Abstract
Protease activated receptor 2 (PAR2) is widely-distributed (lung, liver, kidney, etc.) and expressed by variety of cells (i.e. leukocytes, epithelial cells, endothelial cells, and fibroblast). PAR2 may participate in many pathological processes, such as, inflammation, injury, as well as fibrosis. Therefore, in this study, we tested whether PAR2 would exert a role in acid-induced acute lung injury, E. coli pneumonia, bleomycin-induced acute lung injury and fibrosis. Acid, E. coli, or bleomycin were intratracheally instilled into the lungs of both wildtype and PAR2 knockout mice to detect differences in pulmonary edema, lung vascular permeability, lung fibrosis, and other parameters. Knockout of PAR2 did not affect the extent of pulmonary edema and lung vascular permeability in acid-induced acute lung injury. Also, both activation of PAR2 in the airspaces of the lung and deletion of PAR2 did not alter the magnitude of pulmonary edema and lung vascular permeability in E. coli pneumonia. Finally, PAR2 deficiency did not affect the severity of lung inflammation and lung fibrosis in bleomycin-induced acute lung injury and lung fibrosis models. Thus, PAR2 does not appear to play a critical role in the pathogeneses of experimental acid-induced acute lung injury, E. coli pneumonia, and bleomycin-induced acute lung injury and pulmonary fibrosis in mice.
Keywords: Protease activation receptor 2, acute lung injury, lung fibrosis, pulmonary edema, pneumonia
INTRODUCTION
Acute lung injury (ALI) is a clinical syndrome of acute respiratory failure with a mortality of approximately 40% (Rubenfeld, et al., 2005). The pathological features include severe pulmonary edema in the acute stage and fibrotic lesions in the later phase in some patients (Ware & Matthay, 2000). Considerable experimental and clinical evidence has linked coagulation and inflammation (Coughlin, 2000; Su et al., 2005).
The rationale for testing the role of protease activated receptor 2 (PAR2) in acute lung injury and lung fibrosis is based on several experimental studies. Leukocytes and endothelial cells express PAR2 and activation of PAR2 increases vascular permeability (Kawabata et al., 1998; Vergnolle et al., 1999a), activates NF-κB in microvascular endothelial cells (Nguyen et al., 2003), and induces P-selectin-mediated leukocyte rolling (Lindner et al., 2000). PAR2 is also involved in the induction of serum IL-18 and IL-18-dependent liver injury (Ikawa et al., 2005). Other studies have shown that airway epithelial cells express PAR2 and PAR2 activation inhibits recruitment of PMN into the airways after LPS challenge (Moffatt et al., 2002), protects rats from LPS-induced airway injury (Morello et al., 2005), decreases synthesis of proinflammatory cytokines (IL-1β, IL-12, and IFN-γ) and reduces lethality in colitis (Fiorucci et al., 2001). However, one study indicates that knockout of PAR2 does not protect mice from the lethal effects of endotoxemia (Camerer et al., 2006). In chronic inflammation, PAR2 expression is upregulated (Ferrell et al., 2003). PAR2 is expressed by fibroblast and may involve in fibro-proliferative processes of delayed radiation enteropathy (Wang et al., 2003) and the pathogenesis of IgA nephropathy (IgAN), which is characterized by interstitial fibrosis (Grandaliano et al., 2003).
PAR2 is expressed by variety of cells and involve in many pathological process, such as, inflammation, injury, as well as fibrosis, however, whether PAR2 exerts a role in acid-induced acute lung injury, E. coli pneumonia, bleomycin-induced acute lung injury and fibrosis has not been fully investigated. Therefore, in this study, acid, E. coli, or bleomycin were instilled into the lungs of both wildtype and PAR2 knockout mice to compare the differences in pulmonary edema, lung vascular permeability, lung fibrosis, and other parameters.
MATERIALS AND METHODS
Transgenic mice
PAR2 knockout (ko) mice (PAR2−/−, background was greater than 97% C57BL6) and wildtype (wt) mice (8–10 weeks) were generouly provided by Dr. Shaun Coughlin from Univeristy of California San Francisco. Deficiency in PAR2 was generated by targeted gene disruption as described previously (Lindner et al., 2000; Sambrano et al., 2001). The mice had free access to water and food and were maintained in a standard air-filtered ventilated animal house with 12 h light cycles. In all studies, the primary investigator (XS) was blinded to the mouse genotype until completion of the data analysis. The genotypes of the mice were double confirmed after the results came out. All protocols were approved by the University of California, San Francisco Committee on Animal Research.
Chemicals and reagents
SLIGRL-NH2 (PAR2 activating peptide, PAR2-AP) was synthesized as carboxyl amides and purified by reverse-phase high performance liquid chromatography (AnaSpec, San Jose, CA). The PAR2-AP was dissolved in HEPES-buffered Hanks at concentration of 50 mM. Bleomycin (Nippon Kayaku Co. Ltd., Tokyo, Japan) was dissolved in 0.9 % saline.
Direct visualized instillation (DVI)
To deliver different causative agents (acid, E. coli, and bleomycin) into airspaces, we used a direct visualization instillation method as previously described (Su et al., 2004). Briefly, mice were anesthetized by an ip of ketamine (90 mg/kg) and xylazine (10 mg/kg). The mice were suspended with incisors attached to a ~60° wood support by 3/0 suture. A cold-light source (Dolan-Jenner Industries Inc, Lawrence, MA) with two 25 inch flexible fiber-optic arms allowed transillumination to visualize glottis and vocal cords to deliver the causative agents into the airspaces based on the different animal models.
Acid-induced acute lung injury
An established acid-induced acute lung injury mouse model was used (Song et al., 2000). Briefly, mice were intratracheally instilled with pH 1.0 hydrochloride acid (prepared in 2/3 saline, 1.25 ml/kg) by the DVI method. Before exposure to acid, mice were given an injection of 0.05 μCi 125-I albumin via the right jugular vein. Mice were monitored for 4 hours and sacrificed to process for extravascular lung water and lung vascular permeability.
Preparation of E. coli
E. coli serotype K1 was originally isolated from the blood of a patient with biliary sepsis. The methods used to passage, store and amplify the bacteria have been described elsewhere (Matute-Bello et al., 2001). To count E. coli, we measured the optical density (OD) of the diluted E. coli solutions by a spectrophotometer. An equation Y = 4.2 × 108X0.864 was used to calculate the counts of E. coli. Y represents the count of E. coli, and X is the OD value of the diluted E. coli solution.
E. coli-induced acute pneumonia
As in our prior experiments (Su et al., 2006), mice were intratracheally instilled with 25 μl of 107 cfu E. coli (prepared in 0.9 % saline) by the DVI method. Before exposure to E. coli, mice were given an injection of 0.05 μCi 125I albumin via right jugular vein. Mice were monitored for 4 hours and sacrificed to process lung water and lung vascular permeability. Five minutes before the endpoint of the experiment, arterial blood was withdrawn via left ventricle puncture under anesthesia of inhalation of halothane. The arterial blood gases analyses were measured by a blood gas analyzer (Diamond Diagnostics Inc, Holliston, MA).
Bleomycin-induced acute lung injury and lung fibrosis
Established bleomycin-induced acute lung injury (Pittet et al., 2001) and lung fibrosis (Hao et al., 2001) mouse models were used. The mice were intratracheally instilled with bleomycin (prepared in 0.9 % saline; 2 unit/kg for acute lung injury; 1 unit/kg for lung fibrosis; 1.25 ml/kg) by the DVI method. The mice were monitored for 7 days in acute lung injury model and for 14 days in lung fibrosis model.
Extravascular lung water (ELW) and lung extravascular plasma equivalent (EPE)
The lungs were removed, counted in the γ-counter (Packard, Meriden, CT), weighed, homogenized (after addition of 1 ml distilled water). The blood was collected through right ventricle puncture. The homogenate was weighed and a portion was centrifuged (16,000 × g, 8 min) for assay of hemoglobin concentration in the supernatant. Some portion of homogenate, supernatant and blood was weighed and then desiccated in an oven (60°C for 24 h) for gravimetric determination of extravascular lung water. The lung wet-to-dry weight ratio (lung W/D ratio) was calculated by a standard formula (Su et al., 2005). ELW was calculated by: (lung W/D ratioexperimental × Lung dry weightexperimental − lung W/D rationormal × Lung dry weightnormal) × 1000 (μl). Lung extravascular plasma equivalents (EPE) (index of lung vascular permeability to protein) were calculated as the counts of 125I-albumin in the lung tissue divided by the counts of 125I-albumin in the plasma (Su et al., 2005).
Bronchoalveolar lavage
We used the methods as previously described (Su et al., 2005). The white blood cells were counted by a counter (Beckman Coulter Inc., Fullerton, CA). Protein concentration in the BAL was determined by a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA).
Hydroxyproline assay
Hydroxyproline content in the lungs was measured by the method as previously described (Reddy et al., 1996). The lung parenchyma was homogenized in 1.0 ml of deionized water. Homogenete (25 μl) was mixed with 25 μl of 4 N NaOH, and hydrolyzed by autoclaving at 120°C for 30 min. Chloramine-T reagent (Sigma, St. Louis, MO, 0.056 M, 450 μl) was added to the hydrolyzate, and oxidation was allowed to proceed for 25 min at room temprature. Then, Ehrlich's solution (500 μl) was added and the samples were incubated at 65°C for 20 min. Absorbance was measured at 570 nm in a microplate reader (Dynex Technologies, Sunnyvale, CA). Standard curves were generated for each experiment using reagent hydroxyproline (Sigma, St. Louis, MO) as a standard. The results were expressed as micrograms of hydroxyproline contained per gram lung tissue.
Histology
After euthanasia by inhalation of halothane, the chest and abdomen were rapidly opened, the base of the heart was clamped to prevent escape of the pulmonary blood volume. The thoracic organs were removed en bloc, and 10% formalin was instilled through the trachea at a pressure of 25 cm H2O. After 72 h fixation, lungs were Paraffin-embedded, and 5-μm thick sections were cut and stained with hematoxylin and eosin.
Fibrin deposition in the lung
For fibrin immunostaining, 5 μm-thick sections of frozen lung were fixed in 10% buffered formalin containing 2% acetic acid for 30 min at room temperature. Sections were blocked with PBS containing 10% horse serum (i.e., blocking solution; Vector Laboratories) for 30 min, and this was followed by incubation overnight at 4°C with goat anti-mouse fibrinogen diluted (1:1000, ICN Pharmaceuticals, Aurora, OH) in blocking solution. Next, sections were incubated for 3 h with donkey antigoat secondary antibody in blocking solution for 3 h.
Specific experimental protocols
To test this hypothesis, PAR2−/− and wildtype mice were intratracheally exposed to acid, E. coli or bleomycin. In PAR2−/− mice with acid or E. coli induced acute lung injury, extravascular lung water and lung vascular permeability were measured at 4 h after challenge. In PAR2−/− mice with bleomycin-induced acute lung injury, we carried out histology, measured extravascular lung water, protein concentration and leukocyte counts in the BAL, and hydroxyproline in the lung homogenate.
Statistical analysis
Student's t test or One-way analysis of variance (ANOVA) with post hoc Bonferroni test was used (level set at P < 0.05). Results are presented as mean ± SD.
RESULTS
Effect of PAR2 deficiency on extravascular lung water and lung vascular permeability in acid-induced acute lung injury (ALI)
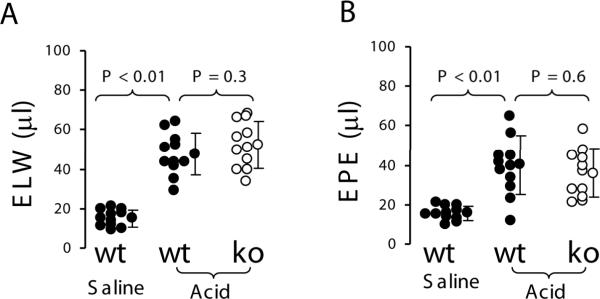
To study whether PAR2 is involved in acid-induced ALI, acid was intratracheally (IT) instilled into the lungs of wildtype (n = 11) and PAR2 knockout mice (n = 11). Wildtype mice (n = 11) were IT instilled with 2/3 saline as control. Four hours later, extravascular lung water and lung vascular permeability in wildtype mice receiving acid were about 3-fold increased compared to wildtype mice receiving saline. However, there were no differences in extravascular lung water and lung vascular permeability between the wildtype and PAR2 knockout mice challenged with acid (Figure 1).
Figure 1.
PAR2 deficiency did not display effect on acid-induced acute lung injury in mouse model. A. Extravascular lung water. B. Lung vascular permeability. Wildtype (n = 11) and PAR2 −/− mice (n = 11) were IT instilled with pH 1.0 hydrochloride acid at dosage 1.25 ml/kg and euthanized at 4 h. Wildtype mice (n = 11) were IT instilled with 2/3 saline as control. Data were pooled in the 4 times of experiments. Data are mean ± SD.
Effect of pretreatment with PAR2 agonist on E. coli pneumonia
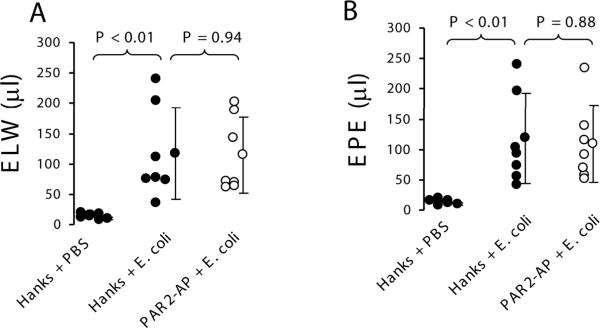
To study whether PAR2 activation alters lung injury in E. coli pneumonia, two groups of wildtype mice (n = 7 each) were instilled with either Hanks or 25 μl of 50 mM PAR2-AP 10 min before exposed to 107 cfu of E. coli. The control wildtype mice (n = 5) were IT instilled with Hanks before challenged with PBS. Four hours later, extravascular lung water and lung vascular permeability were 10-fold increased in wildtype mice receiving E. coli compared to wildtype mice receiving PBS. However, there were no differences in extravascular lung water and lung vascular permeability between wildtype mice receiving Hanks and PAR2-AP challenged with E. coli (Figure 2).
Figure 2.
Activation of PAR2 in the airspaces of the lung did not have impact on extravascular lung water and lung vascular permeability in the E. coli pneumonia model in mice. A. Extravascular lung water. B. Lung vascular permeability. Wildtype mice were IT instilled either Hanks or 25 μl of 50 mM PAR2-AP before challenged with 107cfu E. coli. N = 7 in each group. The control wildtype mice (n = 5) were IT instilled with Hanks before challenged with PBS. Data were pooled in the 3 times of experiments. Data are mean ± SD.
Effect of PAR2 knockout on lung edema, lung vascular permeability, and arterial blood gases in E. coli pneumonia
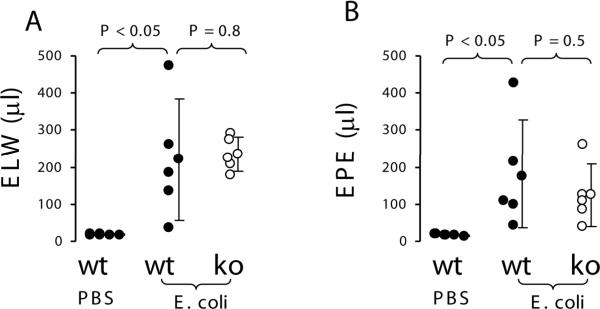
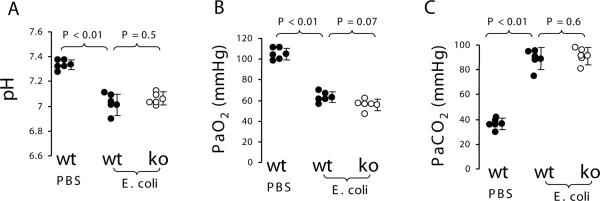
To investigate whether knockout of PAR2 would alter extravascular lung water and lung vascular permeability in E. coli pneumonia, wildtype and PAR2 knockout mice (n = 5, each) were instilled intratracheally with 107 cfu of E. coli. Wildtype mice (n = 3) were IT instilled with PBS as control. Four hours later, extravascular lung water and lung vascular permeability in wildtype mice receiving E. coli were 10~15 fold increased compared to wildtype mice receiving PBS. However, there were no differences in extravascular lung water and lung vascular permeability between the wildtype and PAR2 knockout mice challenged with E. coli (Figure 3). In another set of experiments, wildtype mice receiving IT E. coli developed severe acidosis, hypoxemia, and hypercapnia compared to wildtype mice receiving IT PBS, but there were no differences in these parameters between wildtype and PAR2 knockout mice challenged with E. coli (Figure 4). There were no differences in the numbers of E. coli in the lung homogenate between the two groups of mice (data not shown).
Figure 3.
PAR2 deficiency did not have an impact on E. coli pneumonia model in mice. A. Extravascular lung water. B. Lung vascular permeability. Wildtype (n = 5) and PAR2 −/− (n = 5) mice were instilled with 25 μl of 107 cfu E. coli and sacrificed at 4 h. Wildtype mice (n = 3) receiving IT PBS were used as control. Data were pooled in the 3 times of experiments. Data are mean ± SD.
Figure 4.
PAR2 deficiency did not affect arterial blood gases in E. coli pneumonia model in mice. A. pH. B. PaO2. C.PaCO2. Wildtype (n = 5) and PAR2 −/− (n = 5) mice were IT instilled with 25 μl of 107 cfu E. coli. Wildtype mice (n = 5) receiving IT PBS were used as control. Five minutes before being sacrificed, the mice were anesthetized by inhalation of halothane. The arterial blood was drawn by left ventricle puncture. Data were pooled in the 3 times of experiments. Data are mean ± SD.
Effect of PAR2 deficiency on lung edema in bleomycin-induced acute lung injury
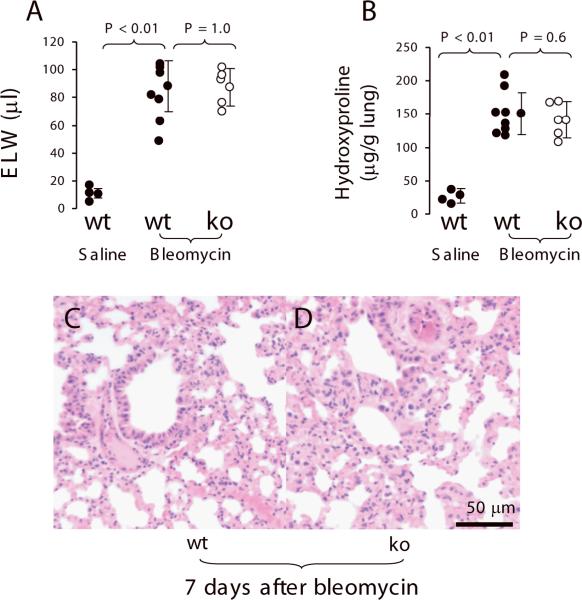
To test whether PAR2 deficiency would reduce extravascular lung water in bleomycin-induced acute lung injury, PAR2 knockout (n = 5) and wildtype mice (n = 8) were instilled with bleomycin (2 unit/kg). The wildtype mice (n = 3) receiving IT 0.9% saline were used as control. Mice were monitored for 7 days. Mortality in the PAR2 knockout mice was 1/5 (20%) and in the wildtype group was 2/8 (25%). The right lung was removed to measure extravascular lung water, and the left lung was used for histology. Extravascular lung water was 6-fold increased in wildtype mice receiving bleomycin compared to wildtype mice receiving saline. But there was no difference in extravascular lung water between wildtype and PAR2 −/− mice receiving bleomycin (Figure 5A). The levels of hydroxyproline in the lung homogenate were 3.5-fold increased in wildtype mice receiving bleomycin compared to wildtype mice receiving saline. However, there was no difference in hydroxyproline of the lung homogenate compared wildtype and PAR2 −/− mice receiving bleomycin (Figure 5B). The severity of pathological changes in the wildtype and PAR2 knockout mice receiving bleomycin were similar (Figure 5C and 5D).
Figure 5.
PAR2 deficiency did not show effect on bleomycin-induced acute lung injury (7 days) in murine model. Wildtype (n = 8) and PAR2 −/− mice (n = 5) were IT exposed with bleomycin at dosage of 2 units/kg. Wildtype (n = 3) receiving IT saline were used as control. A. Extravascular lung water. The right lungs of the dead and the survival (sacrificed at 7 days) were removed to measure extravascular lung water. B. The level of hydroxyproline in the lung homogenate. C, D. Representative histological changes of bleomycin-induced acute lung injury. The left lungs were edematous, consolidate, hemorrhagic, and extensively infiltrated with inflammatory cells. Data were pooled in the 2 times of experiments. Data are mean ± SD.
Effect of PAR2 knockout on bleomycin-induced lung fibrosis
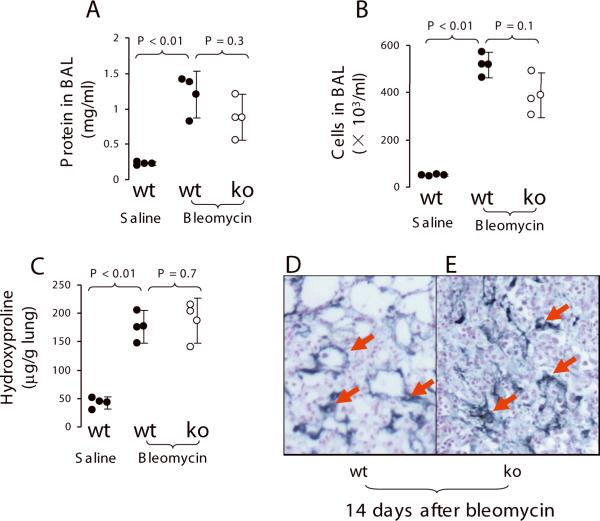
To test whether PAR2 knockout would reduce lung fibrosis over a longer time period, PAR2 knockout (n = 5), and wildtype (n = 3) mice were instilled with bleomycin (1 unit/kg), and then monitored for 14 days. The wildtype mice (n =3) receiving IT 0.9% saline were used as control. Mortality in the PAR2 −/− group was 2/5 (40%) and no mice died in the wildtype. The survivors (n = 3, in each group) were sacrificed at end of the experiment at 14 days. The right lung was lavaged to measure protein concentration and to count leukocytes. After lavage, the lungs were removed for measurement of hydroxyproline. Also, BAL protein concentration and leukocytes were 6~10 fold increased in wildtype mice receiving bleomycin compared to wildtype receiving saline. But, there were no differences in BAL protein concentration and leukocytes between wildtype and PAR2 knockout mice challenged with bleomycin (Figure 6A and 6B). Also, hydroxyproline in lung homogenate was 4.5-fold increased in wildtype mice receiving bleomycin compared to wildtype receiving saline. But, there was no difference in hydroxyproline of lung homogenate between wildtype and PAR2 knockout mice challenged with bleomycin (Figure 6C). Fibrin was prominently accumulated in the lung parenchyma, but the immunoreactivity did not differ in both wildtype and PAR2 knockout mice challenged with bleomycin (Figure 6D, E).
Figure 6.
PAR2 deficiency did not affect bleomycin-induced lung fibrosis. PAR2 −/− (n = 5) and wildtype (n = 3) were IT instilled with bleomycin (1 unit/kg). The wildtype mice (n =3) receiving IT 0.9% saline were used as control. A. Protein concentration in BAL. B. leukocyte counts in BAL in the BAL. C. Hydroxyproline levels in the lung homogenate. D, E. Representative microphotograph of immunohistochemistry staining for fibrin (arrows indicate positive stain, original magnification × 63, scale bar 50 μm). N = 3 in each group. Data were pooled in the 2 times of experiments. Data are mean ± SD.
DISCUSSION
In this study, we tested three different clinically relevant experimental models of lung injury. The main result was that PAR2 deficiency did not affect acid-induced acute lung injury, E. coli pneumonia, or bleomycin-induced acute lung injury and lung fibrosis.
In this study, we have tested whether PAR2 plays a role in different lung injury models in the development phase. Four hours after acid or E. coli challenge, acid-induced acute lung injury and E. coli pneumonia developed. Different from acid-induced ALI and E. coli pneumonia, bleomycin-induced acute lung injury and lung fibrosis models are relatively slow to develop. It takes 7 days to develop severe pulmonary edema and 14 days to develop prominent lung fibrosis.
Aspiration of gastric contents is reported to be associated with a 26–36% incidence of ARDS (Hudson et al., 1995). Acid-induced lung injury is characterized by increased lung vascular permeability and pulmonary edema in this study (Figure 1A and 1B). Aspirated HCl may promote PMN adhesion, activation, and sequestration (Nagase et al., 1999; Folkesson et al., 1995). We also found depletion of neutrophils with vinblastine prevented acid-induced pulmonary edema and reduced lung vascular permeability (data not shown). It has been reported PAR2 activation increases rolling and adherence of leukocytes, especially neutrophils (Vergnolle et al. 1999b; Shpacovitch et al., 2004). Delayed onset of inflammation occurred in PAR2 deficient mice (Lindner et al., 2000). Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms (Su et al., 2005). However, we did not find an effect of PAR2 deficiency on extravascular lung water and lung vascular permeability in acid-induced acute lung injury compared to the wildtype to PAR2 −/− mice. Thus, PAR2 activation does not participate in acid-induced acute lung injury.
There were several reasons to study E. coli pneumonia in PAR2 −/− mice. (i) Acute bacterial pneumonia is the most common cause of clinical acute lung injury (Eisner et al., 2001). (ii) PAR2 activators (tryptase, trypsin) are elevated in pneumonia, which increase neutrophils recruitment (possibly due to tryptase cleaving PAR2) to combat bacterial infections (Huang et al., 2001; Orlowski & Lesser 1989). (iii) Activation of coagulation (high levels of thrombin-antithrombin complexes, soluble tissue factor and factor VIIa) was detected in BALF from infected lungs (Choi, et al., 2004). Also, PAR2 may be activated directly by TF/FVIIa and indirectly by TF/FVIIa-generated FXa (Camerer et al., 2000). However, in the pneumonia model, neither pre-activated PAR2 (Figure 2) or PAR2 deficiency (Figure 3) altered extravascular lung water and lung vascular permeability. Also, there were no differences in E. coli counts in the lung homogenate between the wildtype and PAR2 −/− mice. Thus, PAR2 activation does not appear to determine the severity of gram negative pneumonia. However, there is evidence that PAR2 involves in murine pulmonary Pseudomonal infection (Moraes et al., 2008). In that study, mice were intratracheally challenged with P. aeruginosa and followed for 24 h, a finding that may contribute to different results compared to our studies.
Pulmonary fibrosis induced by intratracheal instillation of bleomycin is an acute lung injury model followed by rapid development of pulmonary fibrosis, which eventually resolves in five to six weeks (Miyazaki et al., 2004). Several prior studies provided evidence that influenced us to study bleomycin-induced acute lung injury and fibrosis, including: (i) TGF-β is a mediator of bleomycin-induced acute lung injury (Pittet et al. 2001), and PAR2 activation induces TGF-β expression (Grandaliano et al., 2003), (ii) the process of injury is accompanied by neutrophil recruitment (Hayashi et al., 2002) and mast cells accumulation, (iii) Tryptase from mast cells can activate PAR2 in the fibroblast to induce fibroproliferative actions (Akers et al., 2000; Frungieri et al., 2002). However, in the bleomycin-induced acute lung injury model, PAR2 deficiency did not alter extravascular lung water (Figure 5A) and hydroxyproline content in the lung homogenate (Figure 5B), and pathological changes (Figure 5D). Moreover, in the bleomycin-induced lung fibrosis model, there were no differences in protein (Figure 6A) and leukocyte counts in the bronchoalveolar lavage (Figure 6B), and hydroxyproline content in the lung homogenate (Figure 6C) fibrin deposition in the lung parenchyma (Figure 6E). These findings suggest that PAR2 activation is not involved in bleomycin-induced acute lung injury and lung fibrosis.
Taken together, PAR2 is activated redundantly by proteases under physiological and pathological conditions. However, based on studies in mice, PAR2 does not play a major role in the pathogeneses of acid-induced acute lung injury, E. coli pneumonia, and bleomycin-induced acute lung injury and pulmonary fibrosis.
ACKNOWLEDGEMENTS
We greatly appreciate Dr. Eric Camerer for his valuable critiques and suggestions. This study was supported by National Heart, Lung, and Blood Institute Grants HL-51854 and HL-51856 (to M.A.M.)
LITERATURE CITED
- Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, McAnulty RJ. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol. 2000;278:L193–201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci U S A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerer E, Cornelissen I, Kataoka H, Duong DN, Zheng YW, Coughlin SR. Roles of protease-activated receptors in a mouse model of endotoxemia. Blood. 2006;107:3912–3921. doi: 10.1182/blood-2005-08-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G, Schultz MJ, van Till JW, Bresser P, van der Zee JS, Boermeester MA, Levi M, van der Poll T. Disturbed alveolar fibrin turnover during pneumonia is restricted to the site of infection. Eur Respir J. 2004;24:786–789. doi: 10.1183/09031936.04.00140703. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Thompson T, Hudson LD, Luce JM, Hayden D, Schoenfeld D, Matthay MA. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Lockhart JC, Kelso EB, Dunning L, Plevin R, Meek SE, Smith AJ, Hunter GD, McLean JS, McGarry F, Ramage R, Jiang L, Kanke T, Kawagoe J. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S, Mencarelli A, Palazzetti B, Distrutti E, Vergnolle N, Hollenberg MD, Wallace JL, Morelli A, Cirino G. Proteinase-activated receptor 2 is an anti-inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci U S A. 2001;98:13936–13941. doi: 10.1073/pnas.241377298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest. 1995;96:107–116. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frungieri MB, Weidinger S, Meineke V, Kohn FM, Mayerhofer A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARgamma: Possible relevance to human fibrotic disorders. Proc Natl Acad Sci U S A. 2002;99:15072–15077. doi: 10.1073/pnas.232422999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandaliano G, Pontrelli P, Cerullo G, Monno R, Ranieri E, Ursi M, Loverre A, Gesualdo L, Schena FP. Protease-activated receptor-2 expression in IgA nephropathy: a potential role in the pathogenesis of interstitial fibrosis. J Am Soc Nephrol. 2003;14:2072–2083. doi: 10.1097/01.asn.0000080315.37254.a1. [DOI] [PubMed] [Google Scholar]
- Hao H, Cohen DA, Jennings CD, Bryson JS, Kaplan AM. Bleomycin-induced pulmonary fibrosis is independent of eosinophils. J Leukoc Biol. 2000;68:515–521. [PubMed] [Google Scholar]
- Hayashi S, Yatsunami J, Fukuno Y, Kawashima M, Miller EJ. Antileukinate, a hexapeptide inhibitor of CXC-chemokine receptor, suppresses bleomycin-induced acute lung injury in mice. Lung. 2002;180:339–348. doi: 10.1007/s00408-002-0106-7. [DOI] [PubMed] [Google Scholar]
- Huang C, De Sanctis GT, O'Brien PJ, Mizgerd JP, Friend DS, Drazen JM, Brass LF, Stevens RL. Evaluation of the substrate specificity of human mast cell tryptase beta I and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- Ikawa K, Nishioka T, Yu Z, Sugawara Y, Kawagoe J, Takizawa T, Primo V, Nikolic B, Kuroishi T, Sasano T, Shimauchi H, Takada H, Endo Y, Sugawara S. Involvement of neutrophil recruitment and protease-activated receptor 2 activation in the induction of IL-18 in mice. J Leukoc Biol. 2005;78:1118–1126. doi: 10.1189/jlb.0305151. [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kuroda R, Minami T, Kataoka K, Taneda M. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br J Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner JR, Kahn ML, Coughlin SR, Sambrano GR, Schauble E, Bernstein D, Foy D, Hafezi-Moghadam A, Ley K. Delayed onset of inflammation in protease-activated receptor-2-deficient mice. J Immunol. 2000;165:6504–6510. doi: 10.4049/jimmunol.165.11.6504. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Frevert CW, Kajikawa O, Skerrett SJ, Goodman RB, Park DR, Martin TR. Septic shock and acute lung injury in rabbits with peritonitis: failure of the neutrophil response to localized infection. Am J Respir Crit Care Med. 2001;163:234–243. doi: 10.1164/ajrccm.163.1.9909034. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Kuwano K, Yoshida K, Maeyama T, Yoshimi M, Fujita M, Hagimoto N, Yoshida R, Nakanishi Y. The perforin mediated apoptotic pathway in lung injury and fibrosis. J Clin Pathol. 2004;57:1292–1298. doi: 10.1136/jcp.2003.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt JD, Jeffrey KL, Cocks TM. Protease-activated receptor-2 activating peptide SLIGRL inhibits bacterial lipopolysaccharide-induced recruitment of polymorphonuclear leukocytes into the airways of mice. Am J Respir Cell Mol Biol. 2002;26:680–684. doi: 10.1165/ajrcmb.26.6.4693. [DOI] [PubMed] [Google Scholar]
- Moraes TJ, Martin R, Plumb JD, Vachon E, Cameron CM, Danesh A, Kelvin DJ, Ruf W, Downey GP. Role of PAR2 in murine pulmonary pseudomonal infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L368–377. doi: 10.1152/ajplung.00036.2007. [DOI] [PubMed] [Google Scholar]
- Morello S, Vellecco V, Roviezzo F, Maffia P, Cuzzocrea S, Cirino G, Cicala C. A protective role for proteinase activated receptor 2 in airways of lipopolysaccharide-treated rats. Biochem Pharmacol. 2005;71:223–230. doi: 10.1016/j.bcp.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Nagase T, Ishii S, Kume K, Uozumi N, Izumi T, Ouchi Y, Shimizu T. Platelet-activating factor mediates acid-induced lung injury in genetically engineered mice. J Clin Invest. 1999;104:1071–1076. doi: 10.1172/JCI7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Coelho AM, Grady E, Compton SJ, Wallace JL, Hollenberg MD, Cenac N, Garcia-Villar R, Bueno L, Steinhoff M, Bunnett NW, Vergnolle N. Colitis induced by proteinase-activated receptor-2 agonists is mediated by a neurogenic mechanism. Can J Physiol Pharmacol. 2003;81:920–927. doi: 10.1139/y03-080. [DOI] [PubMed] [Google Scholar]
- Orlowski M, Lesser M. Lung lymph capillary injury-related protease. Am J Physiol. 1989;257:L202–208. doi: 10.1152/ajplung.1989.257.4.L202. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- Shpacovitch VM, Varga G, Strey A, Gunzer M, Mooren F, Buddenkotte J, Vergnolle N, Sommerhoff CP, Grabbe S, Gerke V, Homey B, Hollenberg M, Luger TA, Steinhoff M. Agonists of proteinase-activated receptor-2 modulate human neutrophil cytokine secretion, expression of cell adhesion molecules, and migration within 3-D collagen lattices. J Leukoc Biol. 2004;76:388–398. doi: 10.1189/jlb.0503221. [DOI] [PubMed] [Google Scholar]
- Song Y, Fukuda N, Bai C, Ma T, Matthay MA, Verkman AS. Role of aquaporins in alveolar fluid clearance in neonatal and adult lung, and in oedema formation following acute lung injury: studies in transgenic aquaporin null mice. J Physiol. 2000;525(Pt 3):771–779. doi: 10.1111/j.1469-7793.2000.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Looney M, Robriquet L, Fang X, Matthay MA. Direct visual instillation as a method for efficient delivery of fluid into the distal airspaces of anesthetized mice. Exp Lung Res. 2004;30:479–493. doi: 10.1080/01902140490476382. [DOI] [PubMed] [Google Scholar]
- Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol. 2005;175:2598–2605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Hollenberg MD, Sharkey KA, Wallace JL. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR2)-activating peptides in the rat paw. Br J Pharmacol. 1999a;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999b;163:5064–5069. [PubMed] [Google Scholar]
- Wang J, Zheng H, Hollenberg MD, Wijesuriya SJ, Ou X, Hauer-Jensen M. Up-regulation and activation of proteinase-activated receptor 2 in early and delayed radiation injury in the rat intestine: influence of biological activators of proteinase-activated receptor 2. Radiat Res. 2003;160:524–535. doi: 10.1667/rr3080. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]