Abstract
Phospholipases are a complex and important group of enzymes widespread in nature, that play crucial roles in diverse biochemical processes and are classified as A1, A2, C, and D. Phospholipases A1 and A2 activities have been linked to pathogenesis in various microorganisms, and particularly in pathogenic protozoa they have been implicated in cell invasion. Kinetoplastids are a group of flagellated protozoa, including extra- and intracellular parasites that cause severe disease in humans and animals. In the present paper, we will mainly focus on the three most important kinetoplastid human pathogens, Trypanosoma brucei, Trypanosoma cruzi, and Leishmania spp., giving a perspective of the research done up to now regarding biochemical, biological, and molecular characteristics of Phospholipases A1 and A2 and their contribution to pathogenesis.
1. Introduction
Kinetoplastids are a group of flagellated protozoans distinguished by the presence, in their single large mitochondrion, of a DNA-containing region known as kinetoplast. These unicellular organisms have a similar genomic organization and cellular structures and undergo morphological changes during their life cycles, being transmitted by different insect vectors. The members of this group include extra- and intracellular parasites that cause severe diseases in humans and animals, as well as various free-living forms. Herein, we will mainly focus on the three most important human pathogens Trypanosoma brucei, Trypanosoma cruzi, and Leishmania spp.
T. brucei and its subspecies are extracellular parasites transmitted by tsetse flies and responsible of human African trypanosomiasis (HAT), also known as African sleeping sickness, and Nagana in cattle. The life cycle of African trypanosomes is complex and represented by extracellular stages found in blood, lymph, and spinal fluid in the mammal [1]. The disease threatens over 70 million people and uncounted numbers of cattle in 36 countries of sub-Saharan Africa, having a devastating impact in human health and economy in affected areas [2]. HAT symptoms occur in two stages; in the first, haemolymphatic phase, parasite invasion of the circulatory and lymphatic systems is associated with severe swelling of lymph nodes. If untreated, the disease overcomes the host's defences and can cause more extensive damage. The second stage, neurological phase, begins when the parasite invades the central nervous system by passing through the blood-brain barrier; without treatment, it is fatal and the damage caused can be irreversible [1].
T. cruzi in contrast, is an intracellular parasite that invades all types of nucleated cells in the mammalian host. This protozoa, transmitted through the faeces of bloodsucking insects of the Triatominae family, enters the mammalian host via damage to the skin and causes Chagas disease in humans, a chronic inflammatory condition characterized by cardiomyopathy, megacolon, and mega-esophagus [3]. The disease is prevalent throughout America and according to WHO estimations, 25 million people are at risk and 10 million are infected worldwide [4]. Chemotherapy against Chagas disease is limited and unsatisfactory. The two available drugs, nifurtimox and beznidazole, are capable of curing at least 50% of recent infections and both produced side effects [5].
The genus Leishmania comprises 20 species of intracellular protozoan that are transmitted by phlebotomine sandflies and infect specifically cells of the mononuclear phagocyte system in mammals. These parasites cause various diseases ranging from self-healing cutaneous leishmaniasis, mucocutaneous leishmaniasis, with partial or total destruction of the mucous membranes, to severe and lethal (if untreated) visceral leishmaniasis, also known as kala-azar. Leishmaniasis is wide spread in Southern Europe, Africa, Asia, and America, threatening 350 million people in 88 countries around the world and represents an important global health problem that results in a significant economic burden [6].
One of the major components of biomembranes present in all living organism are phospholipids (PL), which form the lipid bilayer and serve as hydrophobic anchors of membrane proteins. These compounds can be enzymatically modified by the action of phospholipases (PLAs), with generation of bioactive lipid molecules that can act as second messengers and also modulate the immune response [7–9]. Moreover, PLAs have been considered virulence factors for many pathogenic bacteria like Escherichia coli, Helicobacter pylori, Neisseria spp. Yersinia spp., and so forth [10–14].
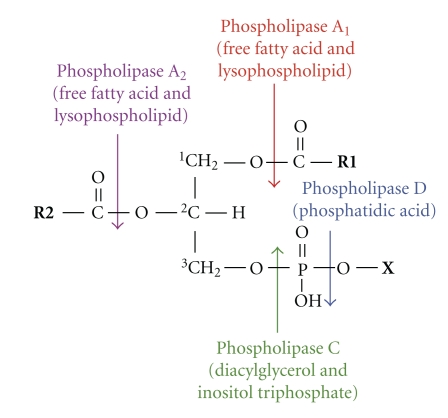
PLAs are a complex and important group of enzymes, widespread in nature, that play crucial roles in diverse biochemical processes and are classified as A1, A2, C, and D, depending on the site of hydrolysis [15]. These enzymes cleave cell membrane and intracellular PL releasing a variety of products such as lysophospholipids (LPL), free fatty acids (FFA), diacylglycerols (DG), choline phosphate, phosphoinositides and phosphatidic acid, among others (Figure 1).
Figure 1.
Hydrolysis of phospholipids by phospholipases. Arrows indicate the sites of attack for hydrolytic cleavage of phospholipases type A1, A2, C, and D. The main products generated by their action are also shown. R1/R2: free fatty acids in sn-1 or sn-2 positions; X: choline, ethanolamine, serine, inositol, and so forth.
Phospholipase A1 (PLA1) (EC 3.1.1.32) specifically hydrolyses acyl groups from PL at the sn-1 position, producing FFA and LPL (Figure 1) [15]. PLA1 activities have been detected in various cell types and tissues from a wide range of organisms by measuring hydrolysis of phosphatidylcholine (PC) to lysophosphatidylcholine (LPC) [15]. But despite their apparent ubiquity and diversity, up to now a limited number of PLA1s have been cloned and characterized [16–18]. Increasing evidence indicates that some PLA1s are capable of generating bioactive lipids, a role traditionally assigned to PLAs A2, C, and D, though the biological relevance of this particular PLA deserves to be deeply studied [16, 19, 20].
At present time, nine PLA1 molecules are known in mammals, being six extracellular and three intracellular enzymes, sharing no sequence homology between them and probably having distinct functions [7]. The extracellular PLA1s belong to the pancreatic lipase gene family, that is conserved in an extensive range of organisms from insects to mammals and have been biochemically characterized and classified, according to their substrate specificities, structures, expression patterns, and possible functions [7]. Some PLA1s have a broad substrate specificity and might also have triacylglycerol lipase activity (EC 3.1.1.3) [21]. Others, such as phosphatidylserine-specific PLA1s from rat platelets and membrane-associated phosphatidic acid PLA1α and PLA1β, show strict substrate specificity [20]. The latter PLA1s have specific roles in producing bioactive LPL, lysophosphatidylserine, and lysophosphatidic acid [7].
Phospholipases A2 (EC 3.1.1.4), in contrast to PLA1, are the most widely studied. Great advances in understanding the structure and function of the superfamily of Phospholipase A2 (PLA2) has occurred in the last decades [9, 22–25]. This superfamily includes fifteen groups, comprising four main types including secreted PLA2 (sPLA2), cytosolic PLA2 (cPLA2), Ca2+-independent PLA2 (iPLA2), and platelet activating factor acetyl hydrolase/oxidized lipid lipoprotein associated PLA2 (LpPLA2) [9]. The classification is based upon the following characteristics: source, secreted, or cytosolic, availability of structural information, molecular weight, cofactors, and inhibitor specificity [9]. sPLA2s have a low molecular weight (∼14 kDa) and contain a large number of disulphide bridges, consistent with their extracellular environment, and require millimolar concentrations of Ca2+ for optimum catalytic activity [9]. cPLA2s have a high molecular weight (∼85 kDa) and preferentially hydrolyze PL containing arachidonic acid, therefore playing a key role in the biosynthesis of eicosanoids, precursors of prostaglandins and thromboxane [26]. Full activation of these enzymes requires Ca2+ binding to an N-terminal C2 domain and phosphorylation on serine residues [23]. iPLA2s have a high molecular weight (85–88 KDa), contain seven to eight ankyrin repeats, one of the most common sequence motif, and the consensus lipase motif GXSXG, being detected mainly in human tissues [27].
Considering the important and various roles that PLAs possess, in the present review, we will summarize the research done to date in Trypanosomes regarding biochemical, biological, and molecular characteristics of PLAs and their contribution to pathogenesis.
2. Biochemical and Biological Characteristics of the Trypanosomatids Phospholipases A
2.1. Phospholipase A1
In 1978, Tizard et al. described the presence of a haemolytic activity in T. congolense, due to the FFA generated by the action of PLA on endogenous PC, meanwhile in the nonpathogenic T. lewisi, no FFA generation was observed and therefore, nonhaemolytic activity was detected [28]. Further, it was determined in four different species of African trypanosomes that Ca2+-independent PLA1 was the predominant PC-degrading activity. The levels of PLA1 varied widely, with very high activity in the pathogenic T. brucei and relatively low activity in the nonpathogenic T. lewisi species [29]. Other authors found that T. brucei bloodstream forms possess high levels of PLA1 activity, whereas in the procyclic culture forms PLA1 specific activity was only 15% of that of bloodstream forms, suggesting an important physiological role for the enzyme in the mammalian stage [30]. Bloodstream trypomastigotes are covered with a dense layer of Variant Surface Glycoprotein (VSG), which protects the parasite from lysis by host complement via the alternative pathway [31]. It has been suggested that the high activity of PLA1 in these forms, may play a role in the acquisition of fatty acids for synthesis of the VSG and also provide a source of myristate that can be employed for remodelling the lipid anchor of the VSG [32].
PLA1 was purified from T. brucei bloodstream forms, where the major portion was found as a soluble activity in the cytosol and the minor as a particle-bound activity associated with lysosomal markers. Both enzymes had optimal activity at acid pH and were activated by Triton X-100 [30]. Although cultured procyclic trypomastigotes also possess PLA1 activity, the levels were significantly reduced compared to bloodstream forms, due to a decrease in soluble PLA, similar levels of lysosomal activity were present in both stages [30]. Other authors reported that PLA1 activity eluted together with a lysophospholipase activity (LPLA) (EC:.3.1.1.5), suggesting that a single enzyme displays both activities [33].
T. brucei PLA1 (TbPLA1) has been recently cloned and expressed as recombinant protein [34]. This intracellular enzyme is localized in the cytosol and has optimal activity at neutral pH and a predicted size of 34.8 kDa. TbPLA1 deacylates choline-containing PLs, with greater efficiency than those containing ethanolamine, inositol, serine, or just phosphorous at the sn-3 position [34]. The enzyme also displayed LPLA activity towards LPC, as previously reported [33].
As regards T. cruzi, we previously determined that when epimastigotes were grown at 28°C and then transferred to 37°C, a fatty acid exchange occurs between PL and neutral lipids [35]. This mechanism of membrane lipid adaptation suggested for the first time the action of PLA activity as part of a deacylation-reacylation cycle in T. cruzi [35]. Other authors reported membrane PLA1 and PLA2 activities in epimastigotes, but acting only on anionic PL such as inositolphospholipids and inositolphosphoceramides [36]. We further determined the presence of a PC-PLA1-degrading activity in all parasite stages, being up to 20-fold higher in the infective amastigotes and trypomastigotes than in the noninfective epimastigotes, as occurs in T. brucei, where the mammalian stages possess the highest levels of PLA1 activity [30, 37]. Interestingly, in both infective stages membrane-bound PLA1 activity was remarkably higher than those detected in organelle bound or soluble fractions [38]. This localization does not appear to have a similar counterpart in T. brucei, where the major proportion of activity (more than 90% of the total) corresponds to a soluble cytosolic fraction [30]. In T. cruzi epimastigotes, in contrast, the enzyme was only detected in lysosomes [37]. It is remarkable that only infective stages secreted PLA1 to the extracellular media [38], similarly to other enzymes that participate in T. cruzi endocytic pathway, such as cruzipain and trans-sialidase [39–42]. We purified T. cruzi PLA1 (TcPLA1) from epimastigote and amastigotes, obtaining in both cases a unique band of ∼38 kDa. These enzymes proved to be independent of the bivalent cations Ca2+, Mn2+, and Mg2+, had an optimum acidic pH, and were activated by Triton X-100. The biochemical characteristics of TcPLA1 activities were similar to those reported for TbPLA1 and other PLA1s from mammalians [43, 44]. As previously demonstrated in T. brucei we also determined the presence of LPLA activity in autolysing parasites [17, 33, 45].
As concerns secreted TcPLA1, we determined during metacyclogenesis, process in which epimastigotes differentiate into the infective metacyclic trypomastigotes, an increase in secreted enzyme activity simultaneously with the appearance of metacyclic forms, as expected [38]. Accordingly, it has been reported that membrane PLA1, A2, and C, may act in remodelling reactions needed for plasma membrane transformation during T. cruzi differentiation; these enzyme activities may be acting in remodelling reactions leading to the anchor of the mature glycoproteins of T. cruzi [46].
In the case of Leishmania spp., preliminary results of our laboratory have also demonstrated in L. braziliensis promastigotes a PLA1 activity hydrolyzing PC. Moreover, we detected by Immunoblot two bands of ∼37 and 41 kDa, using polyclonal antibodies against both TcPLA1 and TbPLA1 [47]. These antibodies were obtained in our laboratory, since no commercial antibodies against any PLA1 were available until this year [48].
2.2. Phospholipase A2
The eukaryotic PLA2 were the first of the PLAs to be recognized. The pancreatic PLA2 has been known to degrade PC since 1878, and at the turn of the century cobra venom was shown by Keyes in 1902 to have haemolytic activity directed towards the membranes of erythrocytes [49]. Secreted and membrane-bound PLA2 activity has been described in Bacteria, Fungi and Protozoa [50–54], but in the case of Trypanosomes just a few reports are available.
PLA2 was isolated from T. congolense; the enzyme appeared to exist in a dimeric form with subunit molecular weights of 16.5 and 18 kDa, had optimum pH of 6.8, and showed specificity for 1,2,dimyristoyl-sn-PC and 1,2,dioleoyl-sn-PC [55]. Inhibition studies implicated a thiol group at the catalytic site of the enzyme, which was stable to heat treatment and possessed haemolytic and anticoagulating properties [55].
In T. brucei bloodstream forms, other authors reported a PLA2 activity that could be stimulated by Ca2+ or by the amphiphilic peptide melittin, and that was responsible of the release of arachidonic acid, a prostaglandins precursor, being a pivotal enzyme in the control of Ca2+ influx [56, 57]. In addition, it was demonstrated in T. brucei procyclic forms, that the arachidonic acid generated endogenously could induce both Ca2+ entry and Ca2+ release from the intracellular compartments acidocalcisomes, suggesting that PLA2 activity participates in T. brucei signalling events [58]. On the other hand, in L. donovani promastigotes and T. cruzi amastigotes, these authors found that arachidonic acid only induced Ca2+ entry, possibly due to low generation of arachidonic acid or to the low amount of releasable Ca2+ in the acidocalcisomes of these cells [58].
Previous reports in T. cruzi suggested that PLA2 could mediate the association between the parasite and macrophages, but the authors did not clearly establish the source of the enzyme [59]. In epimastigotes, it has been described a haemolytic activity that destabilize in vitro red blood cells membranes and that could be attributed to PLA2 activity [60]. However under our experimental conditions using zwitterionic PL such as PC or PE as substrates, no secreted PLA2 was detected in the supernatants of living epimastigotes [38]. In this concern, other authors have described in this protozoa a membrane-bound PLA2 activity acting only on anionic PL such as inositolphospholipids and inositolphosphoceramides [36].
PLA2 degrading activities have been also reported in L. major, and they could be involved in the biosynthesis of lipophosphoglycan, the main macromolecule on the surface of the procyclic promastigote [61]. Other authors showed that parasite pretreatment with a low dose of pachymatismin, a glycoprotein extracted from a marine sponge, increased PLA2 activity, however macrophage invasion was partially inhibited [62].
On the other hand, in L. amazonensis the modification of PL composition of infected macrophages has been described, with increasing levels of LPC, an effect that may reflect indirectly, the action of an endogenous/parasite PLA2 on the macrophage [63]. Furthermore, other studies showed PLA2 activity in supernatants and lysates of L. (L.) amazonensis promastigotes and suggested that this enzyme may be a progression factor for cutaneous leishmaniasis [64].
In summary, there are increasing evidences of the presence and possible roles of PLA2 in the pathogenic Trypanosomes, so far however, these enzymes have not been purified or characterized in deep.
3. Phospholipases A of Trypanosomatids and Pathogenesis
PLA activity has been linked to pathogenesis in various microorganisms such as Escherichia coli, Yersinia spp, Helicobacter pylori, Neisseria spp, Legionella spp., and Campylobacter spp., which cause different disease syndromes; however, the exact mechanism of the PLA action has not been definitively determined [10–14, 65, 66]. PLA toxicity has been associated to cytolytic activity resulting from the accumulation of membrane-destabilizing products or by the extensive destruction of membrane phospholipids [10]. In pathogenic protozoa PLAs have been implicated in cell invasion [54, 67, 68]; in Toxoplasma gondii it has been described that PLA2 inhibition protected human monocytic cells from parasite invasion [53]; in Entamoeba histolytica, PLA2 is one of the several factors related to virulence [54] and in Cryptosporidium parvum the use of PLA2 inhibitors as well as specific anti PLA2 antibodies significantly reduced invasion of human enterocytes [68].
The role of PLA1 in the pathogenesis of African trypanosomiasis has been intensively studied [29, 45, 69, 70]. Hambrey et al. described in the tissue fluids of T. brucei infected rabbits large amounts of PLA1 activity that increased with parasite burden, whereas in blood plasma this activity was also detected, but at a considerably lower level [70]. The enzyme seemed to be of trypanosomal origin, being either secreted by living parasites or released from dying organisms [70]. In intravascular locations PLA1 could contribute to the pathology of trypanosomiasis by causing cell membrane damage and could account for some or all of the connective tissue cell destruction, which is a prominent feature of infections with T. brucei[71].
The high level of PLA1 found in T. congolense and T. brucei, in comparison to other pathogens like Escherichia coli (1000 times fold higher) [33], and its relatively low level in the nonpathogenic rat trypanosome T. lewisi, suggested the importance of the enzyme in the pathology of African trypanosomiasis [28, 29, 45, 69, 72]. Given that T. lewisi and T. congolense are restricted almost entirely to the blood stream of the host, whereas T. brucei develops mainly in the connective tissues [71], it was suggested that PLA1 could help the latter to penetrate blood vessels endothelium and other barriers hindering and contributing to tissue damage [29].
In the pathogenic T. brucei and T. congolense it has been determined that PLA1 activity increased greatly during the autolytic process and large quantities of FFA were accumulated, whereas the non pathogenic T. lewisi failed to increase the enzyme activity even on prolonged autolysis [69]. PLA1 yields FFA and LPC, which is then further degraded by the LPLA to yield more FFA and glycerophosphorylcholine. FFA are cytotoxic and haemolytic as a result of their detergent-like properties [73], and they could account for the immunosuppression and the structural disturbances in lymphoid organs observed in African trypanosomiases [69]. These observations deserve to be updated and deeply studied.
The first evidences related to phospholipid degrading enzymes in T. cruzi, was associated to the inflammatory responses that appear surrounding degenerating amastigote nests in various tissues of Chagas' disease patients [74]. This finding strongly suggested that autolytic processes generate factors, possibly PL-breakdown products, which cause inflammation [11]. In this regard, it was demonstrated that FFA and LPL released from killed trypomastigotes have toxic effects on culture cells [12]. These facts are in agreement with the pathogenic mechanism proposed for African trypanosomiasis [29, 69]. Accordingly, we determined in all T. cruzi stages the rapid and extensive breakdown of endogenous PL in autolysing parasites [37]. A major increase in FFA was observed, significantly higher than the generation of LPC, indicating not only the presence of PLA1 activity but also LPLA activity [37]. We also found that living T. cruzi infective stages were able to hydrolyze LPC, confirming the presence of a LPLA activity (Belaunzarán et al. unpublished observations). It is well known that LPC is potentially toxic for the cells [75, 76] though this activity would thereby contribute significantly to the parasite self-protection against lysocompounds. Similarly, in living T. brucei it has been demonstrated that PLA1 is active against LPL [77]. Other authors reported that bloodstream forms can acquire substantial amounts of exogenous LPL through a pathway consisting of three enzymes associated with the plasma membrane: PLA1, acyl-COA ligase, and LPC acylCOA-acyl transferase [32]. These cytotoxic compounds can change the ionic permeability of the plasma membrane, though they are rapidly metabolized to ensure tolerable levels in the cell. Thus a membrane-bound PLA1 would protect T. brucei against the high levels of plasma LPC [32].
In T. cruzi, we already showed the involvement of PLA1 in the early events of parasite-host cell interaction preceding parasite invasion. We demonstrated that either intact infective parasites or purified PLA1 significantly modified the host cell lipid profile with generation of second lipid messengers (DG, FFA, and LPC) and concomitant protein kinase C activation [38], an enzyme that has been implicated in the upregulation of T. cruzi invasion [78].
With respect to Leishmania spp., it has been observed that LPC, which is scare in the macrophage, increased significantly after infection with L. amazonensis [63]. As LPC and arachidonic acid are the products of PC cleavage by PLA2, the increase in the levels of LPC may suggest the action of the enzyme on the macrophage PC, producing prostaglandin E2 [63]. In this regard, it has been shown that this lipid mediator is increased after 1-2 hours of infection with L. donovani and can exacerbate the infection [79]. Nevertheless, whether the LPC generation was due to parasite PLA2 or to the activation of macrophage PLA2 remains unclear [63]. It is possible that the LPC could also be generated by a PLA1 activity, similarly to that we detected in L. braziliensis [47].
4. Bioinformatic Analysis of the Trypanosomatid Genomes for Phospholipases A
The publication of the genomes of the kinetoplastid parasites T. brucei [80], T. cruzi [81], Leishmania spp. [82], and other related organisms, allowed the scientific community to perform comparative analyses giving insight into the evolutionary similarities/differences among trypanosomatids.
T. brucei PLA1 (Tb.927.1.4830) has been cloned and characterized and the analyses of its protein sequence indicated that this enzyme is not homologous to neutral lipases [34]. The only similarity to them was in the amino acidic sequence that contains a lipase consensus pattern harbouring a conserved GXSXG motif, a marker of the serine hydrolase superfamily [34]. In these enzymes, the catalytic triad is typically constituted by a base residue (Histidine), an acid (Aspartic), and a nucleophile (Serine), belonging to the latter to the GXSXG motif. No eukaryotic homologues of TbPLA1 were found in T. cruzi and Leishmania spp., but orthologues of this enzyme were identified in T. congolense (TcIL3000.1.2010) and T. vivax (TvY486_0102170) [34]. Interestingly, TbPLA1 resembled a putative PLA1 homologue from Sodalis glossinidius, a proteobacterium endosymbiont of tsetse flies. These findings suggested that a T. brucei ancestor acquired the PLA1 gene through horizontal gene transfer after/during its adaptation to a parasitic lifestyle in the insect vector [34].
Regarding T. cruzi, we previously reported the presence in the T. cruzi data base (http://www.tcruzidb.org/) of at least sixteen different genes encoding putative lipases and the identified sequences presented a high degree of similarity among them (70–80%), may be haplotype variants [38]. When we further performed a search in the Kinetoplastid Genomic Resource TriTryDB (http://tritrypdb.org/tritrypdb/) using only the lipase consensus pattern of TbPLA1 and considering the biochemical characteristics of T. cruzi PLA1, the number of putative genes was reduced to eight [38]. One of them (Tc00.1047053510679.100) was cloned and expressed in E. coli, being the recombinant enzyme recognized by both anti-TcPLA1 and anti-TbPLA1 antibodies [48]. The eight sequences are currently under study in our laboratory, to elucidate the identity of each of these genes that codify for T. cruzi PLA1 and to obtain the active recombinant enzyme.
We extended these analyses to Leishmania spp., searching in the TriTryDB database for homologues of T. cruzi PLA1 putative genes and have identified in L. braziliensis, L. infantum, and L. major, three, nine and eight putative genes with the conserved lipase motif, respectively. One of the putative genes from L. braziliensis, LbrM31_V2.2750, was cloned and expressed in E. coli, being the recombinant protein recognized by both anti-TcPLA1 and anti-TbPLA1 antibodies [47]. At present, we are running assays with the aim of obtaining and characterizing the active recombinant enzyme.
The alignment of the protein sequences corresponding to the cloned T. brucei, T. cruzi, and L. braziliensis PLA1 (Tb.927.1.4830, Tc00.1047053510679.100, and LbrM31_V2.2750, resp.), with the putative PLA1s proteins of T. congolense and T. vivax, (TcIL3000.1.2010 and TvY486_0102170), shows that the sequences of T. brucei, T. congolense, and T. vivax are closely related, whereas T. cruzi and L. braziliensis only share with all of them the lipase motif (Figure 2). The fact that T. cruzi and L. braziliensis PLA1 protein sequences do not share significant homologies with African trypanosomes, particularly with TbPLA1, is in agreement with that previously observed by Richmond and Smith [34].
Figure 2.
Multiple sequence alignment of trypanosome PLA1. Alignment of the protein sequences of cloned T. brucei, T. cruzi, and L. braziliensis PLA1 and T. congolense and T. vivax putatives PLA1 was performed with NTI 10 Software (Invitrogen) using the Clustal W algorithm. The lipase consensus pattern is underlined. Letters indicate: identical (red), conserved (blue), similar (green), weakly similar (grey), and nonsimilar (black) amino acids. Leishmania braziliensis PLA1 (TriTrypDB LbrM31_V2.2750), Trypanosoma brucei PLA1 (TriTrypDB Tb.927.1.4830), Trypanosoma congolense putative PLA1 (TriTrypDB TcIL3000.1.2010), Trypanosoma vivax putative PLA1 (TriTrypDB TvY486_0102170), and Trypanosoma cruzi PLA1 (TriTrypDB Tc00.1047053510679.100).
Although PLA2 activity was detected in T. brucei, T. congolense, T. cruzi, L. major, and L. amazonensis years ago, still little is known about the identity of the genes that codify for them [36, 55, 56, 61, 63, 83]. We have performed a search in the TriTrypDB database and identified at least 9 putative PLA2-like proteins in the different Trypanosomes species (Table 1), but at the moment no PLA2 of trypanosomal origin has been identified in the genomes or cloned.
Table 1.
Phospholipase A2 putative genes found in TriTrypDB.
| Gene | Organism | Product | Syntenic | Comments |
|---|---|---|---|---|
| LbrM34_V2.2930 | L. braziliensis | phospholipase A2-like protein, putative | yes | no |
| LinJ35_V3.3070 | L. infantum | phospholipase A2-like protein, putative | yes | no |
| LmjF35.3020 | L. major | phospholipase A2-like protein, putative | yes | no |
| LmxM34.3020 | L. mexicana mexicana | phospholipase A2-like protein, putative | yes | no |
| Tbg972.9.7760 | T. brucei gambiense | phospholipase A2-like protein, putative | yes | no |
| TclL3000.0.00740 | T. congolense | product unspecified | no | no |
| Tc00.1047053510743.60 | T. cruzi CL Brener Esmeraldo-like | phospholipase A2-like protein, putative | yes | no |
| Tc00.1047053510659.250 | T. cruzi CL Brener Non-Esmeraldo-like | phospholipase A2-like protein, putative | yes | no |
| TvY486_0906130 | T. vivax | phospholipase A2-like protein, putative | yes | no |
5. Inhibitors of Trypanosomatid Phospholipases A
As described above, parasite PLAs participate in diverse and relevant cellular processes such as membrane remodelling, modification of membrane permeability, generation of lipid second messengers and parasite invasion. All these facts emphasize the interest of these enzymes as potential chemotherapeutic targets that could contribute to the control of parasite proliferation and survival.
A number of compounds with potential inhibitory activity on parasite PLA1 have been investigated. TbPLA1 activity was inhibited by several heavy metals through an undefined mechanism, being the most potent at lower concentrations cadmium and copper. Iron produced partial to total inhibition depending on the concentrations employed, whereas moderate inhibition was detected in the presence of relatively high concentrations of nickel and zinc [34]. As the active-site residue for TbPLA1 is Serine 131, the active-site serine modifiers iPr2P-F (di-isopropyl fluorophosphate), PMSF (phenylmethylsulfonyl fluoride), and E-600 (diethyl-p-nitrophenyl phosphate) were also assayed. Relatively little inhibition of the enzyme activity was observed but at very high concentration of inhibitors, suggesting that the catalytic triad active site of TbPLA1 is buried inside the enzyme and sheltered by a lid domain, a property shared with other lipases [34].
Other compounds with potential inhibitory activity on TcPLA1 were investigated in our laboratory, including the antimalarial drugs quinine and chloroquine, the antiarrhythmic drugs amiodarone and chlorpromazine, and the local anaesthetics dibucaine, procaine, and xylocaine. Among all of them, only chlorpromazine had an inhibitory effect, but at concentrations that induce cell toxicity [37].
As previously mentioned, in T. brucei Ca2+ influx can be regulated by PLA2 [56, 58]. Various inhibitors of this enzyme such as thioetheramide-PC, manoalide, arachidonyl trifluoromethyl ketone, and aristolochic acid were tested in this protozoa, being the most effective in blocking Ca2+ influx 3-(4-octadecyl)-benzoylacrylic acid (OBAA), a potent inhibitor of secreted PLA2 [56]. On the other hand, T. brucei gambiense and T. brucei brucei PLA2s were inhibited in a noncompetitive fashion when using organotin compounds like fatty acid derivatives of dibutyltin dichloride [83]. In the case of T. cruzi, it has been suggested that quinacrine, which inhibited erythrocyte lyses, blocked PLA2 activity [60].
Concerning Leishmania spp., up to now, there are no reports about the use of specific PLA inhibitors. However, it has been reported that the lysophospholipid analog (LPA) miltefosine, affects lipid metabolism in Leishmania donovani promastigotes, with reduction in PC and enhancement in PE and LPC, a process in which PLAs could participate among other enzymes [84]. The usefulness of lipid biosynthesis inhibitors has gained great interest in the last years to fight parasitic Trypanosomes [5, 85–88]. These compounds, initially developed to be antitumor agents, have proved to be highly effective in the treatment of visceral leishmaniasis [87, 89, 90]. Although their effectiveness is known, the mode of action against this parasite is not completely understood [91]. In T. cruzi, the synergy of the LPAs edelfosine, ilmofosine, and miltefosine with the ergosterol biosynthesis inhibitor, ketoconazole, induced alterations in the plasma membrane, reservosomes, and mitochondrion, indicating that these organelles are potential targets of these drugs, probably through interference with lipid metabolism [92].
Considering that PLAs are present in both, trypanosomes and mammalian host, it will be of relevance to achieve the knowledge of their three-dimensional structures to determine the differences/similarities among them. This would allow the rational design of specific inhibitors that could be employed as potential chemotherapeutic agents in the diseases caused by kinetoplastid pathogens.
6. Concluding Remarks
In summary, as presented in this paper PLAs of pathogen trypanosomes mediate a variety of processes in both protozoan and host cell lipid metabolism, being also considered virulence factors. However, the knowledge of these enzymes is far from complete, though in the future, continued biochemical, biological, and structural research are needed to obtain a full understanding of the molecular mechanism in which these enzymes participate. Unravelling the differences between parasite and host PLAs may contribute, besides, to the design of specific enzyme inhibitors that could be used in the treatment of the neglected diseases that trypanosomes cause.
Acknowledgments
The authors thank Guadalupe Giménez for critical comments on the paper. This work was supported by Universidad de Buenos Aires (UBA), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and Agencia Nacional de Promoción Científica y Tecnológica (FONCYT).
References
- 1.Knirsch DGH. African trypanosomiasis. In: Faro S,SD, editor. Parasitic Diseases. New York, NY, USA: Apple Tree Productions; 2005. [Google Scholar]
- 2. http://www.who.int/mediacentre/factsheets/fs259/en/index.html.
- 3.Brener Z, Andrade ZA, Barral-Neto M. Trypanosoma cruzi e Doença de Chagas. Rio de Janeiro, Brazil: Guanabara Koogan S.A. eds; 2000. O parasito e sua interaçao com os hospedeiros. [Google Scholar]
- 4. http://www.who.int/mediacentre/factsheets/fs340/en/index.html.
- 5.Urbina JA. Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Tropica. 2010;115(1-2):55–68. doi: 10.1016/j.actatropica.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 6. http://www.who.int/leishmaniasis/en/
- 7.Aoki J, Inoue A, Makide K, Saiki N, Arai H. Structure and function of extracellular phospholipase A belonging to the pancreatic lipase gene family. Biochimie. 2007;89(2):197–204. doi: 10.1016/j.biochi.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Gimenez G, Magalhães KG, Belaunzarán ML, et al. Lipids from attenuated and virulent Babesia bovis strains induce differential TLR2-mediated macrophage activation. Molecular Immunology. 2010;47(4):747–755. doi: 10.1016/j.molimm.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. Journal of lipid research. 2009;50:S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Istivan TS, Coloe PJ. Phospholipase A in Gram-negative bacteria and its role in pathogenesis. Microbiology. 2006;152(5):1263–1274. doi: 10.1099/mic.0.28609-0. [DOI] [PubMed] [Google Scholar]
- 11.Snijder HJ, Dijkstra BW. Bacterial phospholipase A: structure and function of an integral membrane phospholipase. Biochimica et Biophysica Acta. 2000;1488(1-2):91–101. doi: 10.1016/s1388-1981(00)00113-x. [DOI] [PubMed] [Google Scholar]
- 12.Bos MP, Tefsen B, Voet P, Weynants V, Van Putten JPM, Tommassen J. Function of neisserial outer membrane phospholipase A in autolysis and assessment of its vaccine potential. Infection and Immunity. 2005;73(4):2222–2231. doi: 10.1128/IAI.73.4.2222-2231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmiel DH, Wagar E, Karamanou L, Weeks D, Miller VL. Phospholipase A of Yersinia enterocolitica contributes to pathogenesis in a mouse model. Infection and Immunity. 1998;66(8):3941–3951. doi: 10.1128/iai.66.8.3941-3951.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorrell N, Martino MC, Stabler RA, et al. Characterization of Helicobacter pylori PidA, a phospholipase with a role in colonization of the gastric mucosa. Gastroenterology. 1999;117(5):1098–1104. doi: 10.1016/s0016-5085(99)70394-x. [DOI] [PubMed] [Google Scholar]
- 15.Dennis EA. Phospholipases. In: Boyer PD, editor. The Enzymes. New York, NY, USA: Academic Press; 1983. pp. 307–353. [Google Scholar]
- 16.Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The defective in anther dehiscence1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell. 2001;13(10):2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richmond GS, Smith TK. A novel phospholipase from Trypanosoma brucei. Molecular Microbiology. 2007;63(4):1078–1095. doi: 10.1111/j.1365-2958.2006.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonoda H, Aoki J, Hiramatsu T, et al. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. Journal of Biological Chemistry. 2002;277(37):34254–34263. doi: 10.1074/jbc.M201659200. [DOI] [PubMed] [Google Scholar]
- 19.Hosono H, Aoki J, Nagai Y, et al. Phosphatidylserine-specific phospholipase A1 stimulates histamine release from rat peritoneal mast cells through production of 2-acyl-1-lysophosphatidylserine. Journal of Biological Chemistry. 2001;276(32):29664–29670. doi: 10.1074/jbc.M104597200. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Aokit J, Nagai Y, et al. Serine phospholipid-specific phospholipase a that is secreted from activated platelets. A new member of the lipase family. Journal of Biological Chemistry. 1997;272(4):2192–2198. doi: 10.1074/jbc.272.4.2192. [DOI] [PubMed] [Google Scholar]
- 21.De Maria L, Vind J, Oxenbøll KM, Svendsen A, Patkar S. Phospholipases and their industrial applications. Applied Microbiology and Biotechnology. 2007;74(2):290–300. doi: 10.1007/s00253-006-0775-x. [DOI] [PubMed] [Google Scholar]
- 22.Dennis EA. The growing phospholipase A superfamily of signal transduction enzymes. Trends in Biochemical Sciences. 1997;22(1):1–2. doi: 10.1016/s0968-0004(96)20031-3. [DOI] [PubMed] [Google Scholar]
- 23.Hirabayashi T, Murayama T, Shimizu T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biological and Pharmaceutical Bulletin. 2004;27(8):1168–1173. doi: 10.1248/bpb.27.1168. [DOI] [PubMed] [Google Scholar]
- 24.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochimica et Biophysica Acta. 2006;1761(11):1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Burke JE, Dennis EA. Phospholipase A2 biochemistry. Cardiovascular Drugs and Therapy. 2009;23(1):49–59. doi: 10.1007/s10557-008-6132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukotrienes and Essential Fatty Acids. 2006;75(3):197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A2: structure and function. Biochimica et Biophysica Acta. 2000;1488(1-2):28–39. doi: 10.1016/s1388-1981(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 28.Tizard IR, Mellors A, Holmes WL, Nielsen K. The generation of phospholipase A and hemolytic fatty acids by autolysing suspensions of Trypanosoma congolense. Tropenmedizin und Parasitologie. 1978;29(1):127–133. [PubMed] [Google Scholar]
- 29.Hambrey PN, Mellors A, Tizard IR. The phospholipases of pathogenic and non-pathogenic Trypanosoma species. Molecular and Biochemical Parasitology. 1981;2(3-4):177–186. doi: 10.1016/0166-6851(81)90098-0. [DOI] [PubMed] [Google Scholar]
- 30.Opperdoes FR, Van Roy J. The phospholipases of Trypanosoma brucei bloodstream forms and cultured procyclics. Molecular and Biochemical Parasitology. 1982;5(5):309–319. doi: 10.1016/0166-6851(82)90038-x. [DOI] [PubMed] [Google Scholar]
- 31.Cross GAM. Identification, purification and properties of clone specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- 32.Bowes AE, Samad AH, Jiang P, Weaver B, Mellors A. The acquisition of lysophosphatidylcholine by African trypanosomes. Journal of Biological Chemistry. 1993;268(19):13885–13892. [PubMed] [Google Scholar]
- 33.Sage L, Hambrey PN, Werchola GM. Lysophospholipase 1 in Trypanosoma brucei. Tropenmedizin und Parasitologie. 1981;32(4):215–220. [PubMed] [Google Scholar]
- 34.Richmond GS, Smith TK. The role and characterization of phospholipase A1 in mediating lysophosphatidylcholine synthesis in Trypanosoma brucei. Biochemical Journal. 2007;405(2):319–329. doi: 10.1042/BJ20070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Florin-Christensen M, Florin-Christensen J, De Isola ED, et al. Temperature acclimation of Trypanosoma cruzi epimastigote and metacyclic trypomastigote lipids. Molecular and Biochemical Parasitology. 1997;88(1-2):25–33. doi: 10.1016/s0166-6851(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 36.Bertello LE, Alves MJM, Colli W, De Lederkremer RM. Evidence for phospholipases from Trypanosoma cruzi active on phosphatidylinositol and inositolphosphoceramide. Biochemical Journal. 2000;345(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- 37.Wainszelbaum M, Isola E, Wilkowsky S, Cannata JJB, Florin-Christensen J, Florin-Christensen M. Lysosomal phospholipase A1 in Trypanosoma cruzi: an enzyme with a possible role in the pathogenesis of Chagas’ disease. Biochemical Journal. 2001;355(3):765–770. doi: 10.1042/bj3550765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belaunzarán ML, Wainszelbaum MJ, Lammel EM, et al. Phospholipase A from Trypanosoma cruzi infective stages generates lipid messengers that activate host cell protein kinase c. Parasitology. 2007;134(4):491–502. doi: 10.1017/S0031182006001740. [DOI] [PubMed] [Google Scholar]
- 39.Aparicio IM, Scharfstein J, Lima APCA. A new cruzipain-mediated pathway of human cell invasion by Trypanosoma cruzi requires trypomastigote membranes. Infection and Immunity. 2004;72(10):5892–5902. doi: 10.1128/IAI.72.10.5892-5902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruppi A, Cerbán FM, Vottero-Cima E. Exoantigens from Trypanosoma cruzi contain cruzipain. Acta Tropica. 1997;63(2-3):141–149. doi: 10.1016/s0001-706x(96)00616-x. [DOI] [PubMed] [Google Scholar]
- 41.Souto-Padron T, Campetella OE, Cazzulo JJ, De Souza W. Cysteine proteinase in Trypanosoma cruzi: immunocytochemical localization and involvement in parasite-host cell interaction. Journal of Cell Science. 1990;96(3):485–490. doi: 10.1242/jcs.96.3.485. [DOI] [PubMed] [Google Scholar]
- 42.Villalta F, Zhang Y, Bibb KE, Burns JM, Lima MF. Signal transduction in human macrophages by gp83 ligand of Trypanosoma cruzi: trypomastigote gp83 ligand up-regulates trypanosome entry through the MAP kinase pathway. Biochemical and Biophysical Research Communications. 1998;249(1):247–252. doi: 10.1006/bbrc.1998.9127. [DOI] [PubMed] [Google Scholar]
- 43.Hostetler KY, Yazaki PJ, Van den Bosch H. Purification of lysosomal phospholipase A. Evidence for multiple isoenzymes in rat liver. Journal of Biological Chemistry. 1982;257(22):13367–13373. [PubMed] [Google Scholar]
- 44.Pete MJ, Ross AH, Exton JH. Purification and properties of phospholipase A1 from bovine brain. Journal of Biological Chemistry. 1994;269(30):19494–19500. [PubMed] [Google Scholar]
- 45.Tizard IR, Nielsen K, Mellors A, Assoku RK. Free fatty acids, lysophospholipases, and the pathogenesis of African trypanosomiasis. Lancet. 1977;2(8028):p. 91. doi: 10.1016/s0140-6736(77)90097-6. [DOI] [PubMed] [Google Scholar]
- 46.Salto ML, Bertello LE, Vieira M, Docampo R, Moreno SNJ, De Lederkremer RM. Formation and remodeling of inositolphosphoceramide during differentiation of Trypanosoma cruzi from trypomastigote to amastigote. Eukaryotic Cell. 2003;2(4):756–768. doi: 10.1128/EC.2.4.756-768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belaunzarán ML, Velandia A, Lammel EM, et al. Identification, cloning and expression of a novel Phospholipase A from Leishmania braziliensis. Biocell. 2010;34:p. 91. [Google Scholar]
- 48.Belaunzarán ML. Fosfolipasa A1 de estadíos infectivos de Trypanosoma cruzi: su relación con la Enfermedad de Chagas. Buenos Aires, Argentina: Universidad de Buenos Aires; 2008. Ph.D. thesis. [Google Scholar]
- 49.Waite M. Phospholipases. In: Vance JE, Vance DE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam, The Netherlands: Elsevier; 1996. pp. 211–236. [Google Scholar]
- 50.Sitkiewicz I, Stockbauer KE, Musser JM. Secreted bacterial phospholipase A2 enzymes: better living through phospholipolysis. Trends in Microbiology. 2007;15(2):63–69. doi: 10.1016/j.tim.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Köhler GA, Brenot A, Haas-Stapleton E, Agabian N, Deva R, Nigam S. Phospholipase A2 and Phospholipase B activities in fungi. Biochimica et Biophysica Acta. 2006;1761(11):1391–1399. doi: 10.1016/j.bbalip.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Molecular Microbiology. 2004;53(5):1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 53.Gomez-Marín JE, El'Btaouri H, Bonhomme A, et al. Involvement of secretory and cytosolic phospholipases a2 during infection of IHP1 human monecytic cells with Toxoplasma gondii. Effect of interferon γ. Parasitology Research. 2002;88(3):208–216. doi: 10.1007/s00436-001-0525-z. [DOI] [PubMed] [Google Scholar]
- 54.González-Garza MT, Castro-Garza J, Cruz-Vega DE, et al. Entamoeba histolytica: diminution of erythrophagocytosis, phospholipase A2, and hemolytic activities is related to virulence impairment in long-term axenic cultures. Experimental Parasitology. 2000;96(2):116–119. doi: 10.1006/expr.2000.4554. [DOI] [PubMed] [Google Scholar]
- 55.Nok AJ, Esievo KAN, Ibrahim S, Ukoha AI, Ikediobi CO. Phospholipase A2 from Trypanosoma congolense: characterization and haematological properties. Cell Biochemistry and Function. 1993;11(2):125–130. doi: 10.1002/cbf.290110208. [DOI] [PubMed] [Google Scholar]
- 56.Eintracht J, Maathai R, Mellors A, Ruben L. Calcium entry in Trypanosoma brucei is regulated by phospholipase A2 and arachidonic acid. Biochemical Journal. 1998;336(3):659–666. doi: 10.1042/bj3360659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ridgley EL, Ruben L. Phospholipase from Trypanosoma brucei releases arachidonic acid by sequential sn-1, sn-2 deacylation of phospholipids. Molecular and Biochemical Parasitology. 2001;114(1):29–40. doi: 10.1016/s0166-6851(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 58.Catisti R, Uyemura SA, Docampo R, Vercesi AE. Calcium mobilization by arachidonic acid in trypanosomatids. Molecular and Biochemical Parasitology. 2000;105(2):261–271. doi: 10.1016/s0166-6851(99)00186-3. [DOI] [PubMed] [Google Scholar]
- 59.Connelly MC, Kierszenbaum F. Modulation of macrophage interaction with Trypanosoma cruzi by phospholipase A2-components of the parasite membrane. Biochemical and Biophysical Research Communications. 1984;121(3):931–939. doi: 10.1016/0006-291x(84)90766-6. [DOI] [PubMed] [Google Scholar]
- 60.Lujan HD, Bronia DH. Intermembrane lipid transfer during Trypanosoma cruzi-induced erythrocyte membrane destabilization. Parasitology. 1994;108(3):323–334. doi: 10.1017/s0031182000076162. [DOI] [PubMed] [Google Scholar]
- 61.Smith TK, Milne FC, Sharma DK, Crossman A, Brimacombe JS, Ferguson MAJ. Early steps in glycosylphosphatidylinositol biosynthesis in Leishmania major. Biochemical Journal. 1997;326(2):393–400. doi: 10.1042/bj3260393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le Pape P, Zidane M, Abdala H, Moré MT. A glycoprotein isolated from the sponge, Pachymatisma johnstonii, has anti-leishmanial activity. Cell Biology International. 2000;24(1):51–56. doi: 10.1006/cbir.1999.0450. [DOI] [PubMed] [Google Scholar]
- 63.Henriques C, Atella GC, Bonilha VL, De Souza W. Biochemical analysis of proteins and lipids found in parasitophorous vacuoles containing Leishmania amazonensis. Parasitology Research. 2003;89(2):123–133. doi: 10.1007/s00436-002-0728-y. [DOI] [PubMed] [Google Scholar]
- 64.Passero LFD, Laurenti MD, Tomokane TY, Corbett CEP, Toyama MH. The effect of phospholipase A2 from Crotalus durissus collilineatus on Leishmania (Leishmania) amazonensis infection. Parasitology Research. 2008;102(5):1025–1033. doi: 10.1007/s00436-007-0871-6. [DOI] [PubMed] [Google Scholar]
- 65.Banerji S, Aurass P, Flieger A. The manifold phospholipases A of Legionella pneumophila—identification, export, regulation, and their link to bacterial virulence. International Journal of Medical Microbiology. 2008;298(3-4):169–181. doi: 10.1016/j.ijmm.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Grant KA, Belandia IU, Dekker N, Richardson PT, Park SF. Molecular characterization of pldA, the structural gene for a phospholipase A from Campylobacter coli, and its contribution to cell- associated hemolysis. Infection and Immunity. 1997;65(4):1172–1180. doi: 10.1128/iai.65.4.1172-1180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saffer LD, Schwartzman JD. A soluble phospholipase of Toxoplasma gondii associated with host cell penetration. Journal of Protozoology. 1991;38(5):454–460. doi: 10.1111/j.1550-7408.1991.tb04816.x. [DOI] [PubMed] [Google Scholar]
- 68.Pollok RCG, McDonald V, Kelly P, Farthing MJG. The role of Cryptosporidium parvum-derived phospholipase in intestinal epithelial cell invasion. Parasitology Research. 2003;90(3):181–186. doi: 10.1007/s00436-003-0831-8. [DOI] [PubMed] [Google Scholar]
- 69.Tizard I, Nielsen KH, Seed JR, Hall JE. Biologically active products from African trypanosomes. Microbiological Reviews. 1978;42(4):661–681. doi: 10.1128/mr.42.4.664-681.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hambrey PN, Tizard IR, Mellors A. Accumulation of phospholipase A in tissue fluid of rabbits infected with Trypanosoma brucei. Tropenmedizin und Parasitologie. 1980;31(4):439–443. [PubMed] [Google Scholar]
- 71.Goodwin LG, Guy MW. Serum and tissue fluid changes caused by Trypanosoma brucei infection in the rabbit. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1973;67(1):p. 12. doi: 10.1016/0035-9203(73)90269-1. [DOI] [PubMed] [Google Scholar]
- 72.Roberts CJ, Tizard IR, Mellors A, Clarkson MJ. Lysophospholipases, lipid metabolism, and the pathogenesis of African trypanosomiasis. Lancet. 1977;2(8049):1187–1188. doi: 10.1016/s0140-6736(77)91589-6. [DOI] [PubMed] [Google Scholar]
- 73.Colley CM, Zwaal RFA, Roelofsen B, Van Deenen LLM. Lytic and non-lytic degradation of phospholipids in mammalian erythrocytes by pure phospholipases. Biochimica et Biophysica Acta. 1973;307(1):74–82. doi: 10.1016/0005-2736(73)90026-6. [DOI] [PubMed] [Google Scholar]
- 74.Tafuri WL. Pathogenesis of Trypanosoma cruzi infections. In: Lumdsen WHR, Evans DA, editors. Biology of the Kinetoplastida. London, UK: Academic Press; 1979. [Google Scholar]
- 75.Prokazova NV, Zvezdina ND, Korotaeva AA. Effect of lysophosphatidylcholine on transmembrane signal transduction. Biochemistry. 1998;63(1):31–37. [PubMed] [Google Scholar]
- 76.Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. American Journal of Physiology. 2007;31(1):5–16. doi: 10.1152/advan.00088.2006. [DOI] [PubMed] [Google Scholar]
- 77.Samad A, Licht B, Stalmach ME, Mellors A. Metabolism of phospholipids and lysophospholipids by Trypanosoma brucei. Molecular and Biochemical Parasitology. 1988;29(2-3):159–169. doi: 10.1016/0166-6851(88)90071-0. [DOI] [PubMed] [Google Scholar]
- 78.Villalta F, Zhang Y, Bibb KE, Pratap S, Burns JM, Lima MF. Signal transduction in human macrophages by gp83 ligand of Trypanosoma cruzi: trypomastigote gp83 ligand up-regulates trypanosome entry through protein kinase C activation. Molecular Cell Biology Research Communications. 1999;2(1):64–70. doi: 10.1006/mcbr.1999.0150. [DOI] [PubMed] [Google Scholar]
- 79.Reiner NE, Malemud CJ. Arachidonic acid metabolism by murine peritoneal macrophages infected with Leishmania donovani: in vitro evidence for parasite-induced alterations in cyclooxygenase and lipoxygenase pathways. Journal of Immunology. 1985;134(1):556–563. [PubMed] [Google Scholar]
- 80.Berriman M, Ghedin E, Hertz-Fowler C, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309(5733):416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 81.El-Sayed NM, Myler PJ, Bartholomeu DC, et al. The genome sequence of Trypanosoma cruzi, etiologic agent of chagas disease. Science. 2005;309(5733):409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- 82.Ivens AC, Peacock CS, Worthey EA, et al. The genome of the kinetoplastid parasite, Leishmania major. Science. 2005;309(5733):436–442. doi: 10.1126/science.1112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shuaibu MN, Kanbara H, Yanagi T, Ameh DA, Bonire JJ, Nok AJ. Phospholipase A2 from Trypanosoma brucei gambiense and Trypanosoma brucei brucei: Inhibition by organotins. Journal of Enzyme Inhibition. 2001;16(5):433–441. doi: 10.1080/14756360109162392. [DOI] [PubMed] [Google Scholar]
- 84.Rakotomanga M, Blanc S, Gaudin K, Chaminade P, Loiseau PM. Miltefosine affects lipid metabolism in Leishmania donovani promastigotes. Antimicrobial Agents and Chemotherapy. 2007;51(4):1425–1430. doi: 10.1128/AAC.01123-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lux H, Heise N, Klenner T, Hart D, Opperdoes FR. Ether-lipid (alkyl-phospholipid) metabolism and the mechanism of action of ether-lipid analogues in Leishmania. Molecular and Biochemical Parasitology. 2000;111(1):1–14. doi: 10.1016/s0166-6851(00)00278-4. [DOI] [PubMed] [Google Scholar]
- 86.Lira R, Contreras LM, Santa Rita RM, Urbina JA. Mechanism of action of anti-proliferative lysophospholipid analogues against the protozoan parasite Trypanosoma cruzi: potentiation of in vitro activity by the sterol biosynthesis inhibitor ketoconazole. Journal of Antimicrobial Chemotherapy. 2001;47(5):537–546. doi: 10.1093/jac/47.5.537. [DOI] [PubMed] [Google Scholar]
- 87.Croft SL, Seifert K, Duchêne M. Antiprotozoal activities of phospholipid analogues. Molecular and Biochemical Parasitology. 2003;126(2):165–172. doi: 10.1016/s0166-6851(02)00283-9. [DOI] [PubMed] [Google Scholar]
- 88.Urbina JA. Mechanisms of action of lysophospholipid analogues against trypanosomatid parasites. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100(1):S9–S16. doi: 10.1016/j.trstmh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 89.Croft SL, Engel J. Miltefosine—discovery of the antileishmanial activity of phospholipid derivatives. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100(1):S4–S8. doi: 10.1016/j.trstmh.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Murray HW. Treatment of visceral leishmaniasis (kala-azar): a decade of progress and future approaches. International Journal of Infectious Diseases. 2000;4(3):158–177. doi: 10.1016/s1201-9712(00)90078-x. [DOI] [PubMed] [Google Scholar]
- 91.Azzouz S, Maache M, Sánchez-Moreno M, Petavy AF, Osuna A. Effect of alkyl-lysophospholipids on some aspects of the metabolism of Leishmania donovani. Journal of Parasitology. 2007;93(5):1202–1207. doi: 10.1645/GE-1086R1.1. [DOI] [PubMed] [Google Scholar]
- 92.Santa-Rita RM, Lira R, Barbosa HS, Urbina JA, de Castro SL. Anti-proliferative synergy of lysophospholipid analogues and ketoconazole against Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae): cellular and ultrastructural analysis. Journal of Antimicrobial Chemotherapy. 2005;55(5):780–784. doi: 10.1093/jac/dki087. [DOI] [PubMed] [Google Scholar]