Research highlights
▸ ERN and Pe were observed in children between 4 and 8 years of age. ▸ Age and gender related differences were observed for the ERN, but not for Pe. ▸ ERN and Pe are associated boldness and attentional control in young children.
Keywords: ERN, Pe, Affective behaviors, Development
Abstract
Despite recent evidence that neural correlates of error monitoring such as the error-related negativity (ERN) and error positivity (Pe) are visible in children sooner than previously thought, little is known about these components early in life. Error-monitoring components can be noninvasively recorded from a very early age and have been proposed as biological markers of risk for psychopathology. Therefore, the current study represents an attempt to examine the presence of these components in a sample of very young children and explore their associations with affect and attentional control.
Fifteen children between ages 4 and 8 participated in two laboratory episodes: interacting with a stranger and completing a computerized flanker task. Shy and bold behaviors were scored during the stranger interaction and parents reported on temperament-based affective behaviors. Both ERN and Pe were visible in children as young as age 4. A trend-level interaction was observed between age and gender in association with ERN amplitudes. Age and gender were unrelated to the Pe. Greater ERN and Pe were associated with better poorer orienting and greater attentional focusing, respectively. Greater Pe was also linked to less observed boldness. Implications for studies of the development of performance monitoring in children are discussed.
1. Introduction
Error monitoring reflects core cognitive control processes that may underlie the regulation of emotions and behavior (Falkenstein et al., 2000, Rothbart et al., 1994). Scalp recorded event-related potentials (ERPs) reflecting error monitoring, including the error-related negativity (ERN; Gehring et al., 1993) and error positivity (Pe; Falkenstein et al., 2000), can be noninvasively recorded from children, adolescents, and adults. Research on the ERN and Pe thus far has largely focused on adult samples, resulting in a lack of understanding of the development of components of error monitoring and their associations with behavior in typically-developing children. Yet, given recent discussions of the ERN as a possible endophenotype for mental health outcomes (e.g. Olvet and Hajcak, 2008), characterizing the normative development of ERPs related to error monitoring will be a crucial step in identifying trajectories of mental health and disorder. The current study represents an attempt to address a gap in the literature by examining the ERN and Pe in a sample of young children and exploring associations with affective behaviors.
1.1. The ERN and Pe
The ERN is typically seen 50–100 ms following an incorrect behavioral response, with maximum amplitudes at frontocentral midline scalp recording sites (Dehane et al., 1994, Falkenstein et al., 1991, Gehring et al., 1993). The ERN is believed to reflect activation in the anterior cingulate cortex (ACC; Dehane et al., 1994, Gehring et al., 2000), which shows increased activity under conditions requiring monitoring of performance and cognitive control (Luu and Pederson, 2004, Posner and Rothbart, 2000). The ERN is believed not only to capture aspects of error detection (Falkenstein et al., 1991), but also conflict detection (Botvinick et al., 2001), reinforcement learning (Holroyd and Coles, 2002), emotional reactions to errors (Luu and Pederson, 2004), and motivation (Gehring and Willoughby, 2002). Thus, the ERN likely reflects a general process of performance monitoring and self regulation (Falkenstein et al., 2000).
A lesser studied index of error monitoring, the error positivity (Pe), is a positive-going slow wave that follows the ERN (Falkenstein et al., 1991, Leuthold and Sommer, 1999) and is also believed to be related to ACC activity (van Veen and Carter, 2002). The Pe has a slightly more posterior scalp distribution than the ERN and may reflect more elaborated and conscious processing of errors (Falkenstein et al., 1991, Falkenstein et al., 2000, Nieuwenhuis et al., 2001), although there continues to be debate about the unique role of the Pe in the detection of errors and monitoring of performance (Overbeek et al., 2005).
1.2. Development of the ERN and Pe
Initially believed not to be visible in children under 12 years of age (Davies et al., 2004), both the ERN and Pe have now been elicited in groups children as young as 5–7 years of age (Santesso et al., 2005, Torpey et al., 2009, Wiersema et al., 2007). This early detection is likely the result of increased use of tasks that have been modified for age appropriateness rather than applying standardized adult tasks to populations of young children (Torpey et al., 2009). Suggestions have been made that greater task difficulty decreases the likelihood of observing ERN and Pe in young children, even when overall performance is unchanged (Hogan et al., 2005, Torpey et al., 2009). Overall, the morphology and distribution of the ERN in children is similar to that seen in adults (Arbel and Donchin, 2010). However, the amplitude of the ERN generally increases with age (Davies et al., 2004, Kim et al., 2007, Ladouceur et al., 2004), likely reflecting maturation of the ACC (Adleman et al., 2002, Caviness et al., 1996).
Some work has implicated different rates of maturation for ERN in boys and girls, with girls reaching peak ERN amplitudes near age 10, 3 years earlier than for boys (Davies et al., 2004). This difference may reflect gender-related variation in the timing of myelination and dendritic pruning during childhood and adolescence (Anderson, 2003, Berenbaum et al., 2003), and has plausible links to the onset of mental illness in the adolescent years (Anderson, 2003).
In contrast to the ERN, the Pe shows little age or gender-related change over time (Davies et al., 2004, Wiersema et al., 2007), which may implicate a less protracted maturational period. Though based on only a few studies, past work supports a link between ERN and Pe, but characterizes them as independent components of the error-monitoring system (Falkenstein et al., 2000, Wiersema et al., 2007). Additional work will be needed to elucidate the nature and impact of differences between the ERN and Pe.
Thus, the ERN and Pe appear to be differentially influenced by age and gender over the course of neural development. However, studies that examine both components in children are rare. Therefore, an additional aim of the present study was to examine age and gender-related differences in the ERN and Pe in early childhood.
1.3. Error monitoring and affective behaviors in children
From a temperament framework, affective behaviors reflect individual differences in propensities for experiencing, expressing, and regulating emotional reactivity. This understanding combines the notion that observed emotional behaviors, such as displays of positive and negative emotions, are perhaps some of the most salient and easily observed facets of temperament (Goldsmith et al., 1987) with the idea that these behavioral displays are based on biologically based individual differences in emotional reactivity and regulation (Rothbart and Derryberry, 1981). A host of temperament research has focused on the affective behaviors of fear and effortful control. At their high-end extremes, these behaviors are believed to be linked to subsequent clinical diagnoses such as anxiety or attention deficit/hyperactivity, respectively. Recent work suggests that these affective behaviors may represent early vulnerabilities to disorder which can be identified prior to clinical diagnosis (Beauchaine et al., 2010, Muris et al., 2001). The use of electrophysiological recordings may enable the detection of such vulnerabilities at the level of neural activity, serving as endophenotypes (Gottesman and Gould, 2003, Olvet and Hajcak, 2008) of putative risk for targeted programs of prevention and intervention.
In adults, the ERN has been associated with numerous forms of affect, such as reward sensitivity (Foti and Hajcak, 2009), error significance (Gehring and Willoughby, 2002, Hajcak et al., 2004, Luu et al., 2000a), general negative affect (Hajcak et al., 2004, Luu et al., 2000a), worry (Hajcak et al., 2003), and depression (Chiu and Deldin, 2007, Holmes and Pizzagalli, 2008). Modulation of the ERN has been linked to individual differences in the control of attention such that greater ERNs are linked to better overall monitoring (Botvinick et al., 2004, Luu et al., 2000b). This is perhaps not surprising given that both the ERN and processes of cognitive control are thought to be under the influence of the ACC (Bush, 2004, Dehane et al., 1994, Luu and Pederson, 2004, MacDonald et al., 2000).
While there is evidence for developmental differences between the ERN and Pe (e.g., Torpey et al., 2009), some studies have replicated associations from the adult literature among components of error monitoring, negative affect, and attention control. For example, more negative ERN amplitudes in children during a flanker task are associated with more anxious behaviors, including a diagnosed anxiety disorder (ages 8–14; Ladouceur et al., 2006), parent-reported obsessive-compulsive behaviors (age 10; Santesso et al., 2006), and a history of behavioral inhibition in infancy (ages 14–16; McDermott et al., 2009). Findings for the Pe in these studies were mixed, with some (e.g., Santesso et al., 2006), but not all work finding links between a greater amplitude Pe and greater anxious behaviors.
Recent work has also shown that deficits in attention control or error monitoring, particularly in combination with negative affect, places children at heightened risk for negative outcomes; these associations are often visible at the neural level (Ladouceur et al., 2010, McDermott et al., 2009). For example, children who have been diagnosed with Attention Deficit Hyperactivity Disorder (ADHD) show attenuated Pe during a go/no-go task (ages 7–13; Wiersema et al., 2007). Similarly, using a Stop Signal task, Liotti et al. (2005) found that children with ADHD showed both poor performance and an attenuated ERN (ages 9–11; Liotti et al., 2005). Similarly, work conducted by Steiben et al. (ages 8–12; Steiben et al., 2007) using a go/no-go task reported an association between attenuated ERN amplitudes and parent and teacher reports of both greater impulsivity and poorer attention control.
1.4. The current study
Given the dearth of research on the development of ERN and Pe in children, the current study was designed to examine the presence of the ERN and Pe in a group of typically-developing young children. To our knowledge, this is the first study to include children as young as age 4 in such an investigation, though findings in a population this young will increase our ability to track normative and aberrant patterns of developing attentional control. Moreover, conducting this work in association with affective behaviors enhances our understanding of the implications associated with aberrant developmental pathways of attention control. Given our use of a carefully selected, age appropriate-task (Torpey et al., 2009), we hypothesized that both the ERN and Pe would be present in children between the ages of 4 and 8, though both would be less frontalized and smaller in amplitude than has been seen in adults. Moreover, given what is known about the changes in amplitude and frontalization across development, we hypothesized that age would be positively correlated with error-related neural activity (i.e., more negative ERN and more positive Pe following errors) in young children, and that this positive correlation would be particularly visible at frontal electrodes. Finally, we hypothesized that ERN and Pe would be associated with children's affective behaviors. Specifically, given the associations between ERN, negative affect, and attention control seen in the literature, we expected that greater ERN and Pe on error trials would be associated greater negativity (i.e., shyness, fear, withdrawal), less positivity (i.e., positive affect, approach), and better attention control (i.e., executive control, orienting, and alerting).
2. Method
2.1. Participants
Thirty-three children (11 females) were recruited to participate in a laboratory visit as part of a study of children's attention and emotions. Participants were recruited through fliers and announcements in a community newsletter. To be eligible for participation, children had to be between 4 and 8 years of age, right-handed, and not taking any stimulant medications. Children were also excluded if they or a member of their immediate family had a history of neurological impairment. The average age of participants was 68.78 months (SD = 15.28). Reflecting the demographics of the area from which they were recruited, the sample was largely middle class (Hollingshead Index: M = 48.62, SD = 12.62). As reported by parents, 29 children (87.9%) were Caucasian, 2 children (6.1%) were African-American, and 1 child (3.0%) was American-Indian; 1 child (3.0%) was reported as being of Hispanic ethnicity.
2.2. Procedure
2.2.1. Laboratory visit
After determining that their child was eligible for participation in the study based on the above criteria, parents who responded to the advertisements were mailed a packet including a consent form and a child temperament questionnaire to be completed and brought to the laboratory visit. Upon arrival to the laboratory, children were fitted with a neural net to be used for EEG data collection. The child then participated in a number of laboratory episodes. This report focuses on two episodes: a conversation with a stranger and a computerized attention task. Families received $20 and children received a small gift as a token of appreciation for their participation.
2.2.2. Conversation with a stranger
While parents waited in an adjacent room, the experimenter led the child into a room and told him/her to wait while the experimenter left to get the next activity ready. When the child had been sitting alone for 10 s, a second experimenter who was unfamiliar to the child entered the room. The stranger approached the child slowly and stayed in the room with him/her for up to 3 min while asking several questions (e.g., “What kinds of games do you like to play?”). Following this, the stranger spoke with the child about the purpose and function of the EEG net (approximately 1 min), stated that it was time for them to leave, and exited the experimental room. Approximately 10 s after the stranger left the experimental room, the experimenter returned and began the ANT task.
2.2.3. Attention Network Test
Children individually completed the child version of the Attention Network Test (ANT; Dennis et al., 2009, Fan et al., 2002, Rueda et al., 2004) on a Dell PC using E-Prime 1.1 (Psychology Software Tools, Inc.: Pittsburg, PA). The experimenter was present throughout testing, but did not provide feedback to participants outside of encouragement to complete the task. Children were seated approximately 10 in. from the computer screen and given a response box to either hold in their lap or place on a table in front of them, whichever was more comfortable.
The experimenter explained the task to each participant using a set of index cards depicting arrays of five fish. Participants were instructed to pay attention only to the fish in the middle of the array (i.e., the target) and to “feed that fish” using the response box. The rightmost button on the response box corresponded to fish depicted as facing rightward; the leftmost button on the response box corresponded to fish depicted as facing leftward. Prior to beginning the practice trials, the experimenter asked participants to indicate which button on the response box corresponded to the correct response for the target arrays depicted on the index cards. When it was clear that participants understood the instructions, they began a set of practice trials.
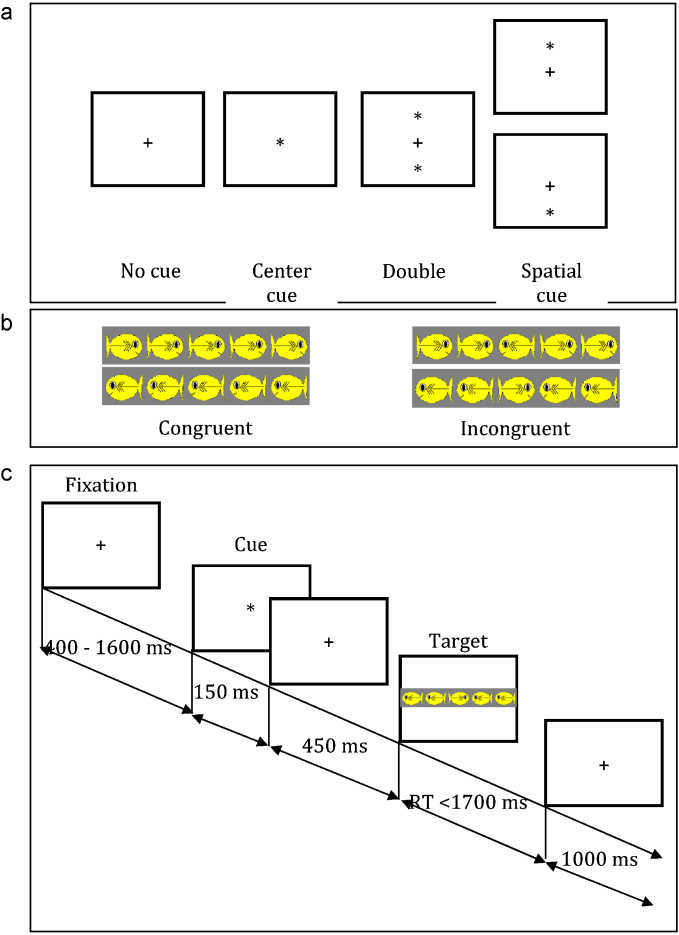
A session of the ANT consisted of a total of 16 practice trials and three experimental blocks of 32 trials. Each trial began with the presentation of a fixation cross for 400 ms. On some trials a warning cue was subsequently presented for 150 ms and represented one of four warning cue conditions: a center cue, a double cue, a spatial cue, or no cue. In the center cue condition, an asterisk was presented at the location of the fixation cross. In the double cue condition, an asterisk appeared at locations of the target both above and below the fixation cross. In the spatial cue condition, a single asterisk appeared in the position of the upcoming target. A fixation period of 450 ms followed the disappearance of the cue. Following this, the target array appeared and remained on the screen until a response was detected or a maximum of 1700 ms elapsed. During congruent trials, the target fish was surrounded by fish facing in the same direction; during incongruent trials, the target fish was surrounded by fish pointing in the opposite direction. Accuracy and reaction time were recorded for each trial. A schematic representation of the task is shown in Fig. 1. Children in the current data set answered an average of 62.33 trials correctly (SD = 17.94) and 19.33 (SD = 10.00) trials incorrectly. Number of correct and incorrect responses were uncorrelated with ERP measures (|r|s < .38, ps > .05).
Fig. 1.
Experimental procedure. (a) The four cue conditions, (b) the four stimuli, and (c) an overview of the procedure.
2.2.4. Electroencephalograph recordings
EEG was continuously recorded during the ANT using a 128-channel dense array Geodesic Sensor Net (Tucker, 1993) and analyzed using Net Station software from Electrical Geodesics, Inc. (EGI, Eugene, OR) at a sampling rate of 500 Hz. Prior to beginning data acquisition, all impedances were reduced to less than 80 kΩ. EEG was recorded using a 0.1–100 Hz bandpass filter. Channels were referenced to the Cz (Channel 129) for acquisition, then rereferenced to the average reference (Bertrand et al., 1985, Tucker et al., 1994) and corrected for polar average reference effects (PARE; Junghöfer et al., 1999) prior to data analysis. Data was highpass filtered at .10 Hz and a lowpass filtered at 35 Hz. Artifacts were screened using automatic detection methods (Net Station, EGI, Inc.) and data was visually inspected. Eye blink and eye movement artifacts (70 μV threshold) and signals exceeding 200 μV were removed during averaging. Across the sample, an average of 8 trials per participant were removed due to eye blinks or eye movements. Channels with excessive noise throughout the experiment were marked as “bad” and excluded from analyses. Children in the current data set had a mean of 41.29 usable correct trials (range = 12–81) and 15.24 usable incorrect trials (range = 10–39) in their final average. The total number of trials included in the correct and incorrect grand averages were 702 and 259, respectively.
2.3. Coding and data reduction
2.3.1. Event-related potentials
The EEG was time-locked to the response, segmented 50 ms prior to and 500 ms following participants’ response, and divided according to type of trial (correct responses vs. incorrect responses). Epochs were baseline corrected for 50 ms preceding the response. Segments containing eye blinks, eye movements, or response times of less than 200 ms were excluded. Bad channels were replaced using spherical spline interpolation of values of neighboring channel (Perrin et al., 1987). This resulted in the replacement of an average of 5.81 channels across all participants.
The time widow for the ERN was identified using a principal components analysis of the grand averaged data. The ERN was defined at the Fz, Cz, and Pz electrode sites as the greatest negative deflection occurring between 52 ms and 134 ms post-response. This difference was then subtracted from the preceding positive peak. This method was selected in order to capture the full degree of the negative deflection and account for possible individual differences in EEG amplitudes prior to the ERN.
The time window for the Pe was also identified using a principal components analysis of the grand averaged data. The Pe was defined at Fz, Cz, and Pz as the average amplitude from 20 ms before to 20 ms after the greatest positive peak occurring between 360 ms and 500 ms post response. The average baseline amplitude from 50 ms to 0 ms prior to response was then subtracted from this value in order to quantify increases in amplitude and account for individual differences in EEG amplitudes prior to the Pe. This method for quantifying the Pe is similar to that used by Nieuwenhuis et al. (2001).
To improve power and signal-to-noise ratio in this relatively small sample of children, we employed a jackknife approach (Miller et al., 1998). This method is an excellent approach for measuring differences in instances where a wide range of variability occurs between participants. The jackknife approach involves calculating N grand-averaged waveforms (where here N = 15), such that each participant is excluded in one of the grand averages. This approach resulted in 15 (N) grand averages comprised of 14 (N − 1) participants. Although the variability due any individual estimates is preserved in the grand average associated with him/her, the use of a grand average reduces the amount of noise in the waveform, making it an optimal technique for use with “noisy” populations, such as children. The significance of statistical estimates can then be tested using the jackknife technique to assess the standard error of the estimate (see Miller et al., 1998 for additional details). The jackknife is a well-reasoned approach that has been demonstrated and validated in a series of simulations. This technique is recommended by ERP experts (e.g., Luck, 2005) and has shown to be a powerful, robust method for use with ERP data, particularly in studies with small sample sizes.
2.3.2. Affective behaviors
Children's affective behaviors were assessed using the Child Behavior Questionnaire Short Form (CBQ-Short; Putnam and Rothbart, 2006). The CBQ short form contains 94 items that assess three broad domains of temperament: surgency, negative affect, and effortful control. These domains include several scales that were selected for the present study based on their associations with negative affect and attention. Namely, the current study focused on the scales of fear (negative affect related to anticipated distress or potential threat), inhibitory control (response planning or suppressing inappropriate responses), Impulsivity (speed of response initiation), and attention focusing (maintaining attentional focus to task at hand). Parents responded to statements using a 6-point Likert scale based on the degree to which scale items are true of their child (1 = extremely untrue of my child, 2 = quite untrue of my child, 3 = slightly untrue of my child, 4 = neither true nor false of my child, 5 = slightly true of my child, 6 = extremely true of my child). As measured by Cronbach's α, reliability for subscales ranged from .70 to .88.
2.3.3. Laboratory-based observations of affect
Videos of the conversation with a stranger were used to assign global ratings of shyness/withdrawal and boldness/approach during the episode for each child. Ratings were assigned for each dimension based on a five-point scale ranging from (1) an absence of the behavior to (5) behavior of the highest intensity and duration. Negative affect was coded as observable negative facial affect and negative vocalizations. This included all negative facial expressions (sadness, fear, etc.) and negative vocalizations such as crying, whimpering and comments about the child's discomfort (Anchors: 1 = no negative affect shown; 5 = display of negative affect that lasts the whole episode, is very intense or results in the episode being stopped). Positive affect was coded as any positive facial affect or positive vocalizations, including smiling, laughing, and excited clapping (Anchors: 1 = no positive affect shown; 5 = display of positive affect that lasts the whole episode, or long displays of intense positive affect). Shyness/withdrawal was coded when the child exhibited inhibited or withdrawn behaviors, such as physically orienting away from stranger, fidgeting, self-stimulation, hiding their face, avoiding interaction, not talking, or abstaining from activity (Anchors: 1 = no shyness/withdrawal shown; 5 = child is extremely shy, freezes, or is totally avoidant or resistant of the stranger throughout the episode). Boldness/approach was coded when the child attempted to interact with the stranger or take control over the episode. This included approaching the stranger and/or initiating conversation or interaction during the episode (Anchors: 1 = no boldness/approach shown; 5 = child takes a lot of initiative in talking to stranger, physically orients toward stranger, seems very comfortable with situation, makes several attempts to engage the stimulus may be controlling the course of the interaction).
All coders were required to achieved a reliability of at least α = .70 with a master coder prior to independent coding. Individual episodes of the conversation with a stranger were double coded and final scores were calculated as the mean of the double codes. Inter-rater reliabilities prior to averaging the scores of the double codes were good (negative affect: α = .50, positive affect: α = .91, shyness/withdrawal: α = .67, boldness/approach: α = .90). Ratings of shyness and boldness during the conversation with a stranger were significantly negatively correlated (r = −.64, p < .01).
2.3.4. Attention control
Scores for three scales of attention control (Fan et al., 2002), alerting, orienting, and executive control, were calculated for each participant based on the response time data recorded during the ANT. Alerting scores were calculated by subtracting the response times (ms) for trials in which the double cue was presented from trials with no cue presentation. Orienting scores were calculated by subtracting the response times for trials in which spatial cues were presented from trials in which a central cue was presented. Executive control scores were calculated by subtracting the response times for congruent trials from response times for incongruent trials. Error trials or trials with response times of less than 200 ms were not included in the calculation of the alerting, orienting, or executive control scores.
2.3.5. Missing data
Participants with fewer than 10 trials of usable EEG data were excluded from ERP averages (Deboer et al., 2005). This resulted in data from 11 children being excluded from ERP averages because there were not enough incorrect trials to create an average and data from one child being excluded because there were not enough correct trials to create an average. Subjects with fewer than 5 trials of usable ANT data were excluded from response time averages. This resulted in two children being excluded from ANT averages because there were not enough incorrect trials to create an average. In addition, two children refused the capping upon arrival to the laboratory and data from two children were lost due to equipment malfunction. Two children were excluded from analyses as they did not appear to engage in or attend to the task. ANT data from one child was lost due to equipment malfunction while EEG data were preserved. Thus, the analyses presented here included complete data from 15 children (7 females). Children included in the final data set did not significantly differ by gender (χ2 = 1.01, p > .10) or by age (t23 = .92, p > .10). Children who were not included in the current analyses were, on average, more shy (t19 = −2.97, p < .01), and showed poorer effortful control (t23 = −2.51, p < .05) and faster response times on the ANT (t23 = −2.10, p < .05) than children who were included in the current data set. Children included in the current analyses did not differ from children without usable data on any other observed or parent-reported measures (|t|s < 1.96, ps > .05).
3. Results
3.1. Descriptive statistics
As is typically seen, mean response times for the ANT differed between correct and error trials (t(23) = 2.63, p < .05); response times on error trials (M = 881.50, SD = 202.74) were significantly faster than response times on correct trials (M = 956.75, SD = 150.62). Age was significantly positively associated the number of correct responses produced (β = .68, t = 4.47, p < .01). The number of correct responses on the ANT was not different for boys and girls (t(23) = −.13, p > .10). Similarly, performance on the ANT was uncorrelated with children's race (r = .27, p > .10) or socioeconomic status (r = −.29, p > .10).
Means and standard deviations for study variables are shown in Table 1. Patterns were consistent with past work, suggesting that both the ERN and Pe were greatest at anterior electrodes.
Table 1.
Means and standard deviations for study variables.
| Variable | N | M | SD |
|---|---|---|---|
| ERN difference at Fz (μv) | 15 | −2.54 | .39 |
| ERN difference at Cz (μv) | 15 | −2.09 | .42 |
| ERN difference at Pz (μv) | 15 | −2.47 | .51 |
| Pe difference at Fz (μv) | 15 | 3.38 | .37 |
| Pe difference at Cz (μv) | 15 | 5.49 | .88 |
| Pe difference at Pz (μv) | 15 | 3.76 | .49 |
| ANT conflict | 25 | 73.02 | 54.03 |
| ANT alerting | 25 | 51.60 | 68.38 |
| ANT orienting | 25 | 13.45 | 77.37 |
| Observed shyness | 21 | 2.05 | .69 |
| Observed boldness | 21 | 3.07 | .91 |
| CBQ fear | 25 | 3.27 | .97 |
| CBQ inhibitory control | 25 | 4.74 | 1.00 |
| CBQ attentional focusing | 25 | 4.87 | 1.22 |
3.2. Testing for the presence of ERN and Pe in young children
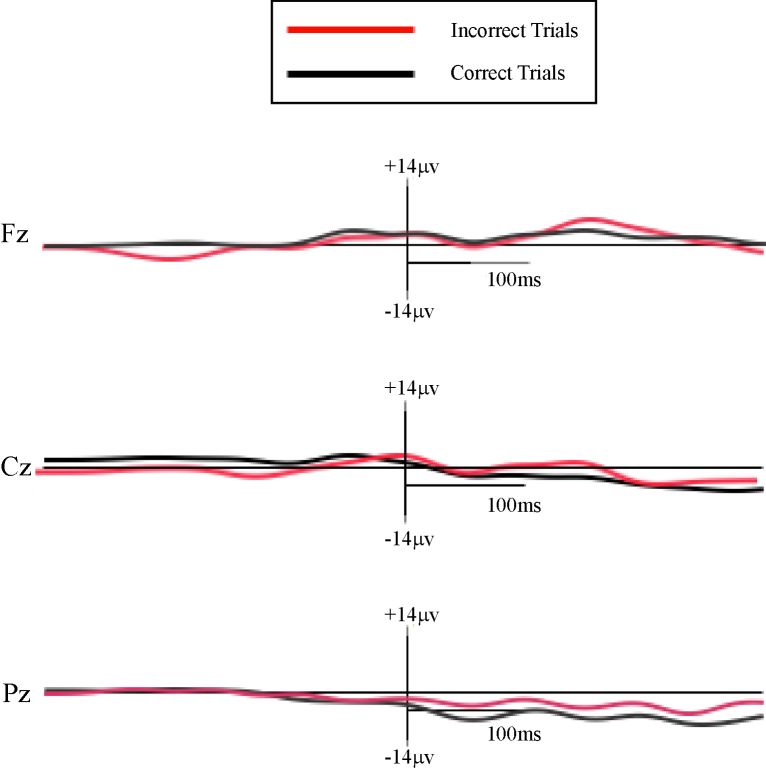
A 2 (Trial Type: correct vs. incorrect) × 3 (Electrode Site: Fz, Cz, Pz) repeated measures ANOVA was used to test for the presence of the ERN. A main effect of Electrode Site suggested that the ERN was more negative at Cz (F = 33.46, p < .01, ) and Pz (F = 92.00, p < .01, ) than at Fz. In addition, a significant main effect of Trial Type suggested that ERN amplitudes were more negative for incorrect than for correct trials (F = 8.18, p < .05, ; Fig. 2). Given this difference in neural activity on correct and incorrect trials, an ERN difference score was created in order to isolate neural activity associated with error commission. This was done by subtracting activity on correct trials from activity on incorrect trials and rescoring the ERN according to the methods described above. This ERN difference score was used in subsequent analyses.
Fig. 2.
Response-locked ERP at Fz, Cz and Pz. Note: Waveforms were high-pass filtered at 15 Hz for display purposes only. Vertical crosshairs represent response event (time = 0).
Similarly, a 2 (Trial Type: correct vs. incorrect) × 3 (Electrode Site: Fz, Cz, Pz) repeated measures ANOVA was used to test for the presence of the Pe. A main effect of Electrode Site (F = 557.87, p < .01, ) suggested that Pe amplitudes were greater at Fz than at Cz (F = 588.84, p < .01, ) and Pz (F = 618.95, p < .01, ). A significant main effect of Trial Type also showed that Pe amplitudes were greater on incorrect than on correct trials (F = 1352.94, p < .01, ). Moreover, a significant interaction between Electrode Site and Trial Type showed a linear decrease in Pe amplitudes during incorrect trials, but not during correct trials (F = 105.36, p < .01, ). Thus, this difference across electrodes appeared to be specific to error trials. Given this difference in neural activity on correct and incorrect trials, a Pe difference score was created in order to isolate neural activity associated with error commission. This was done by subtracting activity on correct trials from activity on incorrect trials and rescoring the Pe according to the methods described above. The Pe difference score was used in subsequent analyses.
3.3. Testing age and gender-related differences in the ERN and Pe
Hierarchical regression analyses were used to test whether age and gender were associated with the ERN and Pe difference scores. In Step 1, children's gender was dummy coded (0 = male, 1 = female) and entered as a predictor of the difference score (ERN or Pe) along with participants’ age in months. The interaction between gender and age was entered in Step 2. The use of regressions rather than ANOVAs to test these effects allowed for the use of the full sample size for each analysis and increased our power to detect significant effects. Separate regressions were used for each electrode site.
Regressions predicting the ERN difference score did not show a significant effect of age (β = −.95, t = 1.44, p > .10) or gender (β = −2.47, t = −1.43, p > .10) at Fz and no significant interaction was observed (β = 2.79, t = 1.43, p > .10). Similarly, there was no significant effect of age (β = −.48, t = −2.43, p > .10) or gender (β = −2.43, t = −1.46, p > .10) and no significant interaction (β = 2.52, t = 1.33, p > .10) at Pz.
A marginally significant interaction emerged between age and gender predicting the amplitude of the ERN difference score at Cz (β = 3.38, t = 1.92, p < .10, ΔR2 = .24). Given the moderate effect size of the interaction and our preexisting hypotheses regarding age and gender this interaction was probed by recoding the gender variable so that boys and girls each served as the reference group for the interpretation of the main effect of age (Aiken and West, 1991). Examining the interaction in this way revealed that for girls, age was marginally associated with the amplitude of the ERN difference score; girls showed a greater difference in ERN amplitude between correct and incorrect trials with age (β = −3.23, t = −2.07, p < .10). However, age was not associated with ERN amplitude at Cz for boys (β = .20, t = .65, p > .10).
There were no significant effects observed at any site for age (Fz: β = .10, t = .14, p > .10, Cz: β = −.31, t = −.44, p > .10, Pz: β = −.06, t = −.09, p > .10) or gender (Fz: β = −.53, t = −.29, p > .10, Cz: β = −.92, t = −.50, p > .10, Pz: β = −1.28, t = −.74, p > .10) in association with Pe amplitude and no significant interactions (Fz: β = .61, t = .29, p > .10, Cz: β = 1.26, t = .60, p > .10, Pz: β = 1.03, t = .53, p > .10).
3.4. Exploring links between the ERN and affective behaviors
Recall that a second aim of the current study was to explore the association of error-related electrical activity in the brain with affective behaviors in early childhood, particularly indices of negative affect and attention. To do this, we examined correlations between the amplitudes of the ERN and Pe difference scores with observed and parent-reported measures of negative affect and attention control. Correlations for the ERN focused on the difference scores at Cz and Pz electrode sites given that these electrodes showed the greatest ERN amplitudes during error trials and given the moderating effect of age at Cz. Similarly, correlations for Pe focused on the difference scores at Fz and Cz given that these electrodes showed the greatest Pe amplitudes on error trials. Recall that the only the CBQ scales of fear, inhibitory control, impulsivity, and attention control were the focus of the present study. Recall also that given the method of calculation of the difference score, a more negative ERN difference score signifies a greater negative deflection on error relative to correct trials. Similarly, a more positive Pe difference score signifies a greater positive deflection on correct vs. incorrect trials. Due to the number of comparisons, only those that reached a significance level of p < .01 are discussed. All correlations are shown in Table 2.
Table 2.
Correlations between neural components of error-monitoring and affective behaviors.
| ERN difference Cz | ERN difference Pz | Pe difference Fz | Pe difference Cz | |
|---|---|---|---|---|
| Alerting | −.05 | −.39 | −.29 | −.38 |
| Orienting | .38 | .70** | .43 | −.21 |
| Executive control | −.14 | .35 | .10 | .19 |
| Observed NA | −.08 | −.07 | .38 | .48 |
| Observed PA | .34 | .23 | −.03 | −.43 |
| Observed SW | −.20 | −.00 | .60* | .65* |
| Observed BA | .25 | .04 | −.43 | −.74** |
| CBQ fear | −.18 | .14 | −.11 | −.45 |
| CBQ inhibitory control | .26 | −.15 | −.54* | .24 |
| CBQ attentional focusing | .09 | −.10 | −.68** | .16 |
Notes: NA = negative affect, PA = positive affect, SW = shyness/withdrawal, BA = boldness/approach. *p < .05, **p < .01.
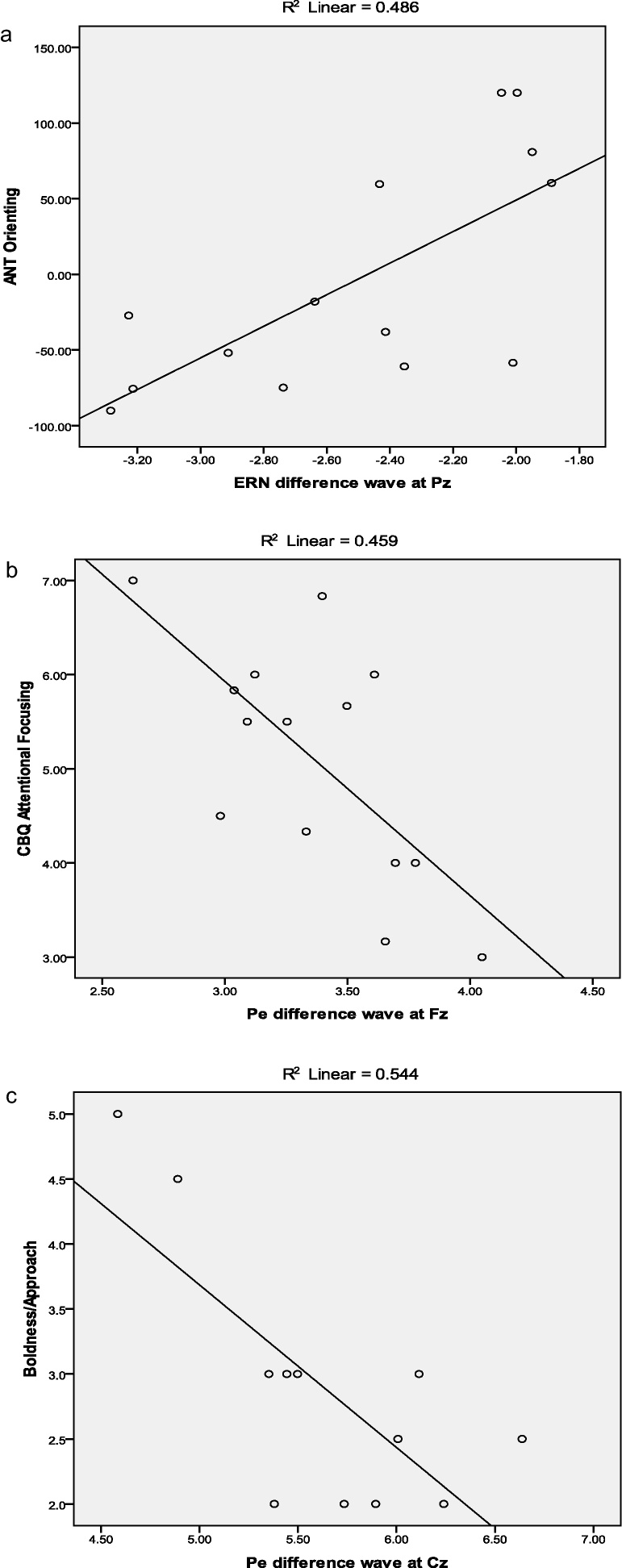
ERN amplitude was not associated with observed behaviors or parent-report measures, but was linked to performance on the ANT. As shown in Fig. 3a, a more negative ERN for incorrect than for correct trials at Pz (i.e., more negative ERN difference wave) was associated with poorer orienting (r = .70).1 In contrast, a more positive Pe for incorrect relative to correct trials (i.e., more positive Pe difference wave) was associated with less parent-reported attention focusing (Fz: r = −.68) and less boldness during the conversation with a stranger (Cz: r = −.74).1 The relation between the Pe difference wave and shyness was in the expected direction at both Fz and Cz, but was not significant at the p < .01 level. Plots of significant correlations are shown in Fig. 3.
Fig. 3.
Correlations of ERN and Pe with (a) orienting, (b) attentional focusing, and (c) boldness/approach.
4. Discussion
The present study extends previous work on the ERN and Pe by examining these ERP components and their associations with affective behaviors in a group of children as young as age 4. Specifically, we found that both the ERN and Pe are discernable in young children between ages 4 and 8. We also found a trend-level association that accounted for 24% of the variance in the amplitude of the ERN difference score suggesting a moderating effect of gender in the link between age and ERN. In contrast, age and gender were unrelated to Pe in young children. Furthermore, ERN and Pe were associated with observed and parent-reported behaviors in children. Greater ERN was associated with poorer attentional orienting in children, while greater Pe was associated with poorer parent-reported attention focusing and less observed boldness in children.
4.1. Age and gender-related changes in error monitoring ERPs
Relative to adults, in whom the ERN has been found to be maximal at frontal scalp regions (Davies et al., 2004, Falkenstein et al., 2000, Gehring et al., 1993), the ERN observed in children in the present study was more broadly distributed. Similar work by Torpey et al. (2009) found a centro-parietal maximum for ERN in children between ages 5 and 7. A shift in maxima from parietal to frontal regions is likely an additional indication that the ERN is maturing in children of this age. Stimulus-locked ERP components of cognitive control, such as the N2 (Lange et al., 1998) have also been linked with neural activity at more posterior sites in children relative to those typically seen in adults (Bunge et al., 2002, Buss et al., 2011). Similar patterns of posterior neural activity have been shown in children during cognitive control tasks using other imaging techniques (Bunge et al., 2002, Ciesielski et al., 2004). Theories of neurodevelopment posit that early-developing areas such as the striatum are involved in cognitive processing before being overtaken by later-maturing, more anterior structures such as the ACC (Bachevalier and Mishkin, 1984, Goldman et al., 1971). This change in primary processing centers may underlie observed patterns of change in posterior to anterior activation during cognitive tasks as children age.
There was a trend in these results such that for girls, being older was associated with a more adult-like pattern of the ERN (i.e., greater differences in amplitude between correct and incorrect trials). Despite empirical support for gender differences in brain maturation, gender is not frequently tested in neuroimaging studies with children. For example, Hogan et al. (2005) reported a similar finding in research with adolescents; their work showed a nonsignificant differentiation of error-related negativity and correct response negativity (CRN) between ages 12 and 18 during a difficult cognitive task. However, because gender effects were not directly assessed, it is unknown whether ability to differentiate between correct and incorrect trials at the level of neural processing differed for girls and boys. Such a distinction may indicate an overall difference in rates of maturation of error-processing components in parallel to differences in rates of maturation of overall brain structure. While data from the current study cannot directly inform this hypothesis, one previous study did show apparent differences in the development of the ERN between girls and boys, with ERN amplitudes peaking earlier in girls than in boys (Davies et al., 2004). This difference in developmental peaks has since been supported in studies using structural MRI (Lenroot et al., 2007).
The significance of gender differences in brain structure and neural development is unclear, perhaps due to inconsistencies in findings, a lack of longitudinal data, and an insufficient understanding of the link between brain structure and function (Blakemore et al., 2009). Moreover, the causal factors underlying these differences remain unknown, leading to speculations that they are products of both biological (Merke et al., 2003) and environmental influences (Maguire et al., 2003). Yet, along with prior work, the current study lends support to the notion that gender should be considered as studies of ERN in children progress.
It is also of note that ERN amplitudes in this sample of children are somewhat smaller than amplitudes that are typically seen in work done with adults. This trend is not uncommon for developmental studies; smaller amplitudes in children may be related to less localized brain activity resulting in part from heightened neuronal density that is still present at this time. Huttenlocher (1990) reported that age 7, neuronal density in the frontal cortex is still 10% above mean-level values for adults. The pruning of cortical neurons and stabilization of synapses continues well into middle childhood. Other work has similarly found that ERP amplitudes increase as components become more stable across development (Davies et al., 2004, Ladouceur et al., 2004, Ladouceur et al., 2007).
In contrast to the ERN, the Pe did not appear to vary with age or gender. This finding expands the age range of children that have shown a Pe during a cognitive task; such findings are in line with the idea that the timing of the development of the Pe is distinct from the ERN (e.g., Hogan et al., 2005). In addition, evidence suggests that the ERN and Pe may be linked to independent neurobiological substrates, such as the mesofrontal/mesolimbic dopaime system, which has been associated with enhanced ERN but not Pe (De Bruijn et al., 2004).
4.2. ERN and Pe are linked to boldness and attention control
To our knowledge, this is the first study to explore links among affective behaviors such as boldness, attention control, and ERP components of error monitoring in young children. Results revealed associations between error-related neural activity in the brain and less efficient attention processing. That is, contrary to study hypothesis, a greater ERN and Pe were related to smaller differences in response times for trials containing cues with spatial information relative to trials with non-informative central cues (i.e., orienting) and poorer attention focusing, respectively. As with other developing ERPs in young children, (e.g., Buss et al., 2011, Jonkman et al., 2003), these data may reflect a lack of processing efficiency in this young group of participants. As previously noted, prior work has suggested that early in life, children can recognize and report making errors, but are not necessarily able to change behavior and improve performance (Zelazo and Müller, 2002). In fact, some research has suggested that these systems of error detection and correction develop separately (van Veen and Carter, 2006). At least one previous study has shown that these networks are continuing to increase to adult levels through age 10 (Rueda et al., 2004). Thus, the ERN and Pe observed here suggest that young children are monitoring their performance; over time they may also become able to use additional cues such to improve performance.
Greater Pe was also related to less boldness during the conversation with a stranger. To the extent that a lack of boldness may reflect hesitancy to approach and cautiousness or vigilance about engaging with new people and situations, this finding replicates previous work linking ERN and Pe to hypervigilance in young children (Ladouceur et al., 2006, McDermott et al., 2009).
4.3. Limitations
Although this study extends the current literature on associations between early components of error monitoring and children's affective behaviors, there are limitations that restrict the conclusions that can be drawn here. First, the relatively small sample size limits the power of the current set of analyses. Replication of these results in a larger sample of children in which age and gender could be directly tested would help to clarify associations presented here. Second, the size of the current sample was further limited by a surprising ceiling effect of performance on the ANT. An unexpected number of children did not err on enough trials to be included in the ERN/Pe analyses. Given past work stressing the importance of task modifications for young children (e.g., Hogan et al., 2005, Torpey et al., 2009), our primary concern was that the task not be overly difficult for young children. However, it appears that modifications not only be considered for comparisons of children and adults, but may also be necessary across stages of early development. Finally, although there are developmental implications in the current findings, the cross-sectional nature of this work limits the degree to which developmental conclusions can be drawn.
5. Conclusions
The current study extends the extant literature by offering evidence that both the ERN and Pe can be elicited in children as young as 4 years of age. In line with research on brain development during childhood, it suggests that there may be age and gender-related differences in the maturation of the ERN and Pe that should be considered in subsequent work. Of additional importance, this work also shows that ERN and Pe are associated with young children's affective behaviors. Our findings are in agreement with the adult literature and suggest that the development of the ERN and Pe have important associations with individual differences in affective behaviors from very early in life.
Footnotes
This research was supported by two grants from the National Institutes of Health (NIH) grant R01 MH075750, awarded to K.B. and grant K01 MH075764, awarded to T.D. This publication was also made possible by Grant RR03037 from the National Center for Research Resources (NCRR) to T.D., a component of the NIH. The first author was supported by a grant from the National Institute of Mental Health (PI: Cole T32 MH070327) during the preparation of this manuscript.
We wish to thank the members of the Emotion Development Lab for their assistance with the collection and scoring of these data, as well as all of the families and children who participated. We also thank the Penn State Social, Life, & Engineering Sciences Imaging Center (SLEIC), Human Electrophysiology Facility.
Partial correlations controlling for age of child are as follows: ERN at Pz with orienting (r = .87, p < .01), Pe at Fz with attention focusing (r = −.62, p < .05), Pe at Cz with boldness (r = .80, p < .01).
References
- Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H., Reiss A.L. A developmental fMRI study of the stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Aiken L.S., West S.G. Sage; Newbury Park, CA: 1991. Multiple Regression: Testing and Interpreting Interactions. [Google Scholar]
- Anderson S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arbel Y., Donchin E. When a child errs: the ERN and Pe complex in children. Psychophysiology. 2010;48:55–63. doi: 10.1111/j.1469-8986.2010.01042.x. [DOI] [PubMed] [Google Scholar]
- Bachevalier J., Mishkin M. An early and late developing system for learning and retention in infant monkeys. Behavioral Neuroscience. 1984;98:770–778. doi: 10.1037//0735-7044.98.5.770. [DOI] [PubMed] [Google Scholar]
- Beauchaine T.P., Hinshaw S.P., Pang K.L. Comorbidity of attention-deficity/hyperactivity disorder and early-onset conduct disorder: biological, environmental, and developmental mechanisms. Clinical Psychology and Practice. 2010;17:327–336. [Google Scholar]
- Berenbaum S.A., Moffat S., Wisniewski A., Resnick S. Neuroendocrinology: cognitive effects of sex hormones. In: de Haan M., Johnson M.H., editors. The Cognitive Neuroscience of Development. Psychology Press; New York: 2003. pp. 207–235. [Google Scholar]
- Bertrand O., Perrin F., Pernier J. A theoretical justification of the average reference in topographic evoked potential studies. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1985;62:462–464. doi: 10.1016/0168-5597(85)90058-9. [DOI] [PubMed] [Google Scholar]
- Blakemore J.E.O., Berenmaum S.A., Liben L.S. Taylor & Francis Group; New York: 2009. Gender Development. [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D.E. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G. Multimodal studies of cingulated cortex. In: Posner M.I., editor. The Cognitive Neuroscience of Attention. Guilford Press; New York: 2004. pp. 207–218. [Google Scholar]
- Buss K.A., Dennis T.A., Brooker R.J., Sippel L.M. An ERP Study of Conflict Monitoring in 4 to 8 Year Old Children: Associations with Temperament. Developmental Cognitive Neuroscience. 2011;1:131–140. doi: 10.1016/j.dcn.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness V.S., Jr., Kennedy D.N., Richelme C., Rademacher J., Filipek P.A. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cerebral Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Chiu P.H., Deldin P.J. Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Ciesielski K.T., Harris R.J., Cofer L.F. Posterior brain ERP patterns related to the go/no-go task in children. Psychophysiology. 2004;41:882–892. doi: 10.1111/j.1469-8986.2004.00250.x. [DOI] [PubMed] [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Deboer T., Scott L.S., Nelson C.A. ERPs in developmental populations. In: Handy T., editor. Event-Related Potentials: A Methods Handbook. MIT Press; Cambridge, MA: 2005. pp. 263–298. [Google Scholar]
- De Bruijn E.R.A., Hulstijn W., Verkes R.J., Ruigt G.S.F., Sabbe B.G.C. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology. 2004;177:151–160. doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- Dehane S., Posner M.I., Tucker D.M. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Dennis T.A., Malone M.M., Chen C.C. Emotional face processing and emotion regulation in children: an ERP study. Developmental Neuropsychology. 2009;34:85–102. doi: 10.1080/87565640802564887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. Effects of cross-modal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., Christ S., Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B.D., Sommer T., Raz A., Posner M.I. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Foti D., Hajcak G. Depression and reduced sensitivity to nonrewards versus rewards: evidence from event-related potentials. Biological Psychology. 2009;81:1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Himle J., Nisenson L.G. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Goss B., Coles M.G.H., Meyer D.E., Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring W.J., Willoughby A.R. The medial frontal cortex and the rapid processing of monitary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Goldman P.S., Rosvokl H.E., Vest B., Galkin T.W. Analysis of the delayed-alternation deficit produced by dorsolateral prefrontal lesions in the rhesus monkey. Journal of Comparative Physiology and Psychology. 1971;77:212–220. doi: 10.1037/h0031649. [DOI] [PubMed] [Google Scholar]
- Goldsmith H.H., Buss A.H., Plomin R., Rothbart M.K., Thomas A., Chess S., Hinde R.A., McCall R.B. What is temperament? Four approaches. Child Development. 1987;58:505–529. [PubMed] [Google Scholar]
- Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:606–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hajcak G., McDonald N., Simons R.F. Anxiety and error-related brain activity. Biological Psychology. 2003;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G., McDonald N., Simons R.F. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hogan A.M., Vargha-Kadem F., Kirkham F.J., Baldeweg T. Maturation of action monitoring from adolescence to adulthood: an ERP study. Developmental Science. 2005;8:525–534. doi: 10.1111/j.1467-7687.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- Holmes A.J., Pizzagalli D.A. Spatio-temporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G.H. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jonkman L.M., Lansbergern M., Stauder J.E. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- Junghöfer M., Elbert T., Tucker D.M., Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology. 1999;110:1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kim E.Y., Iwaki N., Imashioya H., Uno H., Fujita T. Error-related negativity in a visual go/no-go task: children vs. adults. Developmental Neuropsychology. 2007;31:181–191. doi: 10.1080/87565640701190775. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Conway A., Dahl R.E. Attentional control moderates relations between negative affect and neural correlates of action monitoring in adolescence. Developmental Neuropsychology. 2010;35:194–211. doi: 10.1080/87565640903526553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Birmaher B., Axelson D.A., Ryan N.D. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47:1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Carter C.S. ERP correlates of action monitoring in adolescence. Annals of the New York Academy of Sciences. 2004;1021:329–336. doi: 10.1196/annals.1308.040. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D., Dahl R.E., Carter C.S. Development of action monitoring through adolescence into adulthood: ERP and source localization. Developmental Science. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Lange J.J., Wijers A.A., Mulder L.J.M., Mulder G. Color selection and location selection in ERPs: differences, similarities and ‘neural specificity’. Biological Psychology. 1998;48:153–182. doi: 10.1016/s0301-0511(98)00011-8. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Gogtay N., Greenstein D.K., Wells E.M., Wallace G.L., Clasen L.S., Blumenthal J.D., Lerch J., Zijdenbos A.P., Evans A.C., Thompson P.M., Giedd J.N. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthold H., Sommer W. ERP correlates of error processing in spatial S-R compatibility tasks. Clinical Neurophysiology. 1999;110:342–357. doi: 10.1016/s1388-2457(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Liotti M., Pliszka S.R., Perez R., Kothmann D., Woldorff M.G. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Luck S.J. MIT Press; Cambridge: 2005. An Introduction to the Event-related Potential Technique. [Google Scholar]
- Luu P., Collins P., Tucker D.M. Mood, personality, and self monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- Luu P., Flaisch T., Tucker D.M. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P., Pederson S.M. The anterior cingulate cortex: regulating actions in context. In: Posner M.I., editor. Cognitive Neuroscience of Attention. Guilford Press; New York: 2004. pp. 232–242. [Google Scholar]
- MacDonald A.W., Cohen J.D., Stenger V.A., Carter C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulated cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Maguire E.A., Spiers H.J., Good C.D., Hartley T., Frackowiak R.S., Burgess N. Navigation expertise and the human hippocampus: a structural brain imaging analysis. Hippocampus. 2003;13:150–259. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- McDermott J.M., Pérez-Edgar K., Henderson H.A., Chronis-Tuscano A., Pine D., Fox N.A. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merke D.P., Fields J.D., Keil M.F., Vaituzis A.C., Chrousos G.P., Giedd J.N. Children with classic congenital adrenal hyperplasia have decreased amygdala volume: potential prenatal and postnatal hormonal effects. Journal of Clinical Endocrinology and Metabolism. 2003;88:1760–1765. doi: 10.1210/jc.2002-021730. [DOI] [PubMed] [Google Scholar]
- Miller J., Patterson T., Ulrich R. Jackknife-based method for measuring LRP onset latency differences. Psychophysiology. 1998;35:99–115. [PubMed] [Google Scholar]
- Muris P., Schmidt H., Merckelbach H., Schouten E. Anxiety sensitivity in adolescents: factor structure and relationships to trait anxiety and symptoms of anxiety disorders and depression. Behaviour and Research Therapy. 2001;39:89–100. doi: 10.1016/s0005-7967(99)00179-5. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S., Ridderinkhof K.R., Blom J., Band G.P.H., Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Olvet D.M., Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek T.J.M., Nieuwenhuis S., Ridderinkhof K.R. Dissociable components of error processing: on the functional significance of the Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- Perrin F., Pernier J., Bertrand O., Giard M.H., Echallier J.F. Mapping of scalp potentials by surface spline interpolation. Electroencephalography and Clinical Neurophysiology. 1987;66:75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Putnam S.P., Rothbart M.K. Development of short and very short forms of the children's behavior questionnaire. Journal of Personality Assessment. 2006;87:103–113. doi: 10.1207/s15327752jpa8701_09. [DOI] [PubMed] [Google Scholar]
- Rothbart M.K., Ahadi S.A., Hershey K.L. Temperament and social behavior in childhood. Merrill-Palmer Quarterly. 1994;40:21–39. [Google Scholar]
- Rothbart M.K., Derryberry D. Development of individual differences in temperament. In: Lamb M.E., Brown A.L., editors. vol. 1. Erlbaum; Hillsdale, NJ: 1981. pp. 37–86. (Advances in Developmental Psychology). [Google Scholar]
- Rueda M.R., Fan J., McCandliss B.D., Halparin J.D., Gruber D.B., Lercari L.P., Posner M.I. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. ERP correlates of error monitoring in 10-year olds are related to socialization. Biological Psychology. 2005;70:79–87. doi: 10.1016/j.biopsycho.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Santesso D.L., Segalowitz S.J., Schmidt L.A. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Developmental Neuropsychology. 2006;29:431–445. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Steiben J., Lewis M.D., Granic I., Zelazo P.D., Segalowitz S., Pepler D. Neurophysiological mechanisms of emotion regulation for subtypes of externalizing children. Development and Psychopathology. 2007;19:455. doi: 10.1017/S0954579407070228. [DOI] [PubMed] [Google Scholar]
- Torpey D.C., Hajcak G., Klein D.N. An examination of error-related brain activity and its modulation by error value in young children. Developmental Neuropsychology. 2009;36:749–761. doi: 10.1080/87565640903265103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker D.M. Spatial sampling of head electrical fields: the geodesic sensor net. Electroencephalography and Clinical Neurophysiology. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Tucker D.M., Liotti M., Potts G.F., Russell G.S., Posner M.I. Spatiotemporal analysis of brain electrical fields. Human Brain Mapping. 1994;1:134–152. [Google Scholar]
- van Veen V., Carter C.S. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;144:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- van Veen V., Carter C.S. Conflict and cognitive control in the brain. Current Directions in Psychological Science. 2006;15:237–240. [Google Scholar]
- Wiersema J.R., van der Meere J.J., Royers H. Developmental changes in error monitoring: an event-related potential study. Neuropsychologia. 2007;45:1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Zelazo P.D., Müller U. The balance beam in the balance: reflections on rules, relational complexity, and developmental processes. Journal of Experimental Child Psychology. 2002;81:458–465. doi: 10.1006/jecp.2002.2667. [DOI] [PubMed] [Google Scholar]