the redox potential of the porphyrin macrocycles. With the same axial ligand and similar sterics of aryl ring meso substituents, the reactivities of Compound I models with electron-deficient porphyrins were greater than the reactivities of those with electron-rich porphyrin rings [23–25]
In a study of oxidations by oxoiron(IV) porphyrins, our group proposed a disproportionation step in the reaction mechanism where the oxoiron(IV) species disproportionates to give an iron(III) species and a more reactive oxoiron(IV) porphyrin radical cation that is the true oxidant [26]. This conclusion was based on inverted reactivity patterns in regard to the electron demand of the porphyrin ring. Supporting evidence for a disproportionation step included dual-parameter Hammett analyses and the observation of suppressed reactivity with the addition of excess iron(III) species. Herein we report direct spectroscopic observation of conversion of oxoiron(IV) species to the corresponding iron(IV)-oxo porphyrin radical cation in a mixed solution of acetonitrile and water (1:1, v/v). The results support the conclusion that oxoiron(IV) species react by initial disproportionation to give oxoiron(IV) porphyrin radical cations that are true oxidants.
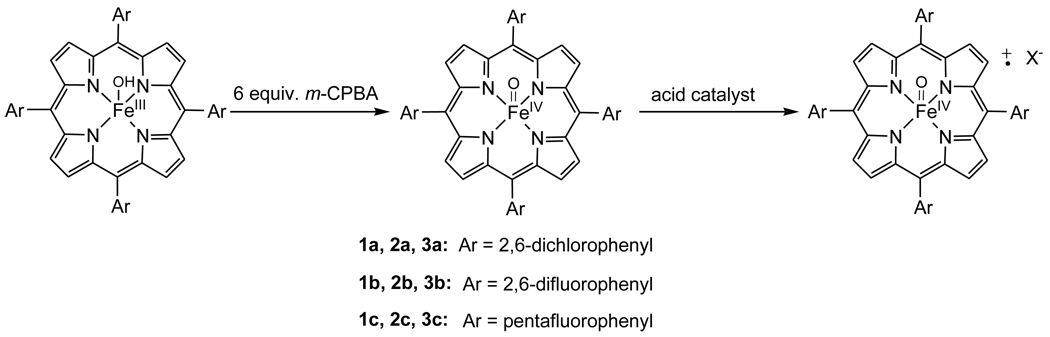
Three porphyrin systems, 5,10,15,20-tetrakis(2,6-dichlorophenyl)porphyrin (TDCPP), 5,10,15,20-tetrakis(2,6-difluorophenyl)porphyrin (TDFPP) and 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin (TPFPP), were studied in this work (Scheme 1). The commercially available free porphyrin ligands with chloride counterions were converted to the iron(III) hydroxide complexes (porphyrin)FeIII(OH) (1), which were then oxidized to oxoiron(IV) porphyrin species (2) according to literature methods [15]. Two equivalents of the terminal oxidant m-chloroperoxybenzoic acid (m-CPBA) were found to be necessary for the complete formation of 2 in acetonitrile solution for all three porphyrin systems as determined by UV-visible spectroscopy [15, 26].
Scheme 1.
A reaction scheme for the oxidation of (porphyrin)FeIII(OH) (1) and acid-catalyzed conversion of oxoiron(IV) porphyrin species (2) to oxoiron(IV) porphyrin radical cations (3).
Attempts to prepare (TDCPP)+˙ FeIV(O)(X) (3a) at room temperature by adding an excess of m-CPBA (2–100 equivalents) to ferric species 1a were not successful in either CH2Cl2 or CH3CN solutions. Addition of two equivalents of m-CPBA to the CH2Cl2 solution of 1a led to axial ligand exchange and formation of a high-spin (TDCPP)FeIII(m-CPBA) (417, 509, 573 nm) [27]. In acetonitrile, addition of the same amount m-CPBA to 1a generated red species (TDCPP)FeIV(O) (2a), which slowly decayed to a mixture of 1a and (TDCPP)FeIII(m-CBA) (414, 505, 577 nm). A large excess of m-CPBA (100 equivalents) could prolong the life-time of 2a because of the regeneration of 2a from ferric porphyrin, but no oxoiron(IV) porphyrin radical cation species was observed.
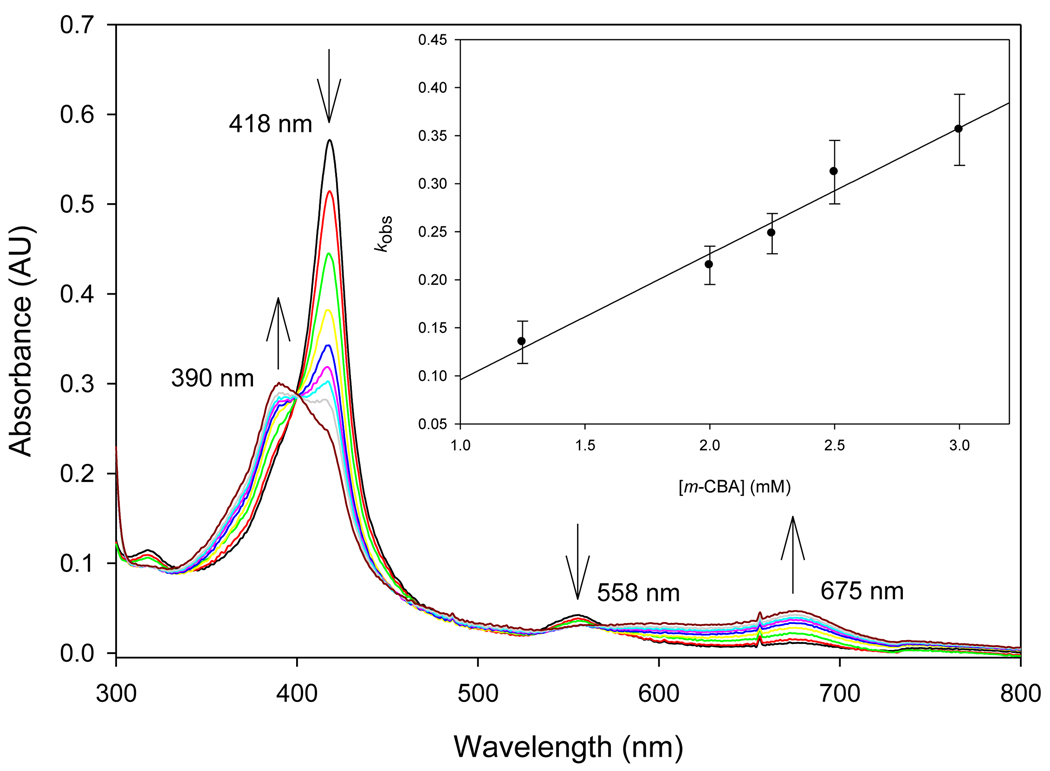
It was known that water can substantially stabilize oxoiron(IV) porphyrin radical cation species [19]. When a mixed solvent of acetonitrile and water (1:1, v/v) was used, the UV-visible spectra of 1a and 2a were the same as those in pure acetonitrile solution as expected for the OH ligation of ferric porphyrin 1a and the neutral character of oxoiron(IV) porphyrin species 2a. Addition of 100 equivalents m-CPBA to either 1a or 2a resulted in formation of a new green species that showed a weak Soret band at 390 nm and a broad Q-band at 550–750 nm (Figure 1) in addition to partial porphyrin degradation. The UV-visible spectrum of the green species was characteristic for oxoiron(IV) porphyrin radical cation and very similar to the reported Compound I spectra of several (TDCPP)+˙FeIV(O)(X) (3a) species generated by oxidizing ferric porphyrins containing weak axial ligands at low temperature [9, 22, 28]. When excess amounts of organic reductants such as styrene or diphenylmethane were added to above solutions of 3a, the UV-visible spectrum returned to that of ferric porphyrin 1a, which was recovered in high yield (>95%). The overall reaction sequence is consistent for the behavior expected for oxoiron(IV) porphyrin radical cation species.
Figure 1.
Conversion of (TDCPP)FeIV(O) (2a) to (TDCPP)+˙FeIV(O)(X) (3a) by excessive m-CBA in CH3CN/H2O (1:1, v/v). Inset: observed rate constants at 675 nm.
Conversion from (TDCPP)FeIV(O) (2a) to (TDCPP)+˙FeIV(O)(X) (3a) was shown not to be due to direct reaction of 2a with excess m-CPBA. A solution of 2a prepared in acetonitrile using six equivalents of m-CPBA was stable, but addition of excess acid m-chlorobenzoic acid (m-CBA) resulted in complete conversion of 2a to 3a with a second-order rate constant of (1.3 ± 0.2) × 102 M−1 s−1 (inset of Figure 1). This result demonstrated that acid could catalyze the disproportionation reaction of the Compound II model 2a to the Compound I model 3a in the presence of six equivalents of m-CPBA. With smaller amounts of oxidant, such as two equivalents of m-CPBA used in the preparation of 2a before addition of m-CBA, incomplete transformation to 3a was observed. Apparently, slow disproportionation occurs in the absence of acid catalysis, and the oxidant is degraded by reaction with solvent.
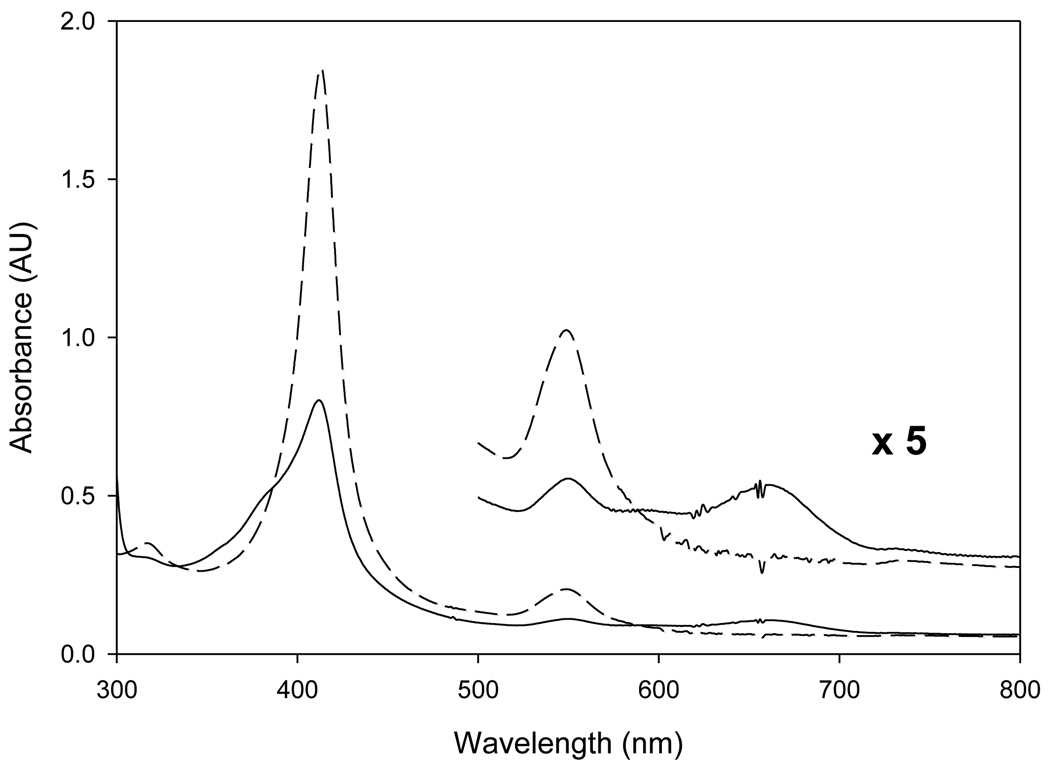
Similar experiments were conducted with the other two porphyrin systems 2b and 2c generated with six equivalents of m-CPBA. Addition of m-CBA (up to 5 mM) to the corresponding CH3CN/H2O (1:1, v/v) solution of oxoiron(IV) porphyrin species 2b and 2c (2 × 10−5 M) at ambient temperature was studied. As shown by the UV-visible spectrum in Figure 2, (TDFPP)FeIV(O) (2b) was not fully converted to the corresponding oxoiron(IV) porphyrin radical cation (TDFPP)+˙FeIV(O) (3b). In addition to the absorbance at 551 nm from 2b [15], a characteristic Q-band at 550–750 nm for Compound I analogue 3b [19] was observed together with broad Soret band. The UV-visible spectrum of most electron-deficient system, TPFPP (3b) did not reveal any detectable amount of conversion of 2c to 3c upon the addition of excess m-CBA.
Figure 2.
UV-visible spectra of (TDFPP)FeIV(O) (2b) before (dashed line) and after (solid line) the addition of excessive amount of m-CBA in CH3CN/H2O (1:1, v/v) solution.
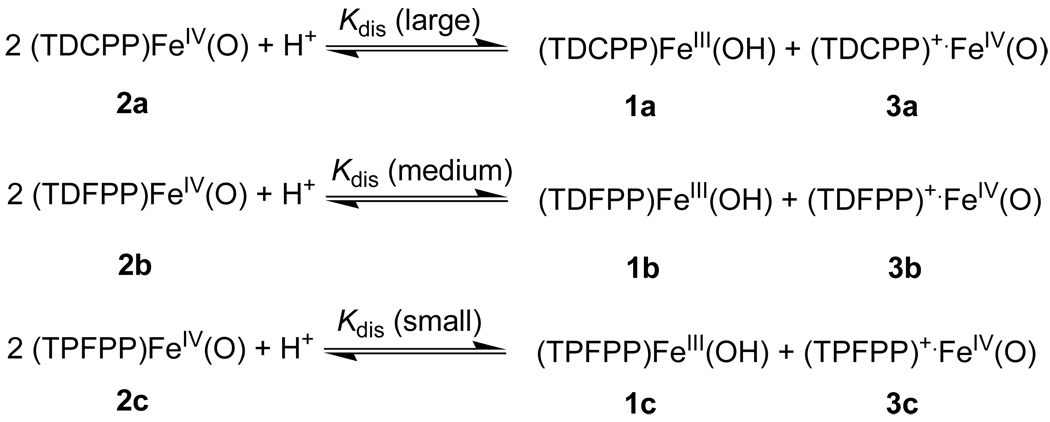
The diverse results for the three oxoiron(IV) porphyrin species 2 upon the addition of m-CBA can be explained by the different disproportionation equilibrium constants Kdis for the three porphyrin systems [29]. By considering the cationic feature of the oxoiron(IV) porphyrin radical cations, one can expect that the order of apparent Kdis values is TDCPP > TDFPP > TPFPP. That is, the strongest electron-withdrawing pentafluorophenyl groups at meso positions disfavor conversion of neutral (2c) to a mixture of cationic species (3c) and ferric species [29–31]. On the contrary, the TDCPP system has the least electron-withdrawing aryl groups on the porphyrin ring for the systems we studied, and 2a undergoes an acid-catalyzed disproportionation reaction to form 3a readily, and 3a is stabilized by water in the polar environment. The observation that (TDCPP)FeIV(O) (2a) oxidizes organic substrates faster than 2b and 2c can be explained by a significantly large Kdis value for 2b resulting in a relatively high concentration of true oxidant 3a [29]. Using previously reported data of suppressing the oxidation reaction by adding excess 1a [26], the equilibrium constant for the disproportionation reaction in TDCPP system is determined to be at the level of ca. 6 × 108 M−1. [32]
Previous research found that porphyrin-iron(IV)-oxo species (2) were more stable in alkaline solutions than in neutral or acidic solutions [11, 33, 34], which might be attributed to slow formation of reactive oxoiron(IV) porphyrin radical cations in the absence of acid catalysts for the disproportionation reactions. The disproportionation mechanism also explains the decreased life-time of 2 in NMR experiments versus UV-visible spectral studies [26]; at higher concentrations of 2 used in NMR experiments, the rate of the disproportionation reaction would be increased such that the lifetime of 2 decreased significantly from several hours in the UV-visible spectral studies to a few minutes in NMR studies.
In summary, direct conversion of oxoiron(IV) porphyrin species (2) to oxoiron(IV) porphyrin radical cations (3) has been observed in acid-catalyzed reactions in a mixed solvent of acetonitrile and water (1:1, v/v) containing excess m-CPBA oxidant. Depending on the electron demand of the substituted aryl groups on the porphyrin macrocycle and the corresponding effects on the disproportionation equilibrium constants, the acid-catalyzed reaction results in different observations for the three porphyrin systems studied here ranging from complete conversion to the Compound I model for 2a to undetectable amounts of the Compound I species for 2c.
Scheme 2.
Different disproportionation equilibrium constants Kdis in three porphyrin systems.
Acknowledgments
This work was supported by grants from the National Science Foundation and the National Institutes of Health (GM-48722 to MN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ortiz de Montellano PR. Cytochrome P450 Structure, Mechanism, and Biochemistry. 3nd ed. New York: Kluwer; 2005. [Google Scholar]
- 2.Sono M, Roach MP, Coulter ED, Dawson JH. Heme-containing oxygenases. Chemical Reviews. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 3.Pan ZZ, Wang Q, Sheng X, Horner JH, Newcomb M. Highly Reactive Porphyrin-Iron-Oxo Derivatives Produced by Photolyses of Metastable Porphyrin-Iron(IV) Diperchlorates. J. Am. Chem. Soc. 2009;131:2621–2628. doi: 10.1021/ja807847q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell SR, Groves JT. A Highly Reactive P450 Model Compound I. J. Am. Chem. Soc. 2009;131:9640–9641. doi: 10.1021/ja903394s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denisov IG, Mak PJ, Makris TM, Sligar SG, Kincaid JR. Resonance Raman Characterization of the Peroxo and Hydroperoxo Intermediates in Cytochrome P450. J. Phys. Chem. A. 2008;112:13172–13179. doi: 10.1021/jp8017875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egawa T, Shimada H, Ishimura Y. Evidence for Compound I Formation in the Reaction of Cytochrome-P450cam with m-Chloroperbenzoic Acid. Biochem. Biophys. Res. Commun. 1994;201:1464–1469. doi: 10.1006/bbrc.1994.1868. [DOI] [PubMed] [Google Scholar]
- 7.Jayaraj K, Terner J, Gold A, Roberts DA, Austin RN, Mandon D, Weiss R, Bill E, Muther M, Trautwein AX. Influence of meso substituents on electronic states of (oxoferryl)porphyrin pi-cation radicals. Inorg. Chem. 1996;35:1632–1640. doi: 10.1021/ic951058v. [DOI] [PubMed] [Google Scholar]
- 8.Groves JT, Haushalter RC, Nakamura M, Nemo TE, Evans BJ. High-valent iron-porphyrin complexes related to peroxidase and cytochrome P-450. J. Am. Chem. Soc. 1981;103:2884–2886. [Google Scholar]
- 9.Mandon D, Weiss R, Jayaraj K, Gold A, Terner J, Bill E, Trautwein AX. Models for peroxidase compound I: generation and spectroscopic characterization of new oxoferryl porphyrin p cation radical species. Inorg. Chem. 1992;31:4404–4409. [Google Scholar]
- 10.Kitagawa T, Mizutani Y. Resonance Raman-Spectra of Highly Oxidized Metalloporphyrins and Heme-Proteins. Coord. Chem. Rev. 1994;135:685–735. [Google Scholar]
- 11.Bell SEJ, Cooke PR, Inchley P, Leanord DR, Lindsay Smith JR, Robbins A. Oxoiron(IV) porphyrins derived from charged iron(III) tetraarylporphyrins and chemical oxidants in aqueous and methanolic solutions. J. Chem. Soc.-Perkin Trans. 1991;2:549–559. [Google Scholar]
- 12.Chin D-H, La Mar GN, Balch AL. Role of ferryl (FeO2+) complexes in oxygen atom transfer reactions. Mechanism of iron(II) porphyrin catalyzed oxygenation of triphenylphosphine. J. Am. Chem. Soc. 1980;102:5945–5947. [Google Scholar]
- 13.Terner J, Palaniappan V, Gold A, Weiss R, Fitzgerald MM, Sullivan AM, Hosten CM. Resonance Raman spectroscopy of oxoiron(IV) porphyrin [pi]-cation radical and oxoiron(IV) hemes in peroxidase intermediates. J. Inorg. Biochem. 2006;100:480–501. doi: 10.1016/j.jinorgbio.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Green MT, Dawson JH, Gray HB. Oxoiron(IV) in chloroperoxidase compound II is basic: Implications for P450 chemistry. Science. 2004;304:1653–1656. doi: 10.1126/science.1096897. [DOI] [PubMed] [Google Scholar]
- 15.Nam W, Park SE, Lim IK, Lim MH, Hong JK, Kim J. First direct evidence for stereospecific olefin epoxidation and alkane hydroxylation by an oxoiron(IV) porphyrin complex. J. Am. Chem. Soc. 2003;125:14674–14675. doi: 10.1021/ja0368204. [DOI] [PubMed] [Google Scholar]
- 16.Groves JT, Gross Z, Stern MK. Preparation and reactivity of oxoiron(IV) porphyrins. Inorg. Chem. 1994;33:5065–5072. [Google Scholar]
- 17.Colclough N, Smith JRL. A Mechanistic Study of the Oxidation of Phenols in Aqueous-Solution by Oxoiron(Iv) Tetra(N-Methylpyridyl)Porphyrins - a Model for Horseradish-Peroxidase Compound-Ii. J. Chem. Soc.-Perkin Trans. 1994;2:1139–1149. [Google Scholar]
- 18.Gold A, Weiss R. High-valent iron porphyrins. J. Porphyrins Phthalocyanines. 2000;4:344–349. [Google Scholar]
- 19.Pan ZZ, Zhang R, Newcomb M. Kinetic studies of reactions of iron(IV)-oxo porphyrin radical cations with organic reductants. J. Inorg. Biochem. 2006;100:524–532. doi: 10.1016/j.jinorgbio.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Nam W, Jin SW, Lim MH, Ryu JY, Kim C. Anionic Ligand Effect on the Nature of Epoxidizing Intermediates in Iron Porphyrin Complex-Catalyzed Epoxidation Reactions. Inorg. Chem. 2002;41:3647–3652. doi: 10.1021/ic011145p. [DOI] [PubMed] [Google Scholar]
- 21.Gross Z, Nimri S. A Pronounced Axial Ligand Effect on the Reactivity of Oxoiron(Iv) Porphyrin Cation Radicals. Inorg. Chem. 1994;33:1731–1732. [Google Scholar]
- 22.Fujii H. Effects of the electron-withdrawing power of substituents on the electronic structure and reactivity in oxoiron(IV) porphyrin p-cation radical complexes. J. Am. Chem. Soc. 1993;115:4641–4648. [Google Scholar]
- 23.Fujii H. Electronic structure and reactivity of high-valent oxo iron porphyrins. Coord. Chem. Rev. 2002;226:51–60. [Google Scholar]
- 24.Goh YM, Nam W. Significant electronic effect of porphyrin ligand on the reactivities of high-valent iron(IV) oxo porphyrin cation radical complexes. Inorg. Chem. 1999;38:914–920. doi: 10.1021/ic980989e. [DOI] [PubMed] [Google Scholar]
- 25.Dolphin D, Traylor TG, Xie LY. Polyhaloporphyrins: Unusual Ligands for Metals and Metal-Catalyzed Oxidations. Acc. Chem. Res. 1997;30:251–259. [Google Scholar]
- 26.Pan ZZ, Newcomb M. Kinetics and Mechanism of Oxidation Reactions of Porphyrin-Iron(IV)-Oxo Intermediates. Inorg. Chem. 2007;46:6767–6774. doi: 10.1021/ic700395j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machii K, Watanabe Y, Morishima I. Acylperoxo-Iron(III) Porphyrin Complexes - a New Entry of Potent Oxidants for the Alkene Epoxidation. J. Am. Chem. Soc. 1995;117:6691–6697. [Google Scholar]
- 28.Sugimoto H, Tung HC, Sawyer DT. The formation, characterization, and reactivity of the oxene adduct of [tetrakis(2,6-dichlorophenyl)porphinato]iron(III) perchlorate in acetonitrile. Model for the reactive intermediate of cytochrome P-450. J. Am. Chem. Soc. 1988;110:2465–2470. [Google Scholar]
- 29.Zhang R, Horner JH, Newcomb M. Laser Flash Photolysis Generation and Kinetic Studies of Porphyrin-Manganese-Oxo Intermediates. Rate Constants for Oxidations Effected by Porphyrin-MnV-Oxo Species and Apparent Disproportionation Equilibrium Constants for Porphyrin-MnIV-Oxo Species. J. Am. Chem. Soc. 2005;127:6573–6582. doi: 10.1021/ja045042s. [DOI] [PubMed] [Google Scholar]
- 30.Groves JT, Stern MK. Synthesis, Characterization, and Reactivity of Oxomanganese(Iv) Porphyrin Complexes. J. Am. Chem. Soc. 1988;110:8628–8638. [Google Scholar]
- 31.Groves JT, Kruper WJ, Jr, Haushalter RC, Butler WM. Synthesis, Characterization, and Molecular Structure of Oxo(porphyrinato)chromium(IV) Complexes. Inorg. Chem. 1982;21:1363–1368. [Google Scholar]
- 32.Keq=vobs[1]/k3[benzyl alcohol][H+][2]2, where vobs[1]=~10−8 M2 s−1 and vobs is the observed reaction rate, k3 is the second-order rate constant for oxidation by 3 (17.5 M−1s−1 from tetramesitylporphyrin was used for the estimation), [benzyl alcohol]=0.1 M, [H+]=10−7 M in a mixed solvent of acetonitrile and water (1:1, v/v) containing no excess m-CPBA, and [2]=10−5 M.
- 33.Rodgers KR, Reed RA, Su YO, Spiro TG. Resonance Raman and Magnetic-Resonance Spectroscopic Characterization of the Fe(I), Fe(II), Fe(III), and Fe(IV) Oxidation-States of Fe(2-Tmpyp)N+(Aq) Inorg. Chem. 1992;31:2688–2700. [Google Scholar]
- 34.Chen SM, Su YO. Electrochemical and Spectral Characterization of Stable Iron(Iv) Tetrakis-5,10,15,20-(N-Methyl-4-Pyridyl)Porphyrin in Aqueous-Solution at Room-Temperature. J. Chem. Soc.-Chem. Commun. 1990:491–493. [Google Scholar]