Abstract
Isoprenylcysteine carboxyl methyltransferase (ICMT) catalyzes the post-translational methylation of C-terminal cysteines of isoprenylated proteins, including small G-proteins and the γ-subunits of heterotrimeric G-proteins. It is widely felt that carboxymethylation promotes efficient membrane association of the methylated proteins and specific protein-protein interactions. In the current study, we tested the hypothesis that ICMT-mediated carboxymethylation of specific proteins (e.g., Rac1) plays a regulatory role in glucose-stimulated insulin secretion (GSIS). Western-blot analysis indicated that ICMT is expressed and predominantly membrane associated in INS 832/13 β-cells. siRNA-mediated knockdown of endogenous expression of ICMT markedly attenuated glucose, but not KCl-induced insulin secretion. These findings were further supported by pharmacological observations, which suggested a marked reduction in glucose-, but not KCl-stimulated insulin secretion by acetyl farnesyl cysteine (AFC), a selective inhibitor of ICMT. In addition, glucose-induced Rac1 activation, a hallmark signaling step involved in glucose-stimulated insulin secretion, was markedly inhibited following pharmacological (AFC) or molecular biological (siRNA-ICMT) inhibition of ICMT. Lastly, we also noticed a marked reduction in glucose-induced acute increase in the generation of reactive oxygen species in INS 832/13 cells pre-treated with AFC or transfected with siRNA-ICMT. Together, these data suggest that ICMT regulates glucose-induced Rac1 activation, generation of reactive oxygen species and insulin secretion in pancreatic β-cells.
Key words: Rac1, ROS, pancreatic islet, carboxymethylation and insulin secretion
Introduction
It has been known for a long time that both monomeric G-proteins (e.g., Rac1 and Cdc42) and the γ-subunits of heterotrimeric G-proteins (Gγ subunits) undergo post-translational modifications, such as isoprenylation and methylation at their C-terminal cysteine residues (often referred to as the CAAX motif).1,2 The first of the four step modification sequence includes incorporation of mevalonic acid-derived farnesyl or geranylgeranyl isoprenoid moiety onto the C-terminal cysteine. This is followed by the proteolytic cleavage of—AAX peptide by the Ras-converting enzyme1 (Rce1) endoprotease of microsomal origin, which leads to methylation of the prenylated cysteine by the carboxyl methyl transferase (ICMT) in the presence of S-adenosyl methionine serving as the methyl donor.3 It is widely accepted that the prenylation and carboxymethylation modification steps increase the hydrophobicity of the candidate proteins for optimal targeting to their relevant membranous sites for the regulation of effector proteins.1–3
While a significant number of recent studies have focused on putative roles of G-protein prenylation in glucose-stimulated insulin secretion (GSIS), very little is known with regard to the potential roles of carboxymethylation in islet function.3 Original studies from our laboratory have attempted to address the roles of carboxymethylation in islet function, including insulin secretion.4,5 Therein, using selective inhibitors of ICMT such as acetyl farnesyl cysteine (AFC), we have been able to demonstrate that Cdc42 and Gγ subunits undergo carboxymethylation in response to glucose in clonal β-cells, normal rat and human islets.4,5 Follow-up studies by Li and coworkers characterized ICMT in insulin-secreting cells for its subcellular localization and regulation by known second messengers of insulin secretion.6 In the current study, we have revisited this area of islet biology to precisely determine the role of carboxymethylation and the identity of methylated proteins to further evaluate their roles in the signaling events leading to insulin secretion.
Along these lines, emerging evidence implicates novel regulatory roles for phagocyte-like NADPH oxidases (Nox) in physiological insulin secretion. For example, using selective inhibitors (e.g., DPI or apocynin) and molecular biological tools (e.g., antisense and siRNAs for Nox subunits), several recent studies have demonstrated “second messenger” roles for Nox-derived reactive oxygen species in glucose-stimulated insulin secretion.7–9 Some of these aspects, including downstream targets for reactive oxygen species signals, have been reviewed by Pi and Collins recently in reference 10. Furthermore, recent studies from our laboratory have also demonstrated a novel regulatory role for Rac1 in Nox-derived generation of reactive oxygen species, thus suggesting that glucose-induced Rac1 activation step might be necessary for Nox-mediated generation of reactive oxygen species and insulin secretion. For example, using selective inhibitors of prenylation (e.g., GGTI-2147), we have demonstrated that post-translational prenylation of Rac1 is important for its regulation of generation of reactive oxygen species.11 Therefore, based on the above evidence and as a logical extension to studies to suggest obligatory roles of ICMT-mediated carboxymethylation of Rac1 function for its subcellular localization and function,12,13 we undertook the current investigation to determine the regulatory roles of ICMT in glucose-induced Rac1 activation, generation of reactive oxygen species and insulin secretion in INS 832/13 cells. We have accomplished this goal by two distinct approaches to compromise the β-cell endogenous ICMT function, via siRNA-mediated knockdown of ICMT expression and pharmacological inhibition of ICMT by AFC. Indeed, data accrued from the current studies underscores the importance of carboxymethylation of Rac1 in glucose-induced Nox activation and associated generation of reactive oxygen species and insulin secretion.
Results
ICMT is expressed in INS 832/13 cells.
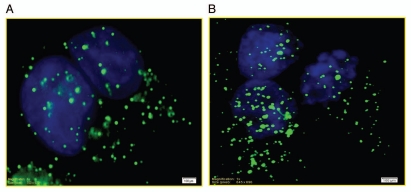
At the outset we determined the immunological localization and subcellular distribution of ICMT in INS 832/13 cells. For this, total particulate and soluble fractions were isolated from INS 832/13 cells by a single step centrifugation method and relative abundance of ICMT was determined in these fractions by western blotting. Data in Figure 1 suggested a predominant membrane association of ICMT in these cells. It should also be noted that we consistently observed a doublet for ICMT on western blots, which might represent a post-translationally modified form of this protein. In the next series of studies we determined the distribution of ICMT in INS 832/13 cells by immunofluorescence method. Data in Figure 2 suggested that ICMT (green) remain diffused throughout the cell under basal [(A) LG; 2.5 mM glucose] conditions. Further, we observed no clear effects of stimulatory glucose [(B) HG; 20 mM glucose] on ICMT distribution in these cells.
Figure 1.
Expression and subcellular distribution of ICMT in INS 832/13 cells. Total particulate and soluble fractions were isolated from INS 832/13 cells by a single step centrifugation method described in Materials and Methods. ICMT expression was determined in these fractions by western blotting. A representative of three blots is shown here.
Figure 2.
Localization of ICMT in INS 832/13 cells by immunofluorescence under basal and glucose-stimulated conditions. INS 832/13 cells were plated on coverslips and cultured overnight in low serum low glucose media prior to incubation with either 2.5 mM (A) or 20 mM glucose (B) for 45 min at 37°C. The cells were fixed in 4% paraformaldehyde solution in PBS for 15 min and permeabilized using 0.2% triton X-100 for 15 min. Fixed cells were examined for ICMT (stained in green) and nuclei (stained in blue) as described under Materials and Methods.
siRNA-mediated knockdown of ICMT attenuates glucose-, but not KCl-induced insulin secretion in INS 832/13 cells.
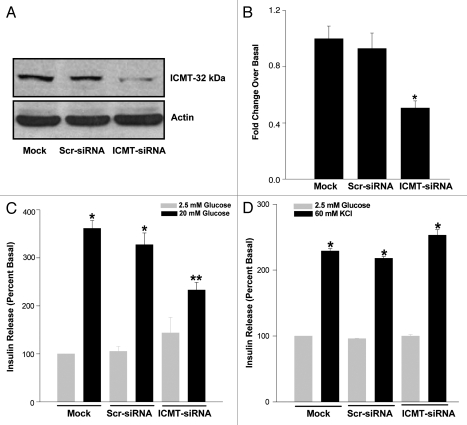
We next investigated potential regulatory roles of ICMT in glucose-induced insulin secretion in these cells. To address this, we knocked down the endogenous expression of ICMT by siRNA methodology. Data in Figure 3A and B indicated more than ∼70 % inhibition in the expression of ICMT following siRNA-ICMT transfection. Data in Figure 3C suggested no significant effects of scrambled siRNA transfection either on basal or glucose-induced insulin secretion (bars 1 vs. 3 and 2 vs. 4). However, transfection of siRNA-ICMT in these cells led to a modest increase in basal secretion (bars 1 or 2 vs. 5), but insulin secretion elicited by stimulatory glucose was significantly reduced in ICMT knocked down cells (bars 2 or 4 vs. 6). This data suggested that activation of ICMT is necessary for glucose-stimulated insulin secretion to occur. We then determined potential requirement for ICMT in insulin secretion elicited by a membrane depolarizing concentration of KCl. Data shown in Figure 3D suggested no significant effects of ICMT knockdown on KCl-induced insulin secretion. Together, data in Figure 3C and D suggest that glucose, but not KCl-evoked insulin secretion is mediated via activation of ICMT.
Figure 3.
Glucose-, but not KCl-stimulated insulin secretion, is attenuated in INS 832/13 cells following siRNA-mediated knockdown of ICMT. INS 832/13 cells were either mock transfected or transfected with scrambled siRNA or siRNA-ICMT at a final concentration of 100 nM and cultured for 24 h. Transfection efficiency was determined by separating equal amounts of proteins on SDS-PAGE and probing with ICMT antibody [(A) representative of three transfections is shown here]. Data in (A) was densitometrically analyzed and expressed as fold change over basal (B). *p < 0.05 compared with mock or scrambled siRNA transfected cells. Further, transfected cells were incubated either with low glucose (LG; 2.5 mM) or high glucose [(C) HG; 20 mM] or a membrane depolarizing concentration of KCl [(D) 60 mM; osmolaity adjusted by lowering NaCl] for 45 min at 37°C. Insulin released into the medium was quantitated by ELISA. Data are expressed as percentage of basal and are mean ′ SEM from three independent determinations. *p < 0.05 vs. respective low glucose controls; **p < 0.05 vs. mock transfected cells.
siRNA-mediated knockdown of ICMT attenuates glucose-induced Rac1 activation in INS 832/13 cells.
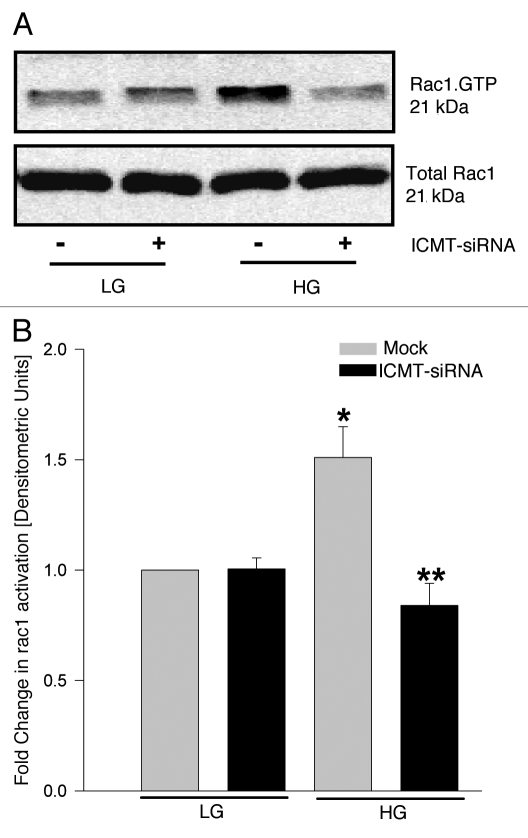
Published evidence from several laboratories, including our own have suggested that activation of Rac1, a small G-protein, is a requisite step in the signaling events leading to glucose-insulin secretion.2,14,15 Furthermore, using inhibitors of post-translational geranylgeranylation (e.g., GGTI-2147) or a dominant negative mutant of the α-subunit of geranylgeranyl transferase, we have demonstrated a requirement for post-translational geranylgeranylation in glucose-induced Rac1 activation and insulin secretion.16 Since Rac1 undergoes carboxymethylation, we investigated if silencing of ICMT affects glucose-induced Rac1 activation. Data shown in Figure 4 demonstrated a significant increase in glucose-induced Rac1 activation (lane 1 vs. 3). siRNA-mediated knockdown of ICMT failed to exert any clear effects on basal Rac1 activation (lane 1 vs. 2), but significantly attenuated glucose-induced Rac1 activation (lane 3 vs. 4). Pooled data from multiple experiments are provided in Figure 4B. Together, these findings suggested a requirement for carboxymethylation for glucose-induced activation of Rac1.
Figure 4.
Depletion of endogenous ICMT markedly attenuates glucose-induced activation of Rac1 in INS 832/13 cells. INS 832/13 cells were transfected with ICMT-siRNA or mock transfected and cultured for 24 h. At confluence, cells were starved overnight and stimulated with either low (2.5 mM) or high (20 mM) glucose for 30 min. The extent of Rac1 activation in these cells was quantitated by PAK-PBD pulldown assay. Total and activated (Rac1.GTP) were determined by western blotting (A) and quantitated by densitometry (B). Data are expressed as fold change in Rac1 activation and are mean ± SEM from three independent determinations. *p < 0.05 vs. mock transfected low glucose; **p < 0.05 vs. mock transfected high glucose.
siRNA-mediated knockdown of ICMT markedly inhibits glucose-induced reactive oxygen species generation in INS 832/13 cells.
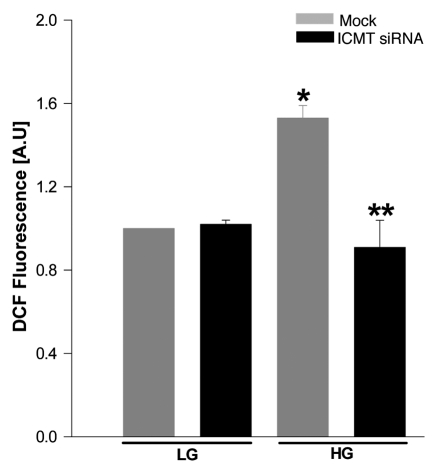
Emerging evidence from multiple laboratories appears to suggest novel second messenger roles for reactive oxygen species in glucose-stimulated insulin secretion.10 It has also been shown that reactive oxygen species generated via the activation of phagocyte-like NADPH oxidase (Nox) plays such regulatory roles in glucose-stimulated insulin secretion since pharmacological (e.g., apocynin or DPI) or molecular biological (e.g., siRNA or antisense for p47phox) inhibition of Nox led to inhibition of glucose-stimulated insulin secretion.8–10 Since Rac1 represents one of the members of Nox holoenzyme,2,18 we investigated if siRNA-mediated knockdown of ICMT exerts any regulatory effects on glucose-induced generation of reactive oxygen species in INS 832/13 cells. Data in Figure 5 suggested no significant effects of ICMT knockdown on basal levels of reactive oxygen species in these cells (bar 1 vs. 2). However, glucose-induced generation of reactive oxygen species was markedly attenuated in cells in which expression of ICMT was knocked down (bar 3 vs. 4). Taken together, these data demonstrated that glucose-induced Rac1 activation (Fig. 4), generation of reactive oxygen species (Fig. 5) and insulin secretion (Fig. 3) are regulated by ICMT in INS 832/13 β-cells.
Figure 5.
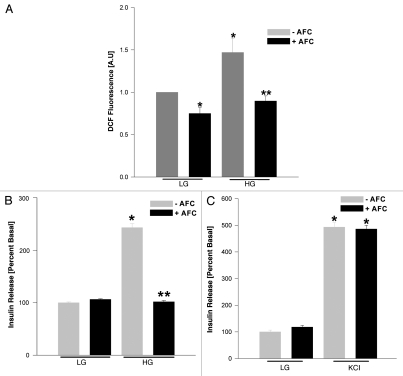
Glucose-induced ROS generation was attenuated in INS 832/13 cells following siRNA-mediated knockdown of ICMT. INS 832/13 cells transfected with ICMT-siRNA (or mock transfected) following which cells were stimulated with low glucose (2.5 mM) or high glucose (20 mM) for 1 h and were incubated with DCHFDA (10 µM; 30 min) and harvested for quantitation of DCF fluorescence. Data expressed as DCF fluorescence and are mean ± SEM from three independent determinations. *p < 0.05 vs. respective low glucose; **p < 0.05 vs. high glucose in mock transfected cells.
Acetyl farnesyl cysteine (AFC), a selective inhibitor of ICMT, attenuates glucose-induced generation of reactive oxygen species and insulin secretion in INS 832/13 cells.
We next confirmed the above data accrued through the use of siRNA-ICMT by a pharmacological approach. In the following studies. we determined the effects of acetyl farnesyl cysteine (AFC), a selective inhibitor of ICMT,4,5 on glucose-induced generation of reactive oxygen species and insulin secretion. Data shown in Figure 6A indicated a modest, but significant inhibition in basal level of reactive oxygen species in these cells following exposure to AFC (Fig. 6; bar 1 vs. 2). However, increase in the level of reactive oxygen species seen in the presence of stimulatory glucose was significantly inhibited by AFC (Fig. 6; bar 3 vs. 4). Furthermore, insulin secretion elicited by stimulatory (Fig. 6B; bar 3 vs. 4), but not basal glucose (Fig. 6B; bar 1 vs. 2), was markedly attenuated by AFC. In addition, in a manner akin to siRNA-ICMT effects, we observed no significant effects of AFC on KCl-induced insulin secretion (Fig. 6C). Together, our above described findings confirm that glucose-, but not KCl-mediated effects on insulin secretion require activation of ICMT. Furthermore, along these lines, we also noticed a significant inhibition of glucose-induced activation of Rac1 by AFC under the conditions it inhibited glucose-induced generation of reactive oxygen species (∼41 ± 10% inhibition by AFC; mean ± SEM from three pull down assays; p < 0.05 vs. diluent) and insulin secretion (additional data not shown). Together, these data further confirm our siRNA-ICMT findings and support our hypothesis that ICMT-mediated carboxymethylation of specific proteins (e.g., Rac1) plays a positive modulatory role in the cascade of events leading to glucose-induced generation of reactive oxygen species and insulin secretion in INS 832/13 cells.
Figure 6.
AFC, a competitive inhibitor of ICMT, attenuates glucose-induced ROS generation and insulin secretion in INS 832/13 cells. INS 832/13 cells were cultured overnight with low-glucose and low-serum medium and then incubated in KRB in the presence of diluent or AFC (100 µM; 1 h) as indicated in the figure. Cells were further stimulated with either low glucose (LG; 2.5 mM) or high glucose (HG; 20 mM) for 1 h in continuous presence or absence of diluent or inhibitor. At the end of stimulation, ROS generation was determined by quantitating DCF fluorescence (A) as described in Figure 5. In a separate set of studies glucose- and KCl-stimulated insulin secretion was quantitated (B and C) under conditions described in Materials and Methods. Data in (B) are mean ± SEM from three independent determinations. *p < 0.05 vs. low glucose without AFC; **p < 0.05 vs. high glucose without AFC whereas data in (C) are mean ± SEM from 12 determinations in each case. *p < 0.05 vs. low glucose without AFC and low glucose with AFC.
Inhibition of ICMT does not affect cell viability.
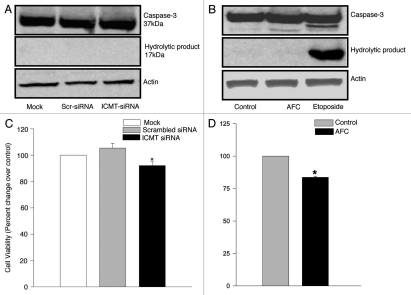
We next investigated potential cytotoxic effects, if any, of ICMT knockdown (via siRNA-ICMT) or inhibition of ICMT activity (by AFC) on INS 832/13 cells. We asked this question to be sure that either inhibition in Rac1 activation, reactive oxygen species generation or insulin secretion seen under these conditions are not due to potential loss in cell viability or cell demise following inhibition of ICMT expression and/or activity. We addressed this by two independent experimental approaches. In the first, we quantitated activation of caspase-3, a hallmark of cellular apoptosis, in both siRNA-ICMT transfected cells and AFC-treated cells. In the second approach, we quantitated the metabolic viability of siRNA-ICMT transfected or AFC-treated cells using the MTT assay. Data shown in Figure 7A and B indicated no caspase 3 activation following siRNA-ICMT transfection or AFC treatment. However, a significant activation of caspase 3 was seen in INS 832/13 cells treated with etoposide, which causes apoptosis in cells via caspase 3 activation. Together, these data in Figure 7A and B suggest no cell death in INS 832/13 cells following inhibition of expression and activity of ICMT. In addition, we observed only a modest inhibition in cell viability as assessed by the MTT in cells following ICMT knockdown via siRNA-ICMT (Fig. 7C) or AFC treatment (Fig. 7D). Together, these findings suggest that the observed inhibition of glucose-induced Rac1 activation, generation of reactive oxygen species and insulin secretion following inactivation of ICMT are specific and do not involve cytotoxic mechanisms.
Figure 7.
ICMT inhibition does not affect cell viability. (A) INS 832/13 cells were mock transfected or transfected either with ICMT siRNA or scrambled siRNA (100 nmol, 24h) or treated with AFC [(B) 100 µM, 1h]. Activated caspase 3 in the lysates was determined by western blot analysis using an antiserum that identifies both the native procaspase and degradative product of caspase 3 using etoposide as a positive control as described under Methods. A representative blot for [(A) n = 2 determinations] and for [(B) n = 3 determinations] is shown here. Actin was used as a loading control. (C) INS 832/13 cells were mock transfected or transfected either with ICMT siRNA or scrambled siRNA (100 nmol, 24h). Cell viability in transfected cells was determined by MTT reduction method as described above. Data are means ± SEM from two independent experiments yielding identical results with n >12 in each group and expressed as percent change over control. * represents p< 0.05 compared with mock or scrambled siRNA transfected cells. (D) INS 832/13 cells treated with AFC (100 µM, 1h) were incubated with MTT (5 mg/mL, 4h) as described in Materials and Methods. Cell viability was determined by quantitating reduction of MTT by metabolically active cells at 570 nm. Data are means ± SEM from two independent experiments yielding identical results with n > 12 in each group and expressed as percent change over control. * represents p < 0.05 compared with control.
Alterations in ICMT expression in in vitro models of gluco-, lipo-, glucolipotoxicity and endoplasmic reticulum stress.
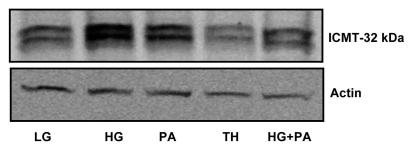
A growing body of evidence implicates that long-term exposure of β-cells to saturated fatty acids (i.e., lipotoxicity), glucose (i.e., glucotoxicity) or both (i.e., glucolipotoxicity) leads to severe metabolic dysfunction and eventual demise of the β-cell.19 Furthermore, exposure of these cells to thapsigargin, leads to endoplasmic reticular stress via depletion of calcium pools culminating in cellular dysfunction.20,21 Therefore, in the last series of these studies we investigated potential alterations in the expression of ICMT in INS 832/13 cells following exposure to palmitate, glucose or thapsigargin. Data shown in Figure 8 indicated a significant increase in the expression of ICMT in cells exposed to gluco-, lipo- or glucolipotoxic conditions. However, no detectable changes were seen in the expression of ICMT protein in thapsigargin-treated cells.
Figure 8.
Expression of ICMT in lysates of INS 832/13 cells under the duress of gluco-, lipo-, glucolipotoxicity and endoplasmic reticulum stress. INS 832/13 cells were plated in six-well plates, grown to 70% confluence and treated with low glucose (LG, 2.5 mM, 48 h), high glucose (HG, 50 mM, 48 h), palmitic acid (PA, 300 µM; 48 h), HG plus PA (48 h) and thapsigargin (TH, 0.5 µM, 9 h). ICMT expression was determined by western blotting. A representative of two blots is shown here. Actin was used as a loading control.
Discussion
Several earlier studies have implicated activation of small G-proteins (e.g., Arf6, Cdc42 and Rac1) in physiological insulin secretion. Such conclusions were drawn from studies involving the use of Clostridial toxins, dominant negative mutants, siRNAs and inhibitors of post-translational modifications, including prenylation, carboxymethylation and palmitoylation (reviewed in ref. 2). To the best of our knowledge, the current study provides the first evidence to implicate carboxymethylation of Rac1 in the signaling cascade leading to glucose-induced ROS generation and insulin secretion. We have presented supporting evidence via two distinct approaches, namely siRNA-mediated knockdown or selective pharmacological inhibition of ICMT, which mediates the carboxymethylation of these signaling proteins.
At least two distinct carboxylmethyl transferases have been identified in insulin secreting cells. The first one is involved in methylating the carboxy terminal leucine (Leu-309) of the catalytic subunit of protein phosphatase 2A; such a signaling step has been implicated in subunit interaction and catalytic activation of the enzyme.22 The second enzyme, which is the focus of the current study, is the ICMT. In a previous study, Li and associates characterized the ICMT in insulin-secreting cells and normal rat islets.6 Such an activity was monitored by quantitating the degree of methylation of AFC by the islet ICMT in the presence of [3H] S-adenosylmethionine as the methyl donor. Subcellular fraction assays revealed that this enzyme activity is enriched in the endoplasmic reticulum.6 Along these lines, using the pharmacological approaches, we have demonstrated that glucose promotes the carboxymethylation of Cdc42, another small G-protein involved in cytoskeletal remodeling and glucose-stimulated insulin secretion.4 It was also demonstrated that the Gγ-subunits also undergo carboxymethylation in a glucose-sensitive manner in clonal β-cells, normal rat islets and human islets.5 Not much has been reported since then with regard to potential functional consequences of carboxymethylation in islet function primarily due to lack of experimental tools (e.g., siRNA) to selectively deplete the expression of ICMT in isolated β-cells. Indeed, data from the current investigation further reinforce our original hypothesis that in addition to prenylation, carboxymethylation of specific G-proteins (e.g., Rac1) plays regulatory roles in physiological insulin secretion. Such regulatory effects may, in part, be due to the ability of methylated Rac1 to increase the activation of Nox and associated generation of reactive oxygen species.
Data accrued in the current studies implicate carboxymethylation as one of the requisite signaling steps for glucose-induced activation by Rac1 in a stimulated β-cell. Moreover, the carboxymethylation of Rac1 appears to be necessary for glucose-induced Nox activation and generation of reactive oxygen species. In this context, using reconstituted systems and the C-terminal Rac1 peptides, Kreck and coworkers have provided experimental support to implicate participatory roles for Rac1 in cell-free activation and assembly of NADPH-oxidase.23 Compatible with these findings are our recent data to implicate inhibition of glucose- or mitochondrial-fuel-induced Nox activation and generation of reactive oxygen species in INS 832/13 cells and normal rat islets by inhibitors of protein prenylation. These studies thus provided evidence for requisite roles for prenylation in the functional regulation of Nox in the islet β-cell.11 Data from the current investigation indicate that in addition to prenylation, the carboxymethylation of specific G-proteins may be necessary for optimal regulation of Nox by glucose. More importantly, our current findings also suggest that carboxymethylation is necessary for glucose-induced activation of Rac1, since pretreatment of isolated β-cells with AFC or selective depletion of ICMT by siRNA markedly attenuated glucose-induced Rac1 activation. These findings are in agreement with recent findings of Cushman and Casey demonstrating inhibition of EGF-induced Rho A and Rac1 activation by cysmethynil, a selective inhibitor of ICMT, in MDA-MD-231 cells.24 Together, based on the above discussion it is concluded that both prenylation and carboxymethylation of Rac1 are necessary for glucose-induced Nox-mediated ROS generation and insulin secretion. It is important to note that palmitoylation of cysteine residues upstream to prenylated and carboxylmethylated Rac1 may not be involved in this signaling cascade at least based on recent studies from Roberts and associates who reported no known consensus palmitoylation motifs for Rac1,25 although this remains to be verified experimentally in the islet β-cell.
Emerging evidence appears to implicate a significant contributory role for Nox in the generation of oxidative stress and the onset of mitochondrial dysfunction in multiple cell types, including the islet β-cell. For example, it has been shown that chronic exposure of isolated β-cells to high concentrations of saturated fatty acids (e.g., palmitate; lipotoxicity), glucose (i.e., glucotoxicity) or both (i.e., glucolipotoxicity) or a mixture of cytokines (e.g., IL-1β, TNFα and IFNγ) culminates in increased oxidative stress, mitochondrial dysfunction and apoptosis in these cells.26–28 Inhibition of protein prenylation of Rac1 by pharmacological approaches (e.g., GGTI-2147) or Rac1 activation by Tiam1, a known guanine nucleotide exchange factor for Rac1 (using NSC23766) markedly attenuated metabolic dysfunction of the β-cell.27,28 Along these lines, data described herein suggest a significant increase in the expression of ICMT under glucolipotoxic conditions. Whether such an increase in the expression translates into increased ICMT activity remains to be verified. Nonetheless, it may be likely that use of selective inhibitors of carboxymethylation might prove to be valuable in preventing oxidative stress induced under the duress of glucolipotoxicity and/or cytokines. These are being studied in our laboratory currently. Based on the data accrued in the current studies we conclude that ICMT regulates glucose-induced Rac1 activation, generation of reactive oxygen species and insulin secretion in pancreatic β-cells.
Materials and Methods
Materials.
siRNA-ICMT (Cat # 43907710) and scrambled siRNA (negative control; Cat # 4390843) were from Ambion. AFC was from Cayman Chemical (Cat # 63270). ECL reagent was from GE Healthcare (Cat # RPN2132). HiPerFect transfection reagent was obtained from Qiagen (Cat # 301705). The rat insulin ELISA kit was from American Laboratory Products (Cat # 80-INSRTH-E01). Rac1 activation assay kit was from Cytoskeleton (Cat # BK035). ICMT antiserum was from Santa Cruz Biotechnology, Inc., (Cat # Sc-130150). DCHFDA (Cat # 35845), thapsigargin (Cat # T9033) and etoposide (Cat # E1383) were from Sigma Aldrich. Alexa-fluor 488 anti-rabbit secondary antibody (Cat # A11008) and Hoechst dye (Cat# 3570) was from Invitrogen molecular probes. Cell proliferation kit (MTT, Cat # 11465007001) was purchased from Roche diagnostics.
Insulin-secreting cells.
INS 832/13 cells were provided by Dr. Chris Newgard (Duke University Medical Center, Durham, NC) and were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum supplemented with 100 IU/ml penicillin and 100 IU/ml streptomycin, 1 mM sodium pyruvate, 2-mercaptoethanol (50 µM) and 10 mM HEPES (pH 7.4). The medium was changed twice weekly and cells were trypsinized and subcloned weekly.
Isolation of total particulate and soluble fractions from INS 832/13 cells.
INS 832/13 cells were homogenized in RIPA buffer (50 mm Tris-HCl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF and protease inhibitor cocktail) and were centrifuged at 105,000x g for 1 h to separate total particulate and soluble fractions. Proteins from individual fraction were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The blots were then probed with antibody raised against ICMT (1:500 dilution) and with rabbit secondary antibody conjugated to horseradish peroxidase. Immune complexes were then detected using the enhanced chemiluminescence kit.
Immunofluorescence studies.
INS 832/13 cells were plated onto coverslips and incubated with (2.5 or 20 mM) glucose for 45 min at 37°C followed by washing in PBS and fixed with 4% paraformaldehyde solution for 15 min at room temperature. They were then permeabilized with 0.2% Triton X-100 for 15 min at room temperature. After blocking with 1% BSA for 1 h, the cells were further incubated with primary antibody ICMT (1:150) in 0.1% BSA solution for 1 h. After extensive washes, the cells were further incubated with secondary antibody Alexa-fluor 488 anti-rabbit (1:1,000) in 0.1% BSA solution for 1 hr at 37°C. Hoechst dye was used to stain for nuclei. The coverslips were then mounted on glass slides containing mounting media (DAKO corporation; Carpinteria, CA) and visualized under an Olympus IX71 inverted fluorescence microscope using a x100 oil-immersion lens.17
siRNA-mediated knockdown of ICMT.
Endogenous expression of ICMT was knocked down by transfecting INS 832/13 cells with ICMT-siRNA. In brief, INS 832/13 cells were plated on 24-well plates and transfection with ICMT-siRNA was performed at 50–60% confluence at a final concentration of 100 nM using HiPerFect transfection reagent. Further, to assess specificity of siRNA, cells were transfected in parallel with non-targeting siRNA (scrambled siRNA; 100 nM). Transfected cells were cultured in complete growth medium for 24 h and efficiency of ICMT knockdown was determined by western blot analysis.
Caspase 3 activity.
Activation of caspase-3 was assessed in cells either transfected with ICMT siRNA or in cells treated with AFC. Cells were harvested and homogenized in sample buffer (0.5% Nonidet P-40, 20 mM HEPES, pH 7.4, 100 mM NaCl and 20 mM DTT and PIC). And ∼30 µg of proteins were resolved by SDS-PAGE (12%) and immunoprobed for caspase-3. Activation of caspase-3 is evidenced by the presence of a hydrolytic product (∼17 kDa). Etoposide (60 µM, 6 h) was used as a standard for apoptotic cell death.
Cell viability assay.
INS 832/13 cells were either treated with AFC (100 µM, 1 h) or transfected with ICMT-specific or scrambled siRNA as described above. Cell viability was determined by incubating AFC treated or ICMT-siRNA transfected cells with 10 µL of stock MTT [4(5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] for 4 h at 37°C. Following dissolution of formazan crystals in solubilization solution, the absorbance was measured at 570 nm using ELISA plate reader.
Insulin release studies.
siRNA-ICMT or scrambled siRNA ransfected cells or AFC-treated cells were cultured overnight in low serum-low glucose-containing media. They were stimulated further either with low or high glucose or potassium chloride in Krebs-Ringer bicarbonate (KRB) buffer, pH 7.4 for periods of time indicated in the text. In studies involving KCl-induced insulin secretion, we noticed that INS 832/13 cells were not responsive to 40 mM KCl in releasing insulin. However, higher KCl concentrations (60 mM) were found to elicit robust insulin release. Therefore, in KCl-stimulated insulin secretion studies, cells were incubated with 60 mM KCl in an osmolarity balanced KRB medium.17
Rac1 activation assay.
The extent of Rac1 activation (i.e., GTP-bound form) was determined using a commercially available kit (Cytoskeleton, Denver, CO).16,27,28 ICMT-siRNA transfected or AFC (100 µM) treated INS 832/13 cells were incubated with low (2.5 mM) or high glucose (20 mM) for 30 min at 37°C. Cell lysates were clarified by centrifugation and p21-activated kinase binding domain (PAK-PBD)-beads were added to the supernatant and mixed gently at 4°C for 1 h. The beads were then centrifuged at x4,000 g for 5 min, rinsed with wash buffer (25 mM Tris, pH 7.5, 30 mM MgCl2, 40 mM NaCl and 150 mM EDTA), and then reconstituted in Laemmli buffer. Proteins were separated on 12% SDS-PAGE and immunoblotted for activated Rac1.
NADPH oxidase activity assay.
This was carried according the method we described recently in reference 27 and 28. In brief, INS 832/13 cells were plated in six-well plates, grown to subconfluence and then either transfected with ICMT-siRNA or treated with AFC (100 µM; 1 h). The cells were washed with PBS and further incubated with 2′,7′-dichloro-dihydrofluorescein diacetate (DCHFDA, 10 µM) for 30 min at 37°C. Cells were then harvested and centrifuged. The pellet was resuspended in PBS and protein concentration was determined using Bradford's assay. Equal amount of proteins were taken and fluorescence was measured at excitation and emission wavelengths of 485 and 530 nm respectively (using Perkin Elmer fluorimeter, Waltham, MA).
ICMT expression profile.
INS 832/13 cells were plated in six-well plates, grown to 70% confluence and treated with low glucose (2.5 mM), high glucose (50 mM), palmitic acid (300 µM), palmitic acid plus high glucose for 48 h or thapsigargin (0.5 µM) for 9 h. Cells were then harvested and centrifuged. The pellet was resuspended in buffer solution (0.5% Nonidet P-40, 20 mM HEPES, pH 7.4, 100 mM NaCl, 20 mM DTT and protease inhibitor cocktail). Equal amount of proteins were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The blots were then probed with antibody raised against ICMT (1:500 dilution) and with rabbit secondary antibody conjugated to horseradish peroxidase. Immune complexes were then detected using the enhanced chemiluminescence kit.
Statistical analysis.
Data are presented as mean ± SEM. Statistical significance differences between values were evaluated by Student's t-test or ANOVA where appropriate. p < 0.05 was considered to be statistically significant.
Acknowledgements
The research work described in this article is supported (to A.K.) by the National Institutes of Health (RO1 74921) and the Department of VA MERIT Review award. A.K. is also the recipient of a Senior Research Career Scientist Award from the Department of VA.
Abbreviations
- AFC
acetyl farnesyl cysteine
- ICMT
isoprenylcysteine methyl transferase
- DCHFDA
2′,7′-dichloro-dihydrofluorescein diacetate
- GSIS
glucose-stimulated insulin secretion
- Nox
phagocyte-like NADPH oxidase
References
- 1.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 2.Kowluru A. Small G proteins in islet beta-cell function. Endocr Rev. 2010;31:52–78. doi: 10.1210/er.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowluru A. Bridging the gap between protein carboxyl methylation and phospholipid methylation to understand glucose-stimulated insulin secretion from the pancreatic beta cell. Biochem Pharmacol. 2008;75:335–345. doi: 10.1016/j.bcp.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, et al. Glucose- and GTP-dependent stimulation of the carboxymethylation of Cdc42 in rodent and human pancreatic islets and pure β-cells: evidence for an essential role for GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest. 1996;98:540–555. doi: 10.1172/JCI118822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowluru A, Li G, Metz SA. Glucose activates the carboxyl methylation of gamma subunits of trimeric GTP-binding proteins in pancreatic beta cells. Modulation in vivo by calcium, GTP and pertussis toxin. J Clin Invest. 1997;100:1596–1610. doi: 10.1172/JCI119684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Kowluru A, Metz SA. Characterization of prenylcysteine methyltransferase in insulin-secreting cells. Biochem J. 1996;316:345–351. doi: 10.1042/bj3160345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan D, Rebelato E, Abdulkader F, Graciano MF, Oliveira-Emilio HR, Hirata AE, et al. Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology. 2009;150:2197–2201. doi: 10.1210/en.2008-1149. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira HR, Verlengia R, Carvalho CR, Britto LR, Curi R, Carpinelli AR. Pancreatic beta-cells express phagocyte-like NAD(P)H oxidase. Diabetes. 2003;52:1457–1463. doi: 10.2337/diabetes.52.6.1457. [DOI] [PubMed] [Google Scholar]
- 9.Uchizono Y, Takeya R, Iwase M, Sasaki N, Oku M, Imoto H, et al. Expression of isoforms of NADPH oxidase components in rat pancreatic islets. Life Sci. 2006;80:133–139. doi: 10.1016/j.lfs.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diab Obes and Metab. 2010;12:141–148. doi: 10.1111/j.1463-1326.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 11.Syed I, Kyathanahalli CN, Kowluru A. Phagocyte-like NADPH oxidase generates ROS in INS 832/13 cells and rat islets: role of protein prenylation. Am J Physiol Regul Integr Comp Physiol. 2011;300:R756–R762. doi: 10.1152/ajpregu.00786.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glomset JA, Farnsworth CC. Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu Rev Cell Biol. 1994;10:181–205. doi: 10.1146/annurev.cb.10.110194.001145. [DOI] [PubMed] [Google Scholar]
- 13.Clarke S, Tamanoi F. Fighting cancer by disrupting C-terminal methylation of signaling proteins. J Clin Invest. 2004;113:513–515. doi: 10.1172/JCI21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Luo R, Kowluru A, Li G. Novel regulation by Rac1 of glucose-and forskolin-induced insulin secretion in INS-1 beta cell. Am J Physiol Endocrinol Metab. 2004;286:818–827. doi: 10.1152/ajpendo.00307.2003. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis- roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122:893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veluthakal R, Kaur H, Goalstone M, Kowluru A. Dominant-negative alpha-subunit of farnesyl- and geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes. 2007;56:204–210. doi: 10.2337/db06-0668. [DOI] [PubMed] [Google Scholar]
- 17.Jayaram B, Syed I, Kyathanahalli CN, Rhodes CJ, Kowluru A. Arf nucleotide binding site opener [ARNO] promotes the sequential activation of Arf6, Cdc42 and Rac1 and insulin secretion in INS 832/13 β-cells and rat islets. Biochem Pharmacol. 2011;81:1016–1027. doi: 10.1016/j.bcp.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 19.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ. Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia. 2009;52:2369–2373. doi: 10.1007/s00125-009-1506-5. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca SG, Burcin M, Gromada J, Urano F. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol. 2009;9:763–770. doi: 10.1016/j.coph.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowluru A. Novel regulatory roles for protein phosphatase-2A in the islet beta cell. Biochem Pharmacol. 2005;69:1681–1691. doi: 10.1016/j.bcp.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Kreck ML, Uhlinger DJ, Tyagi ST, Inge KL, Lambeth JD. Participation of the small molecular weight GTP-binding protein Rac1 in cell-free activation and assembly of the respiratory burst oxidase. J Biol Chem. 1994;269:4161–4168. [PubMed] [Google Scholar]
- 24.Cushman I, Casey PJ. Role of isoprenylcysteine carboxylmethyltransferase-catalyzed methylation in Rho function and migration. J Biol Chem. 2009;284:27964–27973. doi: 10.1074/jbc.M109.025296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, et al. Rho family GTPase modification and dependence on CAAX motif-signaled post-translational modification. J Bio Chem. 2008;283:25150–25163. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, et al. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia. 2007;50:359–369. doi: 10.1007/s00125-006-0462-6. [DOI] [PubMed] [Google Scholar]
- 27.Subasinghe W, Syed I, Kowluru A. Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic beta-cells: evidence for regulation by Rac1. Am J Physiol Regul Integr Comp Physiol. 2011;300:9–11. doi: 10.1152/ajpregu.00421.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syed I, Jayaram B, Subasinghe W, Kowluru A. Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol. 2010;80:874–883. doi: 10.1016/j.bcp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]