Abstract
Understanding mechanisms underlying apoptotic destruction of insulin-secreting cells is critical to validate therapeutic targets for type 1 diabetes mellitus. We recently reported insulin-like growth factor binding protein-3 (IGFBP-3) as a novel mediator of apoptosis in insulin-secreting cells. In light of emerging IGF-independent roles for IGFBP-3, we investigated the mechanisms underlying actions of the novel, recombinant human mutant G56G80G81-IGFBP-3, which lacks intrinsic IGF binding affinity. Using the rat insulinoma RINm5F cell line, we report the first studies in insulin-secreting cells that IGFBP-3 selectively suppresses multiple, key intracellular phosphorelays. By immunoblot, we demonstrate that G56G80G81-IGFBP-3 suppresses phosphorylation of c-raf-MEK-ERK pathway and p38 kinase in time-dependent and dose-dependent manners. SAPK/JNK signaling was unaffected. These data delineate several novel intracellular sites of action for IGFBP-3 in insulin-secreting cells.
Keywords: Insulin-secreting cell, Type 1 diabetes, Insulin-like growth factor binding protein, Apoptosis, Tyrosine kinase
1. Introduction
Apoptotic destruction of insulin-secreting cells is the final common pathway of diverse insults causing type 1 diabetes mellitus in vivo [1-3]. Insulin-like growth factors (IGFs) and their high affinity IGF binding proteins (IGFBPs) comprise a complex super-family of multi-functional proteins regulating cell survival, fate, apoptosis, and metabolism [4]. Dating from the discovery of IGFBPs in the early 1980s, the first revision of the classic somatomedin hypothesis held that IGFBPs functioned solely as passive regulators of IGF bioavailability to the type 1 IGF receptors (heterotetrameric tyrosine kinases on the cell surface) [5]. IGFBP-3, as the most abundant IGFBP species in human serum, represents the prime regulator of IGF half-life in the circulation. Recently, several independent in vitro studies support the emerging concept of IGFBP-3 as a significant regulator of cell survival acting via IGF-independent mechanisms [6,7]. Studies of several mutant IGFBP-3 molecules suggest that the IGF-independent action of IGFBP-3 to promote apoptosis resides in its variable mid-region [8,9], which contains a heparin-like domain.
Triple substitution of glycine at three critical residues in the IGF binding domain of recombinant human (rh) IGFBP-3 eliminates intrinsic IGF binding affinity, while preserving other properties of the native molecule [10]. Global transgenic over-expression of this mutant G56G80G81-rhIGFBP-3 in the mouse resulted in increased brain size and modest increases in circulating IGF-I and IGFBP-3 levels [11]. Several studies with native IGFBP-3 have reported specific IGFBP-3 binding partners in the plasma compartment [12,13], within extracellular matrix [14], and inside the cell [15,16]. Neither a classic IGFBP-3 receptor nor signal transduction pathways restricted to IGFBP-3 action have been identified to date. Recently, we first reported IGFBP-3 as a novel mediator of apoptosis in insulin-secreting cells [17]. To identify the IGF-independent mechanisms of IGFBP-3 in insulin-secreting cells, we studied the actions of G56G80G81-rhIGFBP-3 using the rat insulinoma cell model RIN m5F.
2. Materials and methods
2.1. Cell culture and reagents
Derived from Rattus norvegicus, clonal insulin-secreting RIN-m5F cells were purchased from American Type Tissue Collection (Manassas, VA, USA) [18]. Cells were grown at 37 °C under humidified atmosphere with 5% CO2 and RPMI 1640 medium containing 2 mM l-glutamine, adjusted to 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, 1 mM sodium pyruvate, and 10% fetal bovine serum. Growth media was changed every third day.
pCMV6 vector containing G56G80G81-rhIGFBP-3 was generously provided by Dr. Caroline Buckway of Oregen Health Sciences University (Portland, OR, USA). Monoclonal antibody to tubulin was purchased from Sigma (St. Louis, MO, USA). Polyclonal antibody for IGFBP-3 was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Polyclonal antibodies to phospho-ERK, total ERK, phospho-MEK1/2, MEK1/2, phospho-c-raf, c-raf, phospho-p38, total p38, phospho-SAPK/JNK, and total SAPK/JNK were purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). Secondary antibodies conjugated with horseradish peroxidase were purchased from Jackson Immunologicals (West Grove, PA, USA). ECL for immunoblot development and signal detection was purchased from Amersham Biosciences (Piscataway, NJ, USA). Fluorescein-conjugated goat anti-rabbit IgG and mounting medium for fluorescence with DAPI were purchased from Vector Laboratories, Inc. (Burlingame, CA, USA). Chamber slides were obtained from NalgeNunc International (Rochester, NY, USA). Nitrocellulose membrane (0.2 μm) and Bio-Rad Protein Assay were purchased from Bio-Rad (Hercules, CA, USA). Lipofectamine and plus reagents were purchased from Invitrogen. Cell Death ELISA to detect mono- and oligonucleosome release during apoptosis was purchased from Roche Applied Science (Nonnenwald, Penzberg, Germany).
2.2. Transfection
Cells were seeded in 35 mm2 cell culture dishes, then grown to 50–70% confluence. Cells were washed with phosphate-buffered saline, and 1.5 mL serum-free medium were added to each dish. pCMV vector with or without G56G80G81-rhIGFBP-3 cDNA (up to 4 μg for the dose experiments and 4 μg for the time course experiments), 4 μL lipofectamine reagent and 4 μL plus reagent in 400 μL serum-free medium were then added to each well. After incubation for 5 h, 1.5 mL medium containing 20% serum (to stimulate MAPK cascades) was added in each dish and incubated for the time indicated.
2.3. DNA fragmentation ELISA
Cells were plated at 70% confluence in 96-well plates and allowed to adhere overnight. G56G80G81-rhI-GFBP-3 and empty vector were transfected into cells as described above. To end transfection, the plate was centrifuged at 200g for 10 min to pellet the cells. Apoptosis in the floating and attached cells was quantified by cell death ELISA kit performed according to the manufacturer’s instructions. Briefly, cells resuspended in 200 μL 1X Lysis Buffer were incubated for 30 min at 25 °C. Lysates were centrifuged at 200g for 10 min, and 20 μL of cell lysates were transferred carefully into streptavidin-coated, 96-well plates. Immunoreagent 80 μL was added to each well, and plates were covered with adhesive foil under gentle shaking (300 rpm) for 2 h at 20 °C. Supernatant was thoroughly removed by gentle suction, then each well was rinsed thrice with 25 μL 1X incubation buffer. ABTS solution, 100 μL/well, was added to develop color detected at 405 nm (vs. 490 nm reference).
2.4. Cell growth rate quantitation
The cells were plated in the 6-well plate at about 50% confluence. After 24 h, the cells were transfected with or without 4 μg control vector or GGG-BP-3. After transfection for 24 or 48 h, the cells were trypsinized and counted on a hemacytometer.
2.5. Western blot
Transfection terminated when cells were washed with PBS. Two hundred microlitres of lysis buffer (containing 20 mM Tris, pH 7.5, 1% Nonidet P-40, 150 mM NaCl, 5 mM EDTA, 0.1 mM sodium ortho-vanadate, 1 mM PMSF, and 10 μL/mL aprotinin) were added to each 35 mm2 cell culture dish. Whole cell lysates were collected using the cell scraper. Cell lysates were mixed and incubated on ice for 30 min. Lysates were cleared by centrifugation for 15 min at 13,000g. Protein concentrations were determined by Bio-Rad Protein Assay. Equal amounts of lysates were separated by 15% SDS–PAGE, and resolved proteins were transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat dried milk for 1 h at 25 °C, then incubated with primary antibodies for 3 h at 25 °C. After incubation with horseradish peroxidase-conjugated secondary antibodies, immunoreactive bands were detected by ECL, performed according to the manufacturer’s instructions. After detection of phosphoforms, we stripped membranes with 0.25 mM NaOH for 15 min at 25 °C, then re-probed with antibodies directed against the total specific protein.
2.6. Immunofluorescent microscopy
Cells plated in the chamber slides were grown to 50–70% confluence, then transfected with 0.25 μg DNA using Lipofectamine as described earlier. After two washes with PBS, cells were fixed with 4% paraformaldehyde (pH 7.5) for 20 min at 25 °C. Cells were rinsed twice with PBS and thrice washed with 1 mg/mL sodium borohydrate (5 min/wash) to reduce background. Cells were permeablized with 0.2% Triton X-100 for 5 min at 25 °C. After two washes with PBS, cells were blocked with 2% bovine serum for 1 h at 25 °C. Cells were then incubated with 10 μg/mL anti-IGFBP-3 antibody for 1 h at 25 °C. After two washes with PBS, cells were incubated for 1 h under darkness with fluorescein-conjugated goat anti-rabbit IgG, diluted 400-fold. After two washes in PBS, slides were covered under mounting medium containing DAPI. The 461 nm line from the Ar laser (Leica Inverted confocal microscope) induced fluorescence, signal captured by Himamatsu digital camera and processed with Microscope Control Module Software (QED Imaging, Inc., Pittsburgh, PA, USA).
2.7. Statistic analysis
Data from replicate experiments are expressed as means ± SEM, analyzed by ANOVA and post-hoc Kruskal–Wallis test using Instat (version 2.00; Graph-Pad Software Inc., San Diago, CA, USA). Probability values (p) below 5% were considered significant.
3. Results
3.1. G56G80G81-rhIGFBP-3 induces apoptosis and inhibits growth in RIN cells
Confirming our prior report with the native protein, we observed that mutant G56G80G81-rhIGFBP-3 transfection induced apoptosis within 24 h as detected by oligonucleosome generation (Fig. 1;*p < 0.05 vs. transfection with empty vector). Cell growth was also inhibited in the GGG-rhIGFBP-3 transfected cells compared with the empty vector transfected cells (Fig. 2, p < 0.05 vs. empty vector).
Fig. 1.

Nucleosome ELISA. Cells were transfected with G56G80G81-rhIGFBP-3 or empty vector for 24 or 48 h in the presence of serum (n = 8, *p < 0.05 vs. control by ANOVA).
Fig. 2.

Cell growth rate study. Cells were transfected with empty vector (EV) or GGG-BP-3 for 24 or 48 h. The cells were then trypsinized and counted (p < 0.05 vs. control by ANOVA). Data were from 3 independent experiments.
3.2. Dose-dependent and time-dependent G56G80G81-rhIGFBP-3 transfection
Dramatic, dose-dependent G56G80G81-rhIGFBP-3 synthesis was identified in whole cell lysates (Fig. 3A–B). Of note, mutant G56G80G81-rhIGFBP-3 migrated in a manner identical to native IGFBP-3 as detected by Western immunoblot: 43 and 45 kDa glycosylated doublet bands, a minor fraction in the proteolyzed 29 kDa form. Time-dependent transfection, up to 3 days in the presence of serum, produced consistent increases (not reduction) in IGFBP-3 synthesis (Fig. 3C–D). Confocal microscopy of these transfected cells disclosed immunofluorescent IGFBP-3 localized primarily in the cytoplasm; control cells transfected with empty vector showed no appreciable signal (Fig. 4).
Fig. 3.
Dose- and time-dependent G56G80G81-rhIGFBP-3 transfection. Western blot of whole cell lysates after 24-h transfection using various amount of DNA (A) with densitometric analysis (B) and time course (C,D). Densitometry was normalized with respect to the tubulin control without transfection (**p < 0.01 vs. control by ANOVA). Data were from 3 independent experiments.
Fig. 4.
Confocal microscopy. Cells transfected 24 h with empty vector (A) or G56G80G81-rhIGFBP-3 (B). DAPI staining of nuclear heterochromatin (upper left panels); IGFBP-3 immunostain (upper right panels); light microscopy (lower left panels); DAPI and IGFBP-3 stain overlay (lower right panels).
IGFBP-3 is a secreted protein. The level of BP-3 in the medium was also analyzed by Western blot. It shows that the level of BP-3 is higher in the GGG-rhIGFBP-3 transfected cell medium than the level in the control medium (Fig. 5A–D).
Fig. 5.
Cells were transfected with empty vector (EV) or GGG-BP-3 as indicated for 48 h. Equal amount of medium were analyzed by Western blot (*p < 0.05, **p < 0.01 vs. control by ANOVA). Data were from 4 independent experiments.
3.3. G56G80G81-rhIGFBP-3 inhibits phosphorylation of ERK, MEK, c-raf, p38 kinase, and Akt
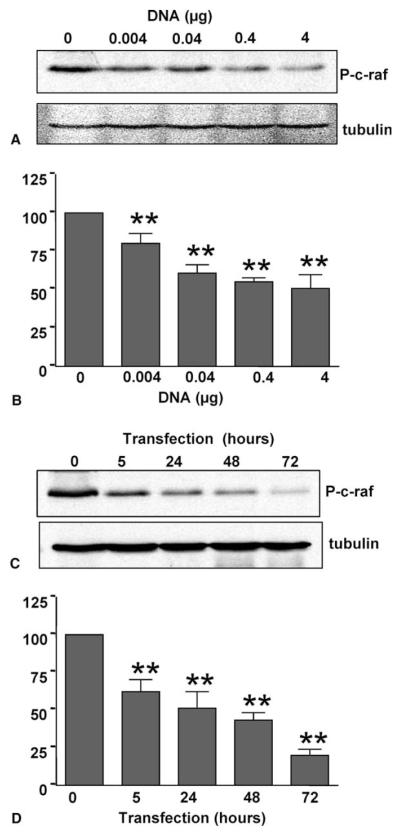
We systematically examined the downstream kinases of the mitogen activated protein kinase (MAPK) cascades due to their central roles for signaling survival over apoptosis. Despite the presence of serum, G56G80G81-rhIGFBP-3 transfection significantly blocked ERK phosphorylation in a dose-dependent (Fig. 6A–B) and time-dependent manner (Fig. 6C–D) by 72 h. The upstream kinases MEK1/2 and c-raf were also inhibited by G56G80G81-rhI-GFBP-3 in both time- and dose-dependent manners (Figs. 7, 8). In the presence of serum, G56G80G81-rhI-GFBP-3 significantly inhibited p38 phosphorylation in a dose-dependent (Fig. 9A–B) and time-dependent manner (Fig. 9C–D). No effect was observed on SAPK/JNK signaling (Fig. 10). Fig. 11 illustrates confirmation of the major actions by G56G80G81-rhI-GFBP-3 vs. mock transfections at the 48-h time point.
Fig. 6.
Western blot for phospho-ERK in whole cell lysates. Dose-range study of 24-h G56G80G81-rhIGFBP-3 transfection (A) with corresponding densitometry normalized to “0” control (B). Time course (C) with corresponding densitometry normalized to “0” control (D) (**p < 0.01 vs. control by ANOVA). Data were from 3 independent experiments.
Fig. 7.
Western blot for phospho-MEK1/2 in whole cell lysates. Dose-range study of 24-h G56G80G81-rhIGFBP-3 transfection (A) with corresponding densitometry normalized to “0” control (B). Time course (C) with corresponding densitometry normalized to “0” control (D) (**p < 0.01 vs. control by ANOVA). Data were from 3 independent experiments.
Fig. 8.
Western blot for phospho-c-raf in whole cell lysates. Dose-range study of 24-h G56G80G81-rhIGFBP-3 transfection (A) with corresponding densitometry normalized to “0” control (B). Time course (C) with corresponding densitometry normalized to “0” control (D) (**p < 0.01 vs. control by ANOVA). Data were from 3 independent experiments.
Fig. 9.
Western blot for phospho-p38 in whole cell lysates. Dose-range study of 24-h G56G80G81-rhIGFBP-3 transfection (A) with corresponding densitometry normalized to “0” control (B). Time course (C) with corresponding densitometry normalized to “0” control (D) (*p < 0.05, **p < 0.01 vs. control by ANOVA). Data were from 3 independent experiments.
Fig. 10.

Western blot for phospho-SAPK/JNK in whole cell lysates. Dose-range study of 24-h G56G80G81-rhIGFBP-3 transfection (A) and time course study (B). A representative blot is from 3 independent experiments.
Fig. 11.

Western blot of whole cell lysates. ERK, c-raf, MEK and p38 phosphorylation were not inhibited by transfection of empty vector (EV), but by G56G80G81-rhIGFBP-3 transfection.
4. Discussion
We have presented the first evidence that IGFBP-3 inhibited specific MAP kinase pathway which may be the potential mechanism underlying IGFBP-3 apoptosis actions. Moreover, we have confirmed that these effects can be independent of this multifunctional protein’s intrinsic IGF binding affinity. In the presence of serum, G56G80G81-rhIGFBP-3 transfection significantly inhibited the phospho-c-raf-MEK-ERK pathway as well as the phospho-p38 in this insulin-secreting line. IGFBP-3 has been shown to block proliferation and to promote apoptosis by inhibiting mitogens acting via cell surface tyrosine kinases, including receptors for vascular endothelial growth factor and the type 1 IGF receptors[17,19-21].
Several novel binding partners for IGFBP-3 have been proposed to explain its IGF-independent actions [20], yet no classic receptor for any IGFBP has been accepted globally. Extracellular signal kinase 1/2 (ERK1/2) at the terminus of the MAPK cascade plays a critical supporting role for cell growth and differentiation [22-25]. Upregulation of ERK1/2 supports β-cell replication in mice induced by neuropeptide Y [26], which indicates ERK1/2 is an important regulation factor in pancreatic β-cell growth. Interrupting this pathway by BP-3 may lead to cell death. However, the relationship between this MAP kinase pathway and BP-3 apoptosis effect need to be evaluated.
p38, another MAP kinase, is activated by diverse cellular stresses, including osmotic shock, inflammatory cytokines, lipopolysaccharides, ultraviolet irradiation, and growth factors [27-31]. The inhibitory action of IGFBP-3 on this distal phosphorelay represents a novel, specific mechanism for its actions. The lack of effect on SAPK/JNK phosphorylation suggests that the effects of IGFBP-3 on MAP kinase regulation are kinase-specific or relay-specific.
Other data obtained from insulin-secreting cell models provide conflicting evidence for the complex roles of such phosphorelays in apoptosis. ERK activation appears necessary for interleukin-1β (IL-1β)-induced apoptosis in human β-cells [32]. The p38 inhibitor, SB203580, counteracts apoptosis induced by the combination of IL-1β and interferon-γ in RINm5F cells [33]. These observations suggest that the balance from multiple signaling transduction pathways determines cell fate, say, survival vs. apoptosis. Our use of transformed cells limits extrapolation to the situation in vivo with human islets. In conclusion, we have presented the first evidence that IGFBP-3 selectively inhibits serum-induced phosphorylation of major MAP kinases in insulin-secreting cells. Further investigations of this novel pathway underlying destruction of insulin-secreting cells are needed and this mechanism may be warranted to validate IGFBP-3 blockade as a novel therapeutic approach to preserve residual β-cell mass in the face of imminent clinical diabetes.
Acknowledgements
G56G80G81-rhIGFBP-3 was generously provided by Dr. Caroline Buckway of Oregen Health Sciences University (Portland, OR, USA). This work was supported in part by NIH Grant K08 DK02876 and the Genentech Clinical Scholar Award of the Lawson Wilkins Pediatric Endocrine Society (to R.F.). The opinions herein are solely those of the authors and do not represent an official position of The State of Texas, the US Army, nor their subordinate agencies.
References
- [1].O’Brien BA, Harmon BV, Cameron DP, Allan DJ. Apoptosis is the mode of beta-cell death responsible for the development of type 1 diabetes in the nonobese diabetic (NOD) mouse. Diabetes. 1997;46:750–757. doi: 10.2337/diab.46.5.750. [DOI] [PubMed] [Google Scholar]
- [2].Kurrer MO, Pakala SV, Hanson HL, Katz JD. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc. Natl. Acad. Sci. USA. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].O’Brien BA, Harmon BV, Cameron DP, Allan DJ. Beta-cell apoptosis is responsible for the development of T1DM in the multiple low-dose streptozotocin model. J. Pathol. 1996;178:176–181. doi: 10.1002/(SICI)1096-9896(199602)178:2<176::AID-PATH433>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- [4].Ferry RJ, Jr., Cerri RW, Cohen P. Insulin-like growth factor binding proteins: new proteins, new functions. Horm. Res. 1999;51:53–67. doi: 10.1159/000023315. [DOI] [PubMed] [Google Scholar]
- [5].Kaplan SA. Somatomedin hypothesis: time for reexamination. Endocrinologist. 2001;11:470–473. [Google Scholar]
- [6].Longobardi L, Torello M, Buckway C, O’Rear L, Horton WA, Hwa V, Roberts CT, Jr., Chiarelli F, Rosenfeld RG, Spagnoli A. A novel insulin-like growth factor (IGF)-independent role for IGF binding protein-3 in mesenchymal chondroprogenitor cell apoptosis. Endocrinology. 2003;144:1695–1702. doi: 10.1210/en.2002-220959. [DOI] [PubMed] [Google Scholar]
- [7].Collard TJ, Guy M, Butt AJ, Perks CM, Holly JM, Paraskeva C, Williams AC. Transcriptional upregulation of the insulin-like growth factor binding protein IGFBP-3 by sodium butyrate increases IGF-independent apoptosis in human colonic adenoma-derived epithelial cells. Carcinogenesis. 2003;24:393–401. doi: 10.1093/carcin/24.3.393. [DOI] [PubMed] [Google Scholar]
- [8].Perks CM, McCaig C, Clarke JB, Clemmons DR, Holly JM. A non-IGF binding mutant of IGFBP-3 modulates cell function in breast epithelial cells. Biochem. Biophys. Res. Commun. 2002;294:988–994. doi: 10.1016/S0006-291X(02)00569-7. [DOI] [PubMed] [Google Scholar]
- [9].Hollowood AD, Stewart CE, Perks CM, Pell JM, Lai T, Alderson D, Holly JM. Evidence implicating a mid-region sequence of IGFBP-3 in its specific IGF-independent actions. J. Cell Biochem. 2002;86:583–589. doi: 10.1002/jcb.10223. [DOI] [PubMed] [Google Scholar]
- [10].Buckway CK, Wilson EM, Ahlsen M, Bang P, Oh Y, Rosenfeld RG. Mutation of three critical amino acids of the N-terminal domain of IGF-binding protein-3 essential for high affinity IGF binding. J. Clin. Endocrinol. Metab. 2001;86:4943–4950. doi: 10.1210/jcem.86.10.7936. [DOI] [PubMed] [Google Scholar]
- [11].Silha JV, Gui Y, Mishra S, Leckstrom A, Cohen P, Murphy LJ. Overexpression of gly56/gly80/gly81-mutant insulin-like growth factor-binding protein-3 in transgenic mice. Endocrinology. 2005;146:1523–1531. doi: 10.1210/en.2004-0905. [DOI] [PubMed] [Google Scholar]
- [12].Oesterreicher S, Blum WF, Schmidt B, Braulke T, Kubler B. Interaction of insulin-like growth factor II (IGF-II) with multiple plasma proteins: high affinity binding of plasminogen to IGF-II and IGF-binding protein-3. J. Biol. Chem. 2005;280:9994–10000. doi: 10.1074/jbc.M411754200. [DOI] [PubMed] [Google Scholar]
- [13].Weinzimer SA, Gibson TB, Collett-Solberg PF, Khare A, Liu B, Cohen P. Transferrin is an insulin-like growth factor-binding protein-3 binding protein. J. Clin. Endocrinol. Metab. 2001;86:1806–1813. doi: 10.1210/jcem.86.4.7380. [DOI] [PubMed] [Google Scholar]
- [14].Liu B, Weinzimer SA, Gibson TB, Mascarenhas D, Cohen P. Type Ia collagen is an IGFBP-3 binding protein. Growth Horm. IGF Res. 2003;13:89–97. doi: 10.1016/s1096-6374(03)00007-8. [DOI] [PubMed] [Google Scholar]
- [15].Lee KW, Liu B, Ma L, Li H, Bang P, Koeffler HP, Cohen P. Cellular internalization of insulin-like growth factor binding protein-3: distinct endocytic pathways facilitate re-uptake and nuclear localization. J. Biol. Chem. 2004;279:469–476. doi: 10.1074/jbc.M307316200. [DOI] [PubMed] [Google Scholar]
- [16].Liu B, Lee HY, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, Cohen P. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis. J. Biol. Chem. 2000;275:33607–33613. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- [17].Shim ML, Katz LEL, Davis J, Dotzler WC, Cohen P, Ferry RJ., Jr. Insulin-like growth factor binding protein-3 is a novel mediator of apoptosis in insulin-secreting cells. Growth Horm. IGF Res. 2004;14:216–225. doi: 10.1016/j.ghir.2003.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gazdar AF, Chick WL, Oie HK, Sims HL, King DL, Weir GC, et al. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc. Natl. Acad. Sci. USA. 1980;77:3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vasylyeva TL, Chen X, Ferry RJ., Jr. Insulin-like growth factor binding protein-3 mediates cytokine-induced mesangial cell apoptosis. Growth Horm. IGF Res. 2005;15:107–114. doi: 10.1016/j.ghir.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- [21].Franklin SL, Ferry RJ, Jr., Cohen P. Rapid insulin-like growth factor (IGF)-independent effects of IGF binding protein-3 on endothelial cell survival. J. Clin. Endocrinol. Metab. 2003;88:900–907. doi: 10.1210/jc.2002-020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- [23].Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- [24].Hill CS, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- [25].Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- [26].Cho YR, Kim CW. Neuropeptide Y promotes β-cell replication via extracellular signal-regulated kinase activation. Biochem. Biophys. Res. Commun. 2004;314:773–780. doi: 10.1016/j.bbrc.2003.12.170. [DOI] [PubMed] [Google Scholar]
- [27].Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- [28].Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- [29].Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- [30].Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- [31].Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- [32].Maedler K, Storling J, Sturis J, Zuellig RA, Spinas GA, Arkhammar PO, Mandrup-Poulsen T, Donath MY. Glucose- and interleukin-1beta-induced beta-cell apoptosis requires Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6.2) selective potassium channel opener in human islets. Diabetes. 2004;53:1706–1713. doi: 10.2337/diabetes.53.7.1706. [DOI] [PubMed] [Google Scholar]
- [33].Saldeen J, Welsh N. p38 MAPK inhibits JNK2 and mediates cytokine-activated iNOS induction and apoptosis independently of NF-KB translocation in insulin-producing cells. Cytokine Network. 2004;15:47–52. [PubMed] [Google Scholar]