Abstract
Synaptic incorporation of NMDA receptors (NMDARs) is regulated by GluN2 subunits with different rules controlling GluN2A- and GluN2B-containing receptors; whereas GluN2B-containing receptors are constitutively incorporated into synapses, GluN2A incorporation is activity-dependent. We expressed electrophysiologically tagged NMDARs in rat hippocampal slices to identify the molecular determinants controlling the mode of synaptic incorporation of NMDARs. Expressing chimeric GluN2 subunits, we identified a putative N-glycosylation site present in GluN2B, but not in GluN2A, as necessary and sufficient to drive NMDARs into synapses in an activity-independent manner. This suggests a novel mechanism for regulating activity-driven changes and trafficking of NMDARs to the synapse.
Introduction
Unique properties allow NMDA-type glutamate receptors to play a critical role during maturation of neuronal circuits, synaptic plasticity, and pathology (Cull-Candy et al., 2001; Bliss et al., 2003; Cline and Haas, 2008).
The NMDA receptor (NMDAR) is an obligate heteromultimer composed of glycine-binding GluN1 subunits and one or more of the glutamate binding GluN2 subunits (A–D), or a combination of GluN2 and GluN3 subunits (Traynelis et al., 2010). GluN1–GluN3 combination may also exist, forming a glycinergic receptor (Chatterton et al., 2002). Glutamate-binding GluN2 subunits control fractional Ca2+ currents (Burnashev et al., 1995; Sobczyk et al., 2005), temporal activation profiles (Erreger et al., 2005), and allow differential interactions with signaling and scaffolding molecules (Barria and Malinow, 2005; Kim et al., 2005). Thus, GluN2 subunit composition affects properties of NMDARs and synaptic plasticity (Barria and Malinow, 2005; Zhou et al., 2007; Foster et al., 2010).
The predominant GluN2 subunits in mammalian hippocampus, GluN2A and GluN2B, exist primarily as diheteromeric GluN1/GluN2A or GluN1/GluN2B complexes. Only a small fraction exists as triheteromeric GluN1/GluN2A/GluN2B receptors (Al-Hallaq et al., 2007). GluN2B is expressed prenatally and is required for normal neuronal pattern formation and viability of the animal (Kutsuwada et al., 1996). In contrast, GluN2A subunit expression and synaptic incorporation increases progressively with age (Monyer et al., 1994; Sheng et al., 1994; Flint et al., 1997; Stocca and Vicini, 1998). This developmental switch in synaptic NMDAR subunit composition from GluN2B- to GluN2A-containing receptors requires synaptic activity or sensory experience and accelerates the decay rate of evoked NMDAR-dependent EPSCs (Yashiro and Philpot, 2008).
However, it is not clear how neuronal activity regulates the subunit composition of synaptic NMDARs. Recent evidence indicates that GluN2 subunits regulate delivery of receptors to synapses with different rules controlling GluN2A- and GluN2B-containing receptors. Synaptic incorporation of GluN2B-containing receptors is constitutive and does not require synaptic transmission. In contrast, GluN2A synaptic incorporation is activity-dependent and requires glutamate binding to NMDARs (Barria and Malinow, 2002).
Here we use electrophysiologically tagged NMDARs expressed in organotypic hippocampal slices to identify the domains determining the mode of synaptic incorporation. Our findings identify molecular differences between GluN2A and GluN2B that determine the mode of synaptic incorporation of NMDARs and suggest a novel mechanism for regulating activity-driven changes and forward trafficking of NMDARs to the synapse.
Materials and Methods
Chimeric subunits
Chimeric subunits were constructed using GFP-tagged GluN2Bwt and GluN2Awt (Barria and Malinow, 2002) and PCR-based methods. All subunits are cloned into the mammalian expression vector pCI (Promega). A description of the chimeras is provided below.
GluN2A-CTBc3 and GluN2B-CTAc3.
The last third of the C terminus of GluN2A (P1222-V1464) and GluN2B (P1219-V1482) were exchanged.
GluN2A-CTB and GluN2B-CTA.
The entire C terminus of GluN2A (L794-V1464) and GluN2B (L795-V1482) were exchanged.
GluN2A intra B.
Mutations N587C, K590D, K592R, A593E, and H595G were introduced in GluN2A-CTB.
GluN2B intra A.
Mutations C588N, D591K, R593K, E594A, and G596H were introduced in GluN2B-CTA.
NTB-GluN2A and NTA-GluN2B.
The entire N terminus of GluN2A (M1-V522) and GluN2B (M1-V523) were exchanged.
B-loop-GluN2A and A-loop-GluN2B.
The N terminus of GluN2A-CTB (M1-K628) and GluN2B-CTA (M1-K629) were swapped.
B-loop-GluN2A sites 1–3.
Predicted individual glycosylation sites present in the B-loop were modified in B-loop-GluN2A as follows: site 1, N675H/ F677Y; site 2, A700P/ E701Y; site 3, M739K.
A-loop-GluN2B site 1.
This predicted glycosylation site, absent in A-loop, was mutated in A-loop-GluN2B to mimic site 1 of GluN2B as follows: site 1, H674N/ Y676F.
A-loop-GluN2B sites 2 and 3.
Predicted individual glycosylation sites were modified in A-loop-GluN2B as follows: site 2, P699A/ Y700E; site 3, K738M.
Slice cultures and transfection
Organotypic hippocampal slices were prepared according to University of Washington guidelines from 6-d-old (p6) Sprague Dawley male and female rats as described previously (Stoppini et al., 1991). After 3–5 d in culture, slices were transfected using a biolistic particle delivery system (Woods and Zito, 2008) and cultured for an additional 48–72 h. A plasmid DNA, ratio 1:1 of GluN1 and GluN2 subunits, was used (70–100 μg each). When necessary, APV (Tocris Bioscience) was added immediately after transfection and replenished every 24 h.
Immunoblot
Homogenates from four slices cultured for different lengths of time were immunoblotted with mouse monoclonal anti-NMDAR2A antibody 2F6–3D5 (provided by Dr. Georg Köhr, Max Plank Institute, Heidelberg, Germany) or anti α-actin antibody (Millipore Bioscience Research Reagents).
Electrophysiology
Whole-cell recordings from CA1 neurons were obtained under visual guidance. The recording chamber was perfused with artificial CSF (ACSF) containing the following: 119 mm NaCl, 2.5 mm KCl, 4 mm CaCl2, 4 mm MgCl2, 26 mm NaHCO3, 1 mm NaH2PO4, 11 mm glucose, 100 μm picrotoxin (Tocris Bioscience), 2 μm 2-chloroadenosine, pH 7.4, gassed with 5%CO2/ 95%O2 at room temperature (20–25°C). Intracellular recording solution contained the following (in mm): 115 cesium methanesulfonate, 20 CsCl, 10 HEPES, 2.5 MgCl2, 2 MgATP, 2 Na2ATP, 0.4 Na3GTP, 10 sodium phosphocreatine, 5 QX-314, and 0.6 EGTA (pH 7.25 and 310 mmol/Kg). Synaptic responses were evoked with bipolar cluster electrodes (FHC) placed over Schaffer collateral fibers.
Synaptic incorporation index is mean current from a 50 ms window 110 ms after the stimulus artifact in evoked EPSCs recorded at −60 mV [recombinant NMDAR (rNMDAR)], normalized to the peak of the EPSC that represents activation of endogenous AMPARs (eAMPARs).
Statistical analysis
All results are represented as mean ± SEM. Statistical differences of these means were determined using Student's t test. Significance was set at p ≤ 0.05.
Results
Synaptic incorporation of GluN2A receptors is activity-dependent
In hippocampal slices prepared from p6 rats, expression of GluN2A is very low and increases rapidly after a week in culture (Fig. 1A). As GluN2A becomes expressed, it is incorporated into synapses, as indicated by the shortening of the decay time of NMDAR-dependent EPSC (Fig. 1B). To reduce the level of neuronal activity acting on NMDARs, we treated organotypic slices with APV, a competitive NMDAR antagonist (Davies and Watkins, 1982), for 3 d before recording. NMDAR-dependent EPSCs from APV-treated slices exhibited longer decay times compared with EPSCs from control, age-matched slices (Fig. 1B), indicating that APV treatment blocked synaptic incorporation of GluN2A receptors. This treatment did not affect expression of the GluN2A subunit (Fig. 1A). These results support the view that incorporation of GluN2A into synapses is regulated by synaptic activity and can be blocked by APV. Similar results have been described using overexpression of electrophysiologically tagged NMDARs in organotypic cultured hippocampal slices (Barria and Malinow, 2002).
Figure 1.
Synaptic incorporation of GluN2A receptors is regulated by synaptic activity. A, Expression of GluN2A in cultured organotypic hippocampal slices. Quantitative immunoblot of endogenous GluN2A from hippocampal slices prepared at p6 and cultured as indicated. Arbitrary units of GluN2A immunoblot are normalized to actin immunoblot (n = 6). Black bar is from slices treated with APV 100 μm for 3 d before harvesting. B, APV blocks incorporation of endogenous GluN2A receptors. Time to half decay of evoked EPSCs recorded at +40 mV in the presence of 2 μm NBQX from CA1 pyramidal cells from organotypic hippocampal slices after 4–5, 7–8, and 11–12 d in culture (DIC) (white bars; n = 9, 10, and 10) and slices treated with 100 μm APV for 3 d before recording (black bars; n = 3, 7, and 10). Inset, representative traces of evoked EPSCs from slices 8 DIC nontreated (Ctrl) and treated with 100 μm APV. Scale bar, 50 ms. *p < 0.05 Student's t test. C, Protein sequence comparison of GluN2A and GluN2B subunits. Left, Topology of GluN2 subunits. Membrane segments M1–M4 are indicated. Right, Comparison of GluN2A and GluN2B amino acid sequences. Alignment was made with AlignX from Vector NTI v11 (Invitrogen). Default pairwise alignment parameters and the blosum62mt2 similarity matrix were used. Specific values are assigned to each residue at a given alignment position in each aligned sequence, depending on whether the residue is identical (+1), similar (0.5), or weakly similar (0.2) to the corresponding residue of the consensus sequence. The N terminus, the C terminus, and four membrane segments are indicated.
To identify domains conferring the activity-dependent or activity-independent modes of synaptic incorporation to GluN2A and GluN2B, respectively, we expressed electrophysiologically tagged NMDARs containing chimeric GluN2 subunits in organotypic hippocampal slices.
A mutant of GluN1 (GluN1 N598R) was used as an electrophysiological tag (etag). This mutation eliminates the normal magnesium blockade of NMDARs observed at hyperpolarized potentials (Burnashev et al., 1992; Single et al., 2000). NMDARs were also optically tagged with GFP to identify transfected CA1 neurons. In transfected cells, evoked EPSCs at hyperpolarized potentials (−60 mV) exhibit a fast component due to the activation of eAMPARs and a slow component that reflects the activation of rNMDARs (Fig. 2A, inset). Previously reported data show that etag-GluN1 does not heteromerize with endogenous GluN2 subunits; therefore, it is inserted into synapses only when it heteromerizes with recombinant GluN2 subunits (Barria and Malinow, 2002). An incorporation index was calculated by measuring the late component of the EPSC (etag-NMDARs) normalized to the peak amplitude of the earlier component (endogenous AMPARs). We used this simple and quantitative assay to identify the domains on GluN2 subunits controlling the mode of synaptic incorporation.
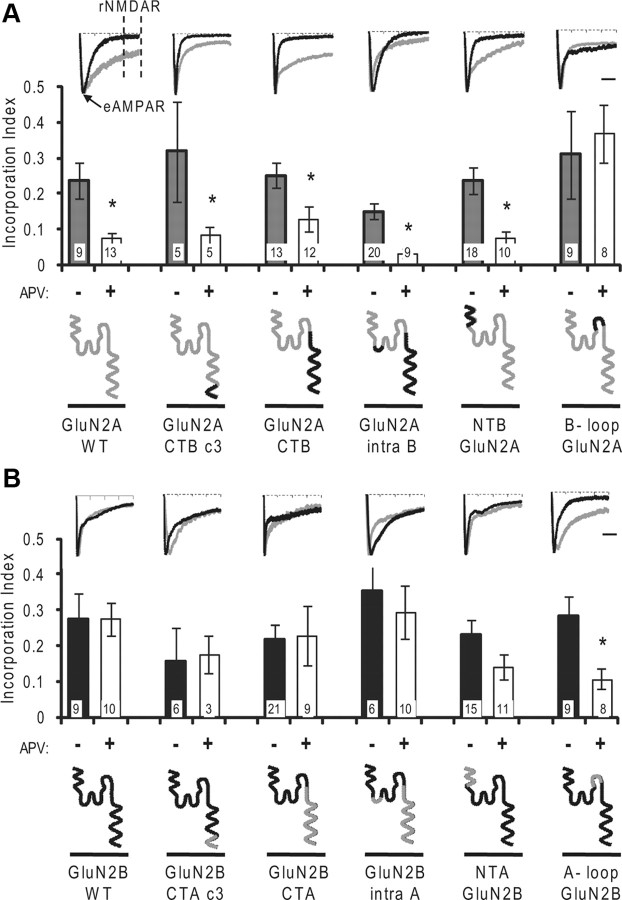
Figure 2.
Synaptic incorporation of GluN2 chimeric subunits. A, GluN2A-based chimeric subunits. Synaptic incorporation index for wild-type and chimeric GluN2A with different domains of GluN2B. Slices were cotransfected with etag-GluN1 and wild-type GluN2A or chimeric GluN2A subunits and expressed in control conditions or 100 μm APV. Incorporation index is calculated as the ratio of the late component (rNMDAR) to the early peak of the response (eAMPAR). Treatment of the slices with APV during the expression period (white bars) blocks synaptic incorporation of wild-type GluN2A (far left). Number of cells as indicated. Insets, Representative EPSCs. Black traces are from slices treated with APV during the expression period. B, GluN2B-based chimeric subunits. Synaptic incorporation index for wild-type GluN2B and chimeric GluN2B with different domains of GluN2A. Treatment of the slices with 100 μm APV during the expression period (white bars) does not block incorporation of wild-type GluN2B (far left). Insets, Representative EPSCs as in A. Scale bars, 50 ms. *p < 0.05 Student's t test.
Molecular determinants of NMDAR synaptic incorporation
At hyperpolarized potentials, endogenous NMDARs are blocked by Mg+2; therefore, as reported before, synaptic incorporation of recombinant etagged GluN2A and GluN2B can be measured as a late current from EPSCs recorded at −60 mV (Fig. 2, far left insets, gray traces). Incubation of slices with APV during the expression period blocks incorporation of recombinant GluN2A-containing receptors but not GluN2B-containing receptors (Fig. 2, far left) (Barria and Malinow, 2002).
GluN2 subunits A and B are highly similar, with 69% of identical or conserved amino acids (Ishii et al., 1993). This similarity is especially high at the four membrane domains (M1–M4) and connecting loops (94%), moderate at the N terminus (80%), and lowest at the C-terminal region (54%) (Fig. 1C).
We first swapped the last portion of the C terminus, which contains a PDZ binding domain and internalization sequences that control stabilization and removal from the plasma membrane (Lavezzari et al., 2004). Swapping this domain had no effect on the insertion behavior. Chimeric GluN2A with the last portion of GluN2B C terminus is not incorporated into synapses when slices are treated with APV (Fig. 2A). Supporting the idea that this portion of GluN2 subunits does not regulate the mode of insertion, chimeric GluN2B with the last portion of GluN2A C terminus incorporates into synapses in a constitutive manner (Fig. 2B). This result suggests that the regulatory domain of synaptic insertion resides away from previously described internalization and synapse stabilization sequences. Swapping the full C terminus of GluN2A and GluN2B also did not alter the mode of synaptic incorporation of these chimeric NMDARs, indicating that the domain controlling the mode of insertion is not located in the intracellular C terminus (Fig. 2).
Two small intracellular loops, one connecting M1 with the re-entering loop that forms the pore of the channel (M2) and the other connecting M2 with M3 (Fig. 1C, topology model), also face the cytosol and therefore could be involved in a cytosolic controlling mechanism. These two loops, 21 and 10 aa long, are identical between GluN2A and GluN2B with the exception of six residues in the larger loop. All six residues in the larger intracellular loop were swapped along with the C terminus to generate chimeric GluN2 subunits with all intracellular domains corresponding to one subunit, while the extracellular domains corresponded to the other. Chimeric GluN2A subunit with all the intracellular domains matching GluN2B was incorporated into synapses and its incorporation was blocked by APV treatment, similar to wild-type GluN2A. This indicates that the domains controlling activity-dependent incorporation must be extracellular. Confirming this, chimeric GluN2B with all the intracellular portions matching those of GluN2A was accordingly incorporated in a constitutive manner and not blocked by APV treatment, similar to wild-type GluN2B (Fig. 2). Thus, our data indicate that the domain determining the mode of synaptic incorporation is not located on GluN2 domains facing the cytosol.
The N terminus and the large loop connecting M3 and M4 face the lumen of the endoplasmic reticulum and transport organelles and the extracellular milieu once the receptor is inserted in the plasma membrane. Replacing the entire N terminus of GluN2A (NTD and S1 domains) with that of GluN2B had no effect on synaptic insertion. Thus, synaptic incorporation of NTB–GluN2A, like wild-type GluN2A, is activity-dependent. Synaptic incorporation of chimeric GluN2B with the N terminus of GluN2A, NTA–GluN2B, was not blocked by APV, indicating that its incorporation is activity-independent, like wild-type GluN2B (Fig. 2B).
The extracellular loop between M3 and M4 is 168 aa long in GluN2A and 169 in GluN2B, with 93% of identity or similarity. Chimeric GluN2A carrying the GluN2B loop (B-loop–GluN2A) is inserted into synapses, but, in contrast to wild-type GluN2A, APV does not block its incorporation. Thus, the M3–M4 loop of GluN2B confers to GluN2A the constitutive mode of synaptic incorporation characteristic of GluN2B receptors (Fig. 2A). In a symmetrical manner, chimeric GluN2B carrying the loop from GluN2A (A-loop–GluN2B) behaves like wild-type GluN2A and APV blocks its incorporation into synapses (Fig. 2B). These experiments indicate that the domain selecting the mode of synaptic incorporation faces the intraluminal side of transport organelles and lies in the loop connecting M3 and M4 and not in the N terminus.
The M3–M4 loop is almost identical between GluN2A and GluN2B subunits with 22 different residues, of which only 12 are nonconservative changes. We looked for conserved sequences that could participate in cellular processes that regulate protein assembly, sorting, or transport to the extracellular membrane. N-glycosylation is a known process involved in regulating proper protein folding, stability, quality control, and post-Golgi trafficking to plasma membranes of polarized cells (Vagin et al., 2009) and predicted glycosylation sites are present on NMDARs subunits (Moriyoshi et al., 1991; Monyer et al., 1992; Ishii et al., 1993; Everts et al., 1997).
Using NetNGlyc server (Blom et al., 2004) we found three predicted sites for N-linked glycosylation in the M3–M4 loop domain of GluN2B located at N675 (site 1), N698 (site 2), and N737 (site 3). Two of these sites, 2 and 3, are also present in GluN2A but have nonconserved substitutions around the asparagines. GluN2A lacked site 1 completely (Fig. 3A).
Figure 3.

Molecular determinant of synaptic incorporation. A, Predicted N-glycosylation sites in M3–M4 loop. Comparison of GluN2A and GluN2B sequences at the predicted N-glycosylation sites (bold). Note the absence of site 1 in GluN2A. B, Mutations of predicted N-glycosylation sites on the B-loop. Synaptic incorporation index of B-loop–GluN2A-based mutants and double-mutant GluN2A H674N/Y676F. Treatment of the slices with 100 μm APV during the expression period (white bars) blocks incorporation when site 1of B-loop is removed (far left). Number of cells as indicated. Insets, Representative EPSCs. Black traces are from slices treated with APV during the expression period. C, Mutations of predicted N-glycosylation sites on the A-loop. Synaptic incorporation index of A-loop–GluN2B-based mutants and double-mutant GluN2B N675H/F677Y. Treatment of the slices with 100 μm APV during the expression period (white bars) does not block incorporation when site 1of B-loop is added (far left). Number of cells as indicated. Insets, Representative EPSCs as in B. Scale bars, 50 ms. *p < 0.05 Student's t test.
In the chimeric GluN2A receptor containing the GluN2B loop, we mutated the residues of site 1 to those present on GluN2A (N675H and F677Y) to eliminate this potential N-glycosylation site. Removal of site 1 in the B-loopp–GluN2A chimera was sufficient to block synaptic incorporation by treatment with APV (Fig. 3B, far left). Conversely, adding site 1 of GluN2B into the A-loop–GluN2B chimera (H674N and Y676F) made the chimera incorporate into synapses in a constitutive manner, the same as wild-type GluN2B (Fig. 3C, far left). Mutations in sites 2 and 3 had no effect on the mode of synaptic incorporation (Fig. 3B,C).
A double mutation in wild-type GluN2B that removes site 1 (N675H/F677Y) removed the constitutive manner of synaptic incorporation of this receptor. Introduction of site 1 onto wild-type GluN2A (H674N/Y676F) caused the receptor to incorporate into synapses in an activity-independent manner (Fig. 3B,A, far right). These data suggest that a predicted glycosylation site at N675 in the M3–M4 loop domain of GluN2B, which is not found in GluN2A, is necessary and sufficient to direct GluN2B-containing receptors to synapses in an activity-independent manner. Absence of this potential N-glycosylation site gives NMDARs a phenotype that requires synaptic activity to incorporate into synapses.
Discussion
Synaptic incorporation of GluN2A-containing receptors requires synaptic activity or sensory experience (for review, see Yashiro and Philpot, 2008). In contrast, GluN2B-containing receptors are incorporated in a constitutive manner (Barria and Malinow, 2002). We used a simple quantitative assay to identify the molecular determinants that segregate GluN2 subunits into activity-dependent and activity-independent trafficking pathways.
The intracellular C termini of GluN2 and GluN1 subunits regulate retention in the endoplasmic reticulum (Wenthold et al., 2003) and synaptic stabilization via PDZ binding domains (Barria and Malinow, 2002; Prybylowski et al., 2002). In addition, the C termini of GluN2 subunits regulate synaptic NMDARs via tyrosine-based internalization signals (Carroll and Zukin, 2002; Prybylowski et al., 2005; Sanz-Clemente et al., 2010). We found that domains facing the cytoplasm do not regulate the mode of synaptic incorporation of NMDARs. Unexpectedly, we found that the extracellular loop between M3 and M4 of GluN2A and GluN2B was sufficient to determine the incorporation phenotype of these GluN2 subunits. Some of the differences in amino acid sequence in the loops occur on or around predicted N-glycosylation sites, a mechanism used by chaperone proteins in the Golgi to identify and move proteins to distinct trafficking vesicles (Martínez-Maza et al., 2001; Nathanson, 2008). GluN2 subunits are glycosylated proteins (Clark et al., 1998) and glycosylation can affect the NMDA receptor's assembly and function (Chazot et al., 1995; Everts et al., 1997; Standley and Baudry, 2000; Gascón et al., 2007). In polarized cells, a single glycosylation site can target proteins to the apical or basolateral membrane. Much of this sorting is thought to occur through interaction with lectins, which recognize glycosylated proteins and can direct their association with lipid rafts (Clark et al., 1998; Vagin et al., 2009).
We identified a putative N-glycosylation site in GluN2B (N675) and H674 of GluN2A, as the molecular determinants controlling the mode of synaptic incorporation. These residues lie in the M3-S2 linker region outside the D2 domain of their respective ligand binding domains. According to the crystal structure of the NMDAR GluN1/GluN2 dimer, these residues lie on the surface in a position potentially accessible to enzymatic modification (Furukawa et al., 2005).
Our data suggest that specific N-glycosylation of the GluN2B subunit could regulate receptor sorting and trafficking within the cell via differential interaction of GluN2 subunits with intraluminal proteins. This could segregate NMDARs into separate trafficking pathways with different requirements to reach the synaptic membrane. Different secretory pathways have also been proposed for AMPA-type glutamate receptors (Malinow et al., 2000) and their assembly and proper intracellular trafficking can be controlled by intraluminal sites (Greger and Esteban, 2007). Segregation of proteins into different secretory pathways could be a general mechanism to position glutamate receptors in different compartments that will react differently to synaptic activity.
Footnotes
This work was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award and National Institutes of Health–National Institute of Neurological Disorders and Stroke Grant R01NS060756 (to A.B.), and Grant PHS NRSA 2T32 GM007270 from National Institute of General Medical Sciences (to G.S.).
References
- Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL, Morris RG. Introduction: long-term potentiation and structure of the issue. Philos Trans R Soc Lond B Biol Sci. 2003;358:607–611. doi: 10.1098/rstb.2003.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. doi: 10.1002/pmic.200300771. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Schoepfer R, Monyer H, Ruppersberg JP, Günther W, Seeburg PH, Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257:1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Chazot PL, Cik M, Stephenson FA. An investigation into the role of N-glycosylation in the functional expression of a recombinant heteromeric NMDA receptor. Mol Membr Biol. 1995;12:331–337. doi: 10.3109/09687689509072435. [DOI] [PubMed] [Google Scholar]
- Clark RA, Gurd JW, Bissoon N, Tricaud N, Molnar E, Zamze SE, Dwek RA, McIlhinney RA, Wing DR. Identification of lectin-purified neural glycoproteins, GPs 180, 116, and 110, with NMDA and AMPA receptor subunits: conservation of glycosylation at the synapse. J Neurochem. 1998;70:2594–2605. doi: 10.1046/j.1471-4159.1998.70062594.x. [DOI] [PubMed] [Google Scholar]
- Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Davies J, Watkins JC. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982;235:378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts I, Villmann C, Hollmann M. N-glycosylation is not a prerequisite for glutamate receptor function but is essential for lectin modulation. Mol Pharmacol. 1997;52:861–873. doi: 10.1124/mol.52.5.861. [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010;30:2676–2685. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Gascón S, García-Gallo M, Renart J, Díaz-Guerra M. Endoplasmic reticulum-associated degradation of the NR1 but not the NR2 subunits of the N-methyl-d-aspartate receptor induced by inhibition of the N-glycosylation in cortical neurons. J Neurosci Res. 2007;85:1713–1723. doi: 10.1002/jnr.21309. [DOI] [PubMed] [Google Scholar]
- Greger IH, Esteban JA. AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol. 2007;17:289–297. doi: 10.1016/j.conb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Martínez-Maza R, Poyatos I, López-Corcuera B, Núñez E, Giménez C, Zafra F, Aragón C. The role of N-glycosylation in transport to the plasma membrane and sorting of the neuronal glycine transporter GLYT2. J Biol Chem. 2001;276:2168–2173. doi: 10.1074/jbc.M006774200. [DOI] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nathanson NM. Synthesis, trafficking, and localization of muscarinic acetylcholine receptors. Pharmacol Ther. 2008;119:33–43. doi: 10.1016/j.pharmthera.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Fu Z, Losi G, Hawkins LM, Luo J, Chang K, Wenthold RJ, Vicini S. Relationship between availability of NMDA receptor subunits and their expression at the synapse. J Neurosci. 2002;22:8902–8910. doi: 10.1523/JNEUROSCI.22-20-08902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Single FN, Rozov A, Burnashev N, Zimmermann F, Hanley DF, Forrest D, Curran T, Jensen V, Hvalby O, Sprengel R, Seeburg PH. Dysfunctions in mice by NMDA receptor point mutations NR1(N598Q) and NR1(N598R) J Neurosci. 2000;20:2558–2566. doi: 10.1523/JNEUROSCI.20-07-02558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley S, Baudry M. The role of glycosylation in ionotropic glutamate receptor ligand binding, function, and trafficking. Cell Mol Life Sci. 2000;57:1508–1516. doi: 10.1007/PL00000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507:13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin O, Kraut JA, Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Renal Physiol. 2009;296:F459–469. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Woods G, Zito K. Preparation of gene gun bullets and biolistic transfection of neurons in slice culture. J Vis Exp. 2008;12:675. doi: 10.3791/675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, Ehninger D, Li GD, Hell JW, Kennedy MB, Silva AJ. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci. 2007;27:13843–13853. doi: 10.1523/JNEUROSCI.4486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]