Abstract
Post traumatic stress disorder (PTSD) is a chronic anxiety disorder initiated by an intensely threatening, traumatic event. There is a great need for more efficacious pharmacotherapy and preventive treatments for PTSD. In animals, corticotropin-releasing factor (CRF) and the CRF1 receptor play a critical role in behavioural and neuroendocrine responses to stress. We tested the hypothesis that CRF1 activation is required for initiation and consolidation of long-term effects of trauma on anxiety-like behaviour in the predator exposure (predator stress) model of PTSD. Male C57BL6 mice were treated with the selective CRF1 antagonist CRA0450 (2, 20 mg/kg) 30 min before or just after predator stress. Long-term effects of stress on rodent anxiety were measured 7 d later using acoustic startle, elevated plus maze (EPM), light/dark box, and hole-board tests. Predator stress increased startle amplitude and delayed startle habituation, increased time in and decreased exits from the dark chamber in the light/dark box test, and decreased risk assessment in the EPM. CRF1 antagonism had limited effects on these behaviours in non-stressed controls, with the high dose decreasing risk assessment in the EPM. However, in stressed animals CRF1 antagonism blocked initiation and consolidation of stressor effects on startle, and returned risk assessment to baseline levels in predator-stressed mice. These findings implicate CRF1 activation in initiation and post-trauma consolidation of predator stress effects on anxiety-like behaviour, specifically on increased arousal as measured by exaggerated startle behaviours. These data support further research of CRF1 antagonists as potential prophylactic treatments for PTSD.
Keywords: Anxiety, consolidation, initiation, CRF1, predator stress, PTSD
Introduction
Fearful and stressful events may precipitate affective psychopathologies (Caspi et al. 2003), including posttraumatic stress disorder (PTSD) (Silver et al. 2002; Yehuda, 2002). Understanding the biological mechanisms supporting development of chronic anxiety after stress exposure will provide novel treatment targets for disorders such as PTSD. New treatment targets are in great demand for this disorder as evidence for robust efficacy of current pharmacological treatments for PTSD is lacking (Institute of Medicine, 2007). One strategy to aid understanding of this neurobiology is the use of predator stress exposure models in the rodent with lasting impact on behaviour. Such models exist and involve either single exposure of a rat or mouse to various forms of predator-specific stimuli, e.g. scent using feline urine (Cohen et al. 2004, 2006), or repeated exposure to scent and visual stimuli plus chronic unstable housing (Zoladz et al. 2008), or single unprotected predator exposure including physical interaction such as chase/escape behaviours (for review see Adamec et al. 2006a).
Although the aforementioned models have similar lasting post-stress effects, our emphasis here is on the consequences of single, intense and brief traumatic stress for the purpose of ease of examination of initiation and consolidation of behavioural changes. Predator stress (PS) in our study involves unprotected exposure of rodents to a cat (Adamec & Shallow, 1993; Adamec et al. 2004c). This PS model may model hyperarousal and comorbid anxiety aspects of PTSD for several reasons (for details see Adamec et al. 2006a). In brief, PS has ecological validity as a robust and innate stressful threat for rodents (Adamec et al. 1998) similar to the type of trauma that can elicit PTSD, e.g. ‘an event that involves actual or threatened death or serious injury’ (APA, 1994, p. 424). Second, duration of anxiogenic effects after PS, as a ratio of lifespan, is comparable to the duration of psychopathology required for a diagnosis of chronic PTSD in humans. Cat exposure increases unconditioned rodent anxiety for ≥3 wk (Adamec & Shallow, 1993). Third, PS has neurobiological face validity in that right amygdala and hippocampal circuitry are implicated in behavioural changes produced by PS (Adamec et al. 2005b), and these areas are consistent with brain areas thought to be involved in PTSD (Rauch et al. 2006). Fourth, in both humans and rodents, features of the stressor predict the level of anxiety, e.g. the less controllable the predator stressor (e.g. fewer escape avenues) the higher the anxiety-like phenotype, similar to PTSD responses to uncontrollable trauma in humans (Adamec, 1997). Fifth, similar lasting changes in startle and habituation of startle are seen in both predator-stressed rodents and humans with PTSD, which offers an ideal measure to test translatability of these findings to patients (Adamec, 1997; Orr et al. 2002) as well as offering some selectivity of the model for PTSD-specific symptoms over generalized anxiety disorder symptoms (APA, 1994; Grillon et al. 2009). Moreover, human females may be more vulnerable to PTSD (Kessler et al. 1995, 2005; Stam, 2007), which is to some extent mimicked in this PS model in mice (Adamec et al. 2006d, 2008b).
Effects of PS undergo processes of initiation and consolidation. For example initiation, but not consolidation, of anxiogenic effects of PS are NMDA-receptor dependent. Systemic administration of NMDA receptor antagonists before, but not after, PS prevents lasting changes in anxiety (Adamec et al. 1999a; Blundell et al. 2005). NMDA receptor blockade in the amygdala before PS also prevents increases in anxiety (Adamec et al. 1999b). Studies of post-stress manipulations reveal that consolidation of effects of PS involve β-noradrenergic, glucocorticoid and mineral corticoid receptor systems (Adamec et al. 2007), 5-HT1A and 5-HT2A receptors (Adamec et al. 2004a,b), as well as protein synthesis (Adamec et al. 2006b).
PS may also act via the corticotrophin-releasing factor (CRF) system, as PS elevates hypothalamic CRF and plasma glucocorticoid levels (Adamec et al. 1998, 2007). CRF acts through two G-protein-coupled receptors, CRF1 and CRF2. CRF1 plays an essential role in the stress response, and in the activation of the hypothalamic–pituitary–adrenal (HPA) axis, controlling neuroendocrine response to environmental stress (Kaplan, 1992). CRF and CRF1 within the brain have also been implicated in anxiety. Genetically modified mice over-expressing CRF are more anxious-like (Groenink et al. 2003; Stenzel-Poore et al. 1994). Nonspecific CRF antagonist treatment in these mice reverses this phenotype (Stenzel-Poore et al. 1994). Moreover, mice lacking CRF1 show an anxiolytic-like profile in tests of anxiety including the elevated plus maze (EPM) (for review see Hauger et al. 2006). CRF1 null mutation mice also show blunted HPA responses to stress (Contarino et al. 1999; Smith et al. 1998; Timpl et al. 1998). Together, these findings suggest CRF1 receptor activation is involved in behavioural and neuroendocrine response to stressors. CRF system abnormalities occur in PTSD and other affective disorders (Risbrough & Stein, 2006). PTSD sufferers exhibit increased CRF concentrations in cerebrospinal fluid, either as a consequence of traumatic stress or as a contributor to vulnerability to PTSD (Baker et al. 1999; Heim et al. 1997; Nemeroff et al. 2006; Sautter et al. 2003). Women with PTSD also show increased ACTH and glucocorticoid response following a CRF injection or acute stressor (Heim et al. 2000). Unsurprisingly, the CRF system and specifically CRF1 have been targeted for pharmacological intervention in stress disorders (Steckler & Dautzenberg, 2006). To this end, small molecule non-peptide antagonists for CRF1 have been developed, although none currently exist for the CRF2 receptor. One such CRF1 antagonist is CRA0450. It is centrally acting, and systemically administered, with little effect on baseline rodent anxiety, but has potent anxiolytic effects in rats stressed prior to anxiety testing (Chaki et al. 2004). These data suggest CRA0450 has potential as an anxiolytic pharmaceutical in stress-precipitated anxiety. The present study tested the hypothesis that CRF1 activation contributes to the initiation and consolidation of lasting anxiogenic effects of a traumatic stressor. Varying doses of CRA0450 were administered before and after predator exposure. If CRF1 enables initiation of stress effects on affect, then CRA0450 given beforehand should reduce lasting anxiogenic effects of PS. If CRF1 participates in the consolidation of these effects, administration of CRA0450 after PS should reduce the lasting anxiogenic effects of PS.
Methods
All procedures involving animals in this study adhered to the guidelines of the Canadian Council on Animal Care, and were approved by the Institutional Animal Care Committee of Memorial University. All efforts were made to minimize pain, stress, and the number of animals used.
Subjects and groups
Male C57BL6 (C57) mice (Charles River, Canada) (n=240) served as subjects. Upon arrival, the mice were aged 4–5 wk, weighing 20–30 g. Subjects were housed individually in clear plastic cages with wire covers (42 cm×25 cm×20 cm) with food and water available ad libitum. Mice were adapted to a reversed 12-h light/dark cycle (lights off at 07:00 hours) for 2 wk prior to experimentation, with handling during the first week and acoustic startle pretesting (see below) during the second week. Handling involved picking the mice up by their tails and holding them on the forearm while gently petting them for 1 min each day. Treatments followed the 2-wk acclimation, followed by 1 wk of rest, and then behavioural testing. Predator-stressed mice were housed in a room separate from mice which were not predator-stressed. Mice were randomly assigned to one of 12 groups (n=20), six were predator-stressed and six were handled controls. Separate groups of stressed and control mice were injected intraperitoneally with either vehicle (0.5 ml Tween-80 in sterile saline, two drops sonicated in 10 ml saline), or CRA0450 (2 or 20 mg/kg suspended in 0.5 ml vehicle). Injections were given either 30 min prior to handling or PS, or 11 min after handling or 1 min after a 10-min cat exposure. Two mice from each of the 12 groups were tested weekly in 10 batches of 24 with a new batch arriving every week. Therefore the study was conducted over a 14-wk period, with testing of each batch taking about 4 wk with equal representation of all groups in each batch.
PS and handling
At the time of treatment, subjects in the PS conditions were placed in the cat exposure room (1.52 m × 1.83 m; 110 lx lighting at the floor from two overhead fluorescent lights) along with a cat for 10 min as described elsewhere (Adamec et al. 2004c). Mice were allowed to move freely within the room. A single cat was used for all exposures, counterbalancing the order of injected groups. The cat played with the mice but did not injure them. At the end of the 10-min test, mice were returned to their home cages as described previously (Adamec et al. 2004c). Handled controls were handled again for 1 min on the day of treatment. The behaviour of the cat and mouse was videotaped to quantify the stress experience. Cat behaviours examined were: latency to approach, and time spent near the mouse; latency to sniff and frequency of sniffing the mouse, and frequency of pawing and biting. Time near the mouse was scored when the cat was within 1 ft of the mouse determined by 1 ft square floor markings. The mouse response to cat approach was scored as active, passive, and escape defensive responses as described previously (Adamec et al. 2004c, 2006d).
Behavioural testing
Following the PS manipulation, mice were left alone in their cages for 6 d. Then responses to the light/dark box, EPM, hole-board, and acoustic startle tests were recorded over 2 d. Mice were first exposed to the light/dark box (08:00–10:00 hours) followed by the hole-board and EPM tests (10:00–13:00 hours) on day 1. The acoustic startle response was recorded on day 2 (08:00–12:00 hours). Except for the acoustic startle, all tests were videotaped. Times of testing were counter-balanced among groups. Further details of apparatus and testing procedures appear elsewhere (Adamec et al. 2004c, 2006d). All apparatuses were cleaned between tests (5% alcohol).
The light/dark box consisted of two chambers joined by a short tunnel, one light (floor illumination of 750 lx) and one dark chamber (unlit). Mice were placed in the light chamber at the start of the test and their activity was videotaped for 5 min, they were then returned to their home cages. Behavioural measures included time spent in each chamber, number of entries into each chamber (defined as having all four paws in the chamber) and number of fecal boli.
The hole-board and EPM were illuminated with red overhead lights. Illumination levels were: 470 lx at the light bulb; and an unmeasurable light intensity at the floor of the testing apparatuses. The hole-board test was performed in an open-topped box, with walls rising 20 cm above the floor of the box. The floor of the box was elevated above the ground with four holes located in each corner. White masking tape outlined the centre of the box, forming a square 4 cm from the walls of the box. Mice were placed in the centre of the floor at the beginning of each trial and videotaped for 5 min. Behaviours measured were: head dips, rears, number of fecal boli, and time spent in the centre as well as near the walls of the box. Head dips were defined as sticking the head into one of the four holes. Rears were scored when the mouse raised itself up on its hind legs, forepaws leaving the ground but not grooming. Mice were considered to be in the centre when the full body was within the centre area, and near the wall when all four feet were within the 4 cm area between the masking tape and the wall. After the hole-board test, mice were transferred by the tail to the EPM.
The EPM consisted of four interconnected arms arranged in the shape of a plus sign, with two opposite ‘open’ arms and two ‘closed’ arms. Transparent plastic walls surrounded the perimeter of the closed arms, while the open arms had a 0.2 cm high lip. Mice were placed in the centre facing the same open arm at the start of each 5-min trial. Behaviours quantified were time of risk assessment, entries and time spent in open and closed arms, and centre head dips (dipping the head below the arms of the maze while in the centre area joining the four arms). Mice were considered in an arm if all four feet were on the arm. Risk assessment was scored when two hind paws were in a closed arm with nose pointed towards one of the open arms. Relative risk assessment (time) was calculated as a ratio of time spent in the closed arms. Ratio time and ratio entry were the standard measures of anxiety. Ratios were calculated as total time in the open arms divided by the total time in any arm for ratio time, and number of entries in the open arms divided by number of entries into any arm for ratio entry. Smaller ratios indicate greater anxiety (File, 1987).
Startle testing occurred in a San Diego Instruments startle chamber. One week prior to treatments, all mice were exposed to pre-startle testing over 3 d, according to the methods of Hebb et al. (2003). Pre-startle testing was done in a dark chamber. This involved acclimating mice to the startle apparatus with a background of 50 dB white noise for 5 min. Then mice were exposed to 10 pulses of 50-ms bursts of white noise of 105 dB amplitude rising out of a background of 50 dB of white noise with a 30-s inter-trial interval. Startle response was measured over a 150-ms recording window by computer. Post-treatment startle testing used the same parameters as the pre-treatment testing except there were 20 trials (10 light and 10 dark occurring randomly). For light trials, lights in the startle chamber came on for 2.95 s prior to the startle stimulus, and remained on with the startle stimulus for an additional 50 ms. The light intensity in the chamber at the level of the mouse was 300 lx. For all startle tests, peak startle amplitude was calculated by computer for each trial, for later analysis as described elsewhere (Adamec et al. 2006d).
Since cat exposure delays habituation of the startle response in rodents (Adamec, 1997), rate of decline of startle response over trials was calculated as a measure of startle habituation. Measures of peak startle amplitudes for each of the 20 trials were averaged over mice of the same group, with the habituation of light and dark startle considered separately. The averaged peak startle amplitudes over the 10 light and 10 dark trials were fitted as previously described (Adamec et al. 2008a) using the Table Curve 4.0 program (Jandel, USA) to an exponential decline function of the form: Y=Y0+ae−t/τ, where Y and Y0 are peak startle amplitudes, a is a constant, e is the base of the natural logarithm, t is startle trial, and τ is the trial constant. The trial constant is the number of trials required for peak startle amplitude to decline to 37% of the maximal value, a measure of the rate of habituation. Estimates of τ and their standard errors acquired from the fitted exponential were used to compare τ values among each of the groups using planned two-tailed t tests (p<0.05). The scoring of videotapes was performed blind.
Statistics
Except for startle data, all analyses of behaviour were done using a three-way analysis of variance (ANOVA) assessing effects of treatment (handling or PS), time of injection (before or after treatment), and dose (0, 2 or 20 mg/kg). Pre-treatment startle data were analysed using a four-way ANOVA across treatment, time of injection, dose and startle trials. Because of deviation from normality, post-treatment startle data were analysed using one-way non-parametric ANOVA on medians (Kruskal–Wallis) comparing combined handled groups, which did not differ, with predator-stressed groups given 0, 2 or 20 mg/kg CRA0450.
Results
Startle
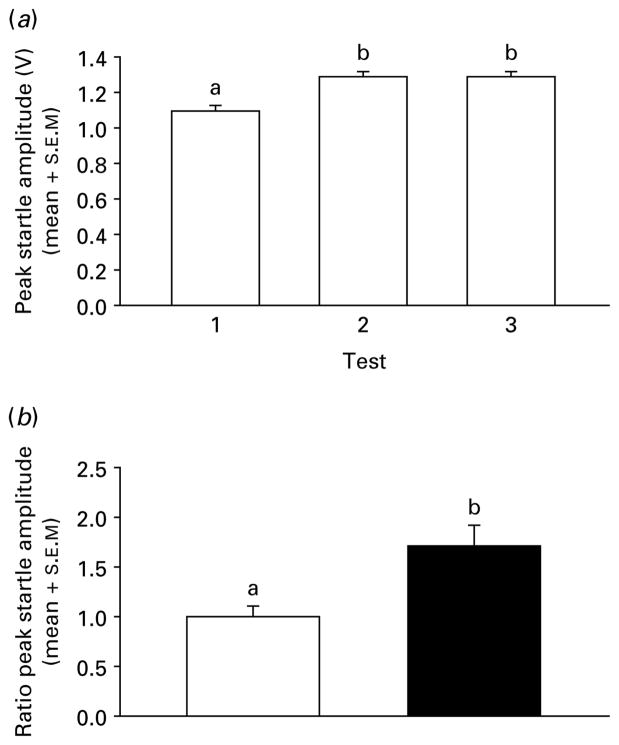
There was a main effect of testing condition (testing over days) on pre-stress peak startle amplitude with no other effects of grouping factors [F(2, 449)=15.22, p<0.001], indicating that at pre-stress all groups were similar for baseline startle values. Mean contrasts (Tukey–Kramer, p<0.05) indicated a startle sensitization from test 1 to tests 2 and 3 (Fig. 1a). Therefore test 3 served as a baseline for post-stress startle analyses. Post-treatment peak startle amplitudes were expressed as a trial×trial ratio of pre-treatment test-3 peak startle amplitudes. Light and dark startle trials were analysed separately. First, post-treatment ratio peak startle amplitude was compared across stressed and handled vehicle groups. Ratio startle response of predator-stressed mice was greater than handled controls [t(78)=3.15, p<0.003; Fig. 1b], and showed an increase over pre-startle response following cat exposure [startle amplitude ratio >1.0; t(38)=3.58, p<0.001]. Handled mice showed no changes in startle response over pre-startle following handling, with a startle amplitude ratio which did not differ from 1.0 [t(38)=0.04, p>0.05].
Fig. 1.
(a) Pre-test peak startle amplitudes (mean+S.E.M.) averaged over trials 1–3 and mice (n=240 per mean). Means with the same letter do not differ, means with different letters differ. (b) Mean (+S.E.M.) peak startle amplitudes as a ratio of test 3 pre-test startle amplitude (ratio peak startle amplitudes) for handled (□) and predator-stressed (■) mice (n=80 per mean) given vehicle. Data are collapsed over injection timing (before and after treatment) and light and dark startle trials. Means with different letters differ.
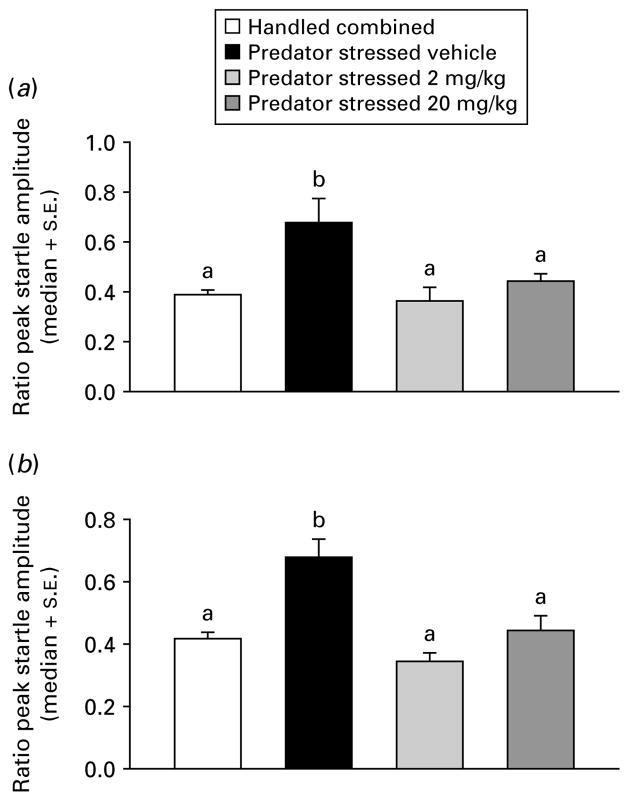
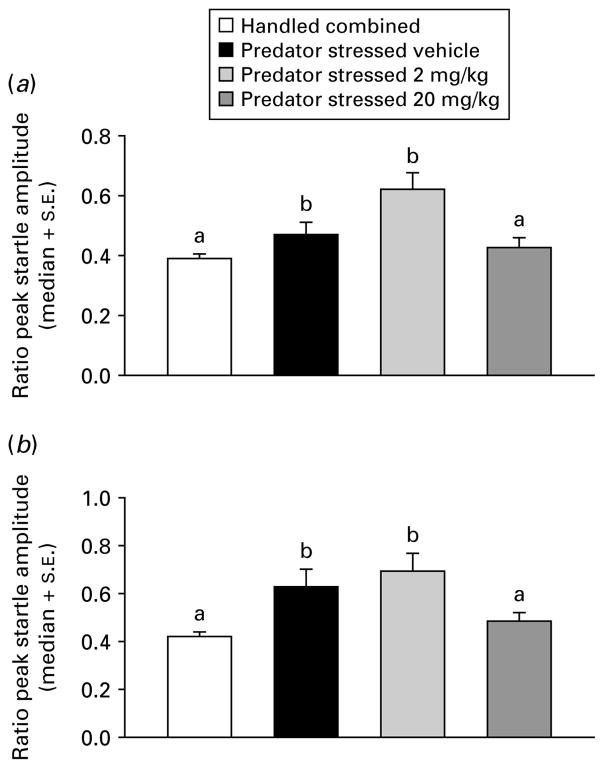
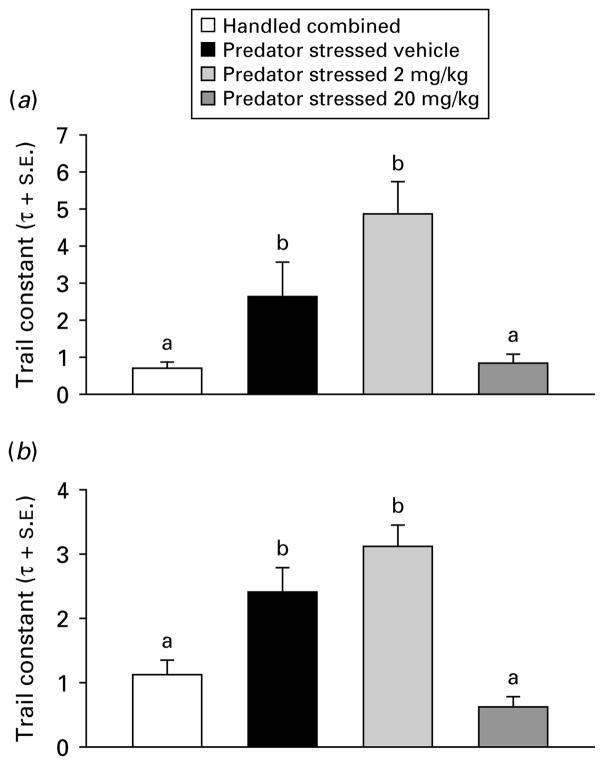
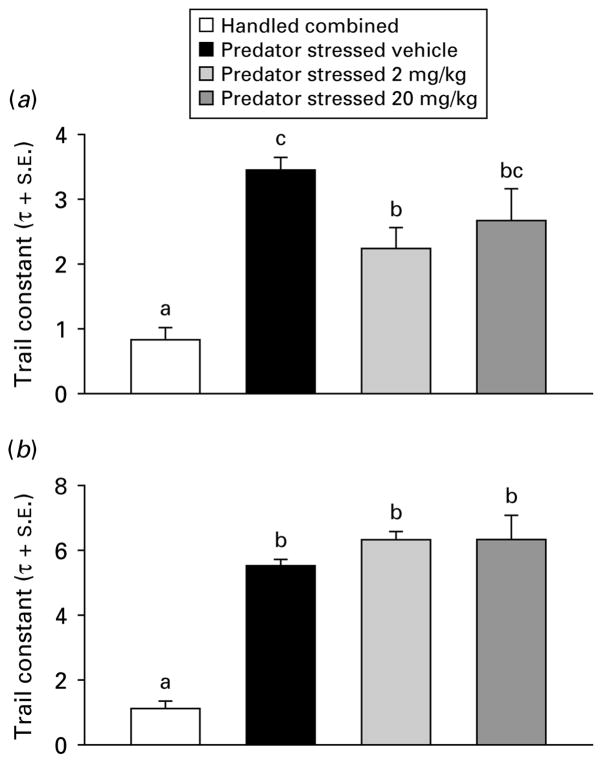
Due to non-normality in the startle data (Omnibus test >2601, p<0.0001), one-way analysis of medians (Kruskal–Wallis) were used for subsequent analyses. Handled treatment groups did not differ on any factors (all p>0.05), therefore they were combined into a single group (n=80). The PS groups were compared to the combined handled group across dose (0, 2, or 20 mg/kg) for time of injection (before or after) and ambience (light or dark) in separate dose-effect analyses. Injection before PS with CRA0450 (2 or 20 mg/kg) prevented the increase in startle seen in vehicle-injected stressed mice in the dark [χ2(3)=17.97, p<0.001] and light [χ2(3)=24.34, p<0.001] median contrasts (Kruskal–Wallis multiple z test, p<0.05; Fig. 2). In mice injected after treatment, the high dose (20 mg/kg) of CRA0450 prevented the increase in startle seen in vehicle-injected stressed mice in the dark [χ2(3)=27.37, p<0.001] and in the light [χ2(3)=39.90, p<0.001] median contrasts (Kruskal–Wallis multiple z test, p<0.05; Fig. 3). Effects of treatments on habituation of startle response were examined using the trial constant (τ) calculated on ratio peak startle amplitudes (all fits were good, d.f. adjusted r2 >0.89, all p<0.01). Stress-induced increases in τ (delays in habituation) returned to handled baseline levels only in mice injected before stress with a high dose of CRA0450 (20 mg/kg) [Fig. 4; startled in the light: t(18)=2.84, p<0.02; and the dark: t(18)=2.09, p<0.03). Stress also delayed habituation (increased τ) in mice injected with vehicle after PS [t(18)=14.05, p<0.001; Fig. 5]. CRA0450 following PS decreased τ at both doses to a level between handled mice and stressed mice given vehicle when startled in the dark [t(18)=3.13, p<0.01]. CRA0450 had no effect on light startle habituation in mice injected following PS (Fig. 5).
Fig. 2.
Median (+S.E.) ratio peak startle amplitudes in (a) dark and (b) light for groups injected prior to treatment. Groups are combined (given vehicle or drug, n=60) handled controls and predator-stressed mice given vehicle or CRA0450 (n=20/group). Medians with the same letter do not differ, means with different letters differ.
Fig. 3.
Median (+S.E.) ratio peak startle amplitudes in (a) dark and (b) light for groups injected after treatment. Groups are combined (given vehicle or drug, n=60) handled controls and predator-stressed mice given vehicle or CRA0450 (n=20/group). Medians with the same letter do not differ, means with different letters differ.
Fig. 4.
Trial constants (τ+S.E.) of startle habituation in (a) dark and (b) light derived from groups injected prior to treatment. Groups are combined (given vehicle or drug, n=60) handled controls and predator-stressed mice given vehicle or CRA0450 (n=20/group). Values of τ with the same letter do not differ, τ values with different letters differ.
Fig. 5.
Trial constants (τ+S.E.) of startle habituation in (a) dark and (b) light derived from groups injected after treatment. Groups are combined (given vehicle or drug, n=60) handled controls and predator-stressed mice given vehicle or CRA0450 (n=20/group). Values of τ with the same letter do not differ, τ values with different letters differ. Values of τ with two letters fall between and do not differ from τ values with single letter.
Light/dark box test
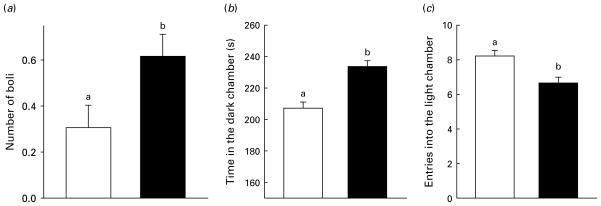
Three-way ANOVAs revealed only treatment effects and no dose or injection time effects in the light/dark box test. PS increased the time spent in the dark chamber, and reduced entries into the light chamber [F(1, 228)=12.93, p<0.001; Fig. 6]. The number of fecal boli were also significantly higher in cat-exposed mice [F(1, 228)=5.39, p<0.022; Fig. 6].
Fig. 6.
Mean+S.E.M. of number of (a) boli, (b) time in the dark chamber, and (c) entries into the light chamber as a function of treatment group in the light/dark box test. For a given plot, means with different letters differ. Data are collapsed across drug treatments to describe main effect of predator stress (□, handled control, n=120; ■, predator-stressed, n=120).
Hole-board test
There were no dose or injection-time effects on any behaviour in the hole-board test. Predator-stressed mice showed fewer head dips than handled controls [treatment effect: F(1, 228)=3.67, p<0.057, or t(228)=1.92, p<0.029, one-tailed test; means±S.E.M.; handled vs. stressed: 4.5±0.25 vs. 3.8+0.27].
EPM test
Relative time risk
There was a marginal treatment×dose interaction [F(2, 228)=2.75, p<0.066; data not shown]. PS reduced risk in vehicle-injected mice. Low and high drug doses, given either before or after treatment, returned relative time risk in PS mice to levels seen in handled mice given vehicle or 2 mg/kg CRA0450. However, handled mice given 20 mg/kg CRA0450 showed a reduced level of risk assessment compared to vehicle-treated handled controls [mean contrasts: all t(228)=2.35, p<0.02]. Of relevance to these findings, centre head dips, a measure of exploratory behaviour, were reduced in PS mice [F(1, 228)=4.39, p<0.038] and were normalized by the 20 mg/kg treatment [F(2, 228)=4.44, p<0.013; mean contrasts: Tukey–Kramer, p<0.05]. To determine if exploratory behaviour differences were contributing to the drug effects on risk assessment measures, a three-way ANCOVA for treatment, dose and injection time was performed, removing the effects of centre head dips, and hole-board head dips on relative time risk. The treatment×dose interaction was strengthened, and the pattern of mean contrasts remained the same [F(2, 226)=3.14, p<0.05; mean contrasts: all t(226)=2.35, p<0.02; data not shown].
Ratio time and entry
There were no effects of PS on these measures [all F(1, 228) and F(2, 228)<1.0, p>0.50; data not shown]. These ratios are typically reduced in PS C57 mice (Adamec et al. 2006c, d, 2008a), although not in stressed CFW mice (Adamec et al. 2004c). It is not clear what caused the discrepancy in ratio time and entry findings between this study and previous work with C57 mice. One possibility is an anxiogenic stress of injection effect in handled mice which had a lasting effect on decreasing ratio time and entry values to the range of PS mice. Support for this explanation follows. EPM ratio time and entry data from previous work in the Adamec laboratory (Adamec et al. 2006d) were compared to present data. Data compared were from male C57 mice of the same age, tested at the same time of day from the same supplier and treated in the same way as mice in the present study. The only exception was that mice in the previous study were not injected. Handled or PS mice (n=30/group) from the previous study were compared to handled or PS mice that were injected with vehicle before or after stress (n=40/group) in the present study. Comparisons were done with Kruskal–Wallis median tests due to deviations of data from normality (Omnibus tests>24.21, p<0.001). Median ratio time and entry of vehicle-injected handled mice in the present study were less than handled mice not injected in the previous study [all χ2(1)>6.45, p<0.02; 0.33 vs. 0.22 median ratio time and 0.33 vs. 0.24 median ratio entry for no injection vs. vehicle-injected mice, respectively]. In contrast, PS mice in the two studies did not differ on either measure [all χ2(1)<1.02, p>0.30].
Cat exposure
PS groups did not differ on any measure of cat or mouse behaviour [F(5, 119)≥2.15, p≥0.06; data not shown].
Discussion
This study examined the effects of CRA0450, a selective CRF type 1 receptor antagonist, in the PS model of PTSD. Cat exposure, an ecologically valid traumatic stress, produced lasting affective changes in mice. PS affected rodent anxiety across a number of behavioural tests, including acoustic startle, risk assessment in the EPM, and response to the light/dark box, consistent with previous studies (Adamec et al. 2004c, 2006c, d, 2008a, b). When administered before PS, CRA0450 significantly blocked baseline (dark) startle, startle under bright light and reversed stress-induced slowing of startle habituation. When administered after PS, these effects were less pronounced and only observed in the high-dose (20 mg/kg) group. CRA0450 treatment did not block PS-induced reductions in light/dark box exploration. PS did not affect open-arm exploration of the EPM but did reduce measures of risk assessment, which was reversed by CRA0450.
These data suggest that the strongest effects of CRA0450 were in blocking initiation and consolidation of stress effects on startle. With regard to initiation, CRA0450 injected prior to PS blocked stress enhancement of ratio peak startle amplitude and delay of startle habituation. Both doses were equally effective in blocking stress effects on startle amplitude, although only a high dose blocked stress effects on habituation. This difference may reflect differing mechanisms modulating stress effects on startle amplitude and startle habituation in mice, as has been suggested in the rat (Adamec et al. 2005a). The data also suggest more CRF1 occupancy and/or increased duration of occupancy is normally required to initiate stress-induced delay of habituation. Overall, these findings indicate an enabling role for CRF1 in mouse startle enhancement consistent with previous work showing that CRF1 antagonism or null mutation blocks acute startle potentiation produced by CRF or conditioned fear (Risbrough et al. 2003, 2004, 2009). Consolidation of stress effects on startle was more difficult to blockade. Higher doses of CRA0450 after stress were required to normalize startle amplitude, with marginal effects of either dose on startle habituation in the dark, or no effects on habituation in the light. The fact that only the high dose post-trauma was effective at blocking increased startle in dark and light and attenuating trauma-induced reduction in habituation may indicate that startle-related CRF mediated cell signalling, producing lasting change in startle was well underway during the stress, therefore receptor antagonism was less effective after receptor activation and/or the length of time required to most effectively block CRF1 during consolidation was not achieved with this one-time dosing design. Further studies are required to examine if more prolonged treatment with CRF1 antagonists after trauma can result in full reversal of all predator exposure effects. It is expected that occupancy was close to maximal at the high dose (>70% of forebrain and >60% of pituitary; Chaki et al. 2004). Another less likely possibility is receptor binding to other than CRF1 receptors is involved in higher dose effects on startle. At higher doses, CRA0450 may bind to σ1 opioid receptors, although little is known about their role in stress-induced modulation of startle (Chaki et al. 2004). It is also possible that CRF2 receptors contribute to the long-term behavioural adaptation post-stress. CRF2 plays a role in consolidation of stress effects (Bakshi et al. 2002; Henry et al. 2006; Todorovic et al. 2007); has an additive effect with CRF1 on stress and CRF-induced increases in startle, and may play a role in startle habituation (Risbrough et al. 2003, 2004, 2009). Given these data, studies examining the potential role of CRF2 receptors in PS effects on habituation also seem warranted.
CRA0450 given before or after stress did not alter light/dark box behaviour, suggesting CRF1 is not involved in initiation or consolidation of lasting stress effects on behaviour in this test. In contrast, CRF1 antagonists are reported to reduce baseline or immediate swim-stress-induced anxiety in the light/dark box test (Griebel et al. 1998; Okuyama et al. 1999). It is possible that CRF1 efficacy in this task depends on the stressor type. Alternatively, none of the previous studies examined impact of CRF1 antagonism on lasting effects of stress in the light/dark box test; taken together with the present data these findings suggest that CRF1 participates in acute but not long-term effects of stress on anxiety in this test.
There were no differences between treatment groups on behavioural measures of activity (rearing) in the hole-board test. Therefore, various effects on anxiety-like behaviours in the present study were not due to changes in activity. On the other hand, head dip measures of exploratory tendency in the hole-board (head dips) and EPM (center head dips) tests were lowered by PS. Such effects of PS on exploration are rare and as indicated in the results may represent additive effects of stress of injections and cat exposure. Nevertheless, these effects were independent of impact of stress and drug on other measures of anxiety in the EPM test. Risk assessment measures in the EPM were found to be reduced by PS, and this effect was reversed by CRA0450 administration; however, the interpretation of these effects is less clear without a PS effect on overall open-arm exploration. The lack of a PS effect on EPM measures in this cohort may be due to the relatively low exploration of open arms (e.g. anxiety baseline) of the handled control rats, as they exhibited only 20% time exploring the open arms, which may have resulted in a floor effect in which further reductions in anxiety are not measurable. As the EPM was the last test of the day previous exposure to the hole-board and light/dark box tests may have reduced exploratory motivation, thus increasing avoidance behaviours across all groups in the following EPM test. This high anxiety-like behaviour across groups may also have been due to injection stress as described in the results. The EPM has been shown to be a highly variable task both across and within laboratories (Crabbe et al. 1999), thus these small changes in methodological procedures may account for this lack of PS effects in this study.
The neural mechanism of CRF1 contributions to PS effects are unknown; however, there are some clues. We have shown that glutamate (NMDA receptor-dependent) signalling in the basolateral amygdala (BLA) is critical to the long-term effects of predator exposure on anxiety-like behaviour (Adamec et al. 1999a, b, 2005a; Blundell & Adamec, 2007; Blundell et al. 2005). In this context it is of interest that prolonged CRF receptor activation of the rodent BLA (over 5 d) has a lasting anxiogenic effect and also a lasting effect in increasing excitability of BLA cells (Rainnie et al. 2004; Shekhar et al. 2005) in a NMDA receptor-dependent manner (Rainnie et al. 2004). Moreover, CRF1 activation has a lasting effect in increasing the response of neurons in the BLA to afferent stimulation in vitro (Ugolini et al. 2008), an effect which could enhance neuroplastic change in BLA and fearfulness. Taken together, these data suggest that CRF1 activation during and after PS facilitates lasting changes in amygdala function, resulting in increased responding to stressful stimuli. CRF1 blockade was less effective after predator exposure on startle potentiation, suggesting that CRF1 activation in startle circuitry induces second-messenger cascades that become irreversible by CRF1 antagonism over time after stress. To identify alternate post-trauma treatment strategies, future studies should examine what second-messenger signals induced by CRF1 activation contribute to the lasting effects of predator exposure on anxiety. Studies of the effects of CRF and CRF1 actions in BLA implicate CAM kinase II (Rainnie et al. 2004) or PKC (Ugolini et al. 2008).
In conclusion, the present data indicate an enabling role for CRF1 activation in initiation and consolidation of long-term effects of PS on anxiety-like behaviours. From a clinical standpoint, these data support further study of CRF1 antagonists as potential prophylactic treatments to prevent lasting effects of severe stress on anxiety.
Acknowledgments
This work was supported by CIHR grants to Dr R. Adamec (ROP91548) and to Dr Risbrough (MH074697). We are grateful to Chris Muir, Waleed Abdel-Razek, and Lesley-Ann Stapleton for technical assistance, and Dr Shigeo Morimoto, Ph.D., Head of Medicinal Research Laboratories, Taisho Pharmaceutical Co., Ltd, Tokyo, Japan for the gift of CRA0450.
Footnotes
Statement of Interest
None.
References
- Adamec R. Transmitter systems involved in neural plasticity underlying increased anxiety and defense – implications for understanding anxiety following traumatic stress. Neuroscience and Biobehavioral Reviews. 1997;21:755–765. doi: 10.1016/s0149-7634(96)00055-3. [DOI] [PubMed] [Google Scholar]
- Adamec R, Bartoszyk GD, Burton P. Effects of systemic injections of Vilazodone, a selective serotonin reuptake inhibitor and serotonin 1A receptor agonist, on anxiety induced by predator stress in rats. European Journal of Pharmacology. 2004a;504:65–77. doi: 10.1016/j.ejphar.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Adamec R, Blundell J, Burton P. Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiology and Behavior. 2005a;86:75–91. doi: 10.1016/j.physbeh.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Adamec R, Blundell J, Strasser K, Burton P. Mechanisms of lasting change in anxiety induced by severe stress. In: Sato N, Pitman R, editors. PTSD: Brain Mechanisms and Clinical Implications. Tokyo: Springer-Verlag; 2006a. pp. 61–81. [Google Scholar]
- Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behavioural Brain Research. 2006c;170:126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Adamec R, Creamer K, Bartoszyk GD, Burton P. Prophylactic and therapeutic effects of acute systemic injections of EMD 281014, a selective serotonin 2A receptor antagonist on anxiety induced by predator stress in rats. European Journal of Pharmacology. 2004b;504:79–96. doi: 10.1016/j.ejphar.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiology and Behavior. 2006d;88:12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Adamec R, Head D, Soreq H, Blundell J. The role of the read through variant of acetylcholinesterase in anxiogenic effects of predator stress in mice. Behavioural Brain Research. 2008a;189:180–190. doi: 10.1016/j.bbr.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Adamec R, Holmes A, Blundell J. Vulnerability to lasting anxiogenic effects of brief exposure to predator stimuli: sex, serotonin and other factors – relevance to PTSD. Neuroscience and Biobehavioral Reviews. 2008b;32:1287–1292. doi: 10.1016/j.neubiorev.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec R, Kent P, Anisman H, Shallow T, Merali Z. Neural plasticity, neuropeptides and anxiety in animals – implications for understanding and treating affective disorder following traumatic stress in humans. Neuroscience and Biobehavioral Reviews. 1998;23:301–318. doi: 10.1016/s0149-7634(98)00032-3. [DOI] [PubMed] [Google Scholar]
- Adamec R, Muir C, Grimes M, Pearcey K. Involvement of noradrenergic and corticoid receptors in the consolidation of the lasting anxiogenic effects of predator stress. Behavioural Brain Research. 2007;179:192–207. doi: 10.1016/j.bbr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Adamec R, Strasser K, Blundell J, Burton P, McKay DW. Protein synthesis and the mechanisms of lasting change in anxiety induced by severe stress. Behavioural Brain Research. 2006b;167:270–286. doi: 10.1016/j.bbr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Adamec R, Walling S, Burton P. Long-lasting, selective, anxiogenic effects of feline predator stress in mice. Physiology and Behavior. 2004c;80:401–410. doi: 10.1016/j.physbeh.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Adamec RE, Blundell J, Burton P. Neural circuit changes mediating lasting brain and behavioral response to predator stress. Neuroscience and Biobehavioral Reviews. 2005b;29:1225–1241. doi: 10.1016/j.neubiorev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Adamec RE, Burton P, Shallow T, Budgell J. NMDA receptors mediate lasting increases in anxiety-like behavior produced by the stress of predator exposure – implications for anxiety associated with posttraumatic stress disorder. Physiology and Behavior. 1999a;65:723–737. doi: 10.1016/s0031-9384(98)00226-1. [DOI] [PubMed] [Google Scholar]
- Adamec RE, Burton P, Shallow T, Budgell J. Unilateral block of NMDA receptors in the amygdala prevents predator stress-induced lasting increases in anxiety-like behavior and unconditioned startle – effect on behavior depends on the hemisphere. Physiology and Behavior. 1999b;65:739–751. doi: 10.1016/s0031-9384(98)00225-x. [DOI] [PubMed] [Google Scholar]
- Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiology and Behavior. 1993;54:101–109. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Baker DG, West SA, Nicholson WE, Ekhator NN, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:585–588. doi: 10.1176/ajp.156.4.585. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. Journal of Neuroscience. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J, Adamec R. The NMDA receptor antagonist CPP blocks the effects of predator stress on pCREB in brain regions involved in fearful and anxious behavior. Brain Research. 2007;1136:59–76. doi: 10.1016/j.brainres.2006.09.078. [DOI] [PubMed] [Google Scholar]
- Blundell J, Adamec R, Burton P. Role of NMDA receptors in the syndrome of behavioral changes produced by predator stress. Physiology and Behavior. 2005;86:233–243. doi: 10.1016/j.physbeh.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, et al. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. European Journal of Pharmacology. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Matar MA, Loewenthal U, et al. Anisomycin, a protein synthesis inhibitor, disrupts traumatic memory consolidation and attenuates posttraumatic stress response in rats. Biological Psychiatry. 2006;60:767–776. doi: 10.1016/j.biopsych.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Matar MA, Zeev K, et al. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology. 2004;29:1962–1970. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, et al. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Research. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- File SE. The contribution of behavioural studies to the neuropharmacology of anxiety. Neuropharmacology. 1987;26:877–886. doi: 10.1016/0028-3908(87)90065-7. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ. Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154,526 in anxiety models in rodents. Comparison with diazepam and buspirone. Psychopharmacology (Berlin) 1998;138:55–66. doi: 10.1007/s002130050645. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink L, Pattij T, de JR, van der GJ, et al. 5-HT1A receptor knockout mice and mice overexpressing corticotropin-releasing hormone in models of anxiety. European Journal of Pharmacology. 2003;463:185–197. doi: 10.1016/s0014-2999(03)01281-0. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS and Neurological Disorders – Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb ALO, Zacharko RM, Dominguez H, Laforest S, et al. Changes in brain cholecystokinin and anxiety-like behavior following exposure of mice to predator odor. Neuroscience. 2003;116:539–551. doi: 10.1016/s0306-4522(02)00710-8. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. Journal of the American Medical Association. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. The role of early adverse life events in the etiology of depression and posttraumatic stress disorder – focus on corticotropin-releasing factor. Annals of the New York Academy of Sciences. 1997;821:194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. Journal of Neuroscience. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- Kaplan NM. The adrenal glands. In: Griffen JE, Ojeda SR, editors. Textbook of Endocrine Physiology. New York: Oxford University Press; 1992. pp. 247–275. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, et al. Posttraumatic stress disorder: a state-of-the-science review. Journal of Psychiatric Research. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Chaki S, Kawashima N, Suzuki Y, et al. Receptor binding, behavioral, and electrophysiological profiles of nonpeptide corticotropin-releasing factor subtype 1 receptor antagonists CRA1000 and CRA1001. Journal of Pharmacology and Experimental Therapeutics. 1999;289:926–935. [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic stress disorder. Psychiatric Clinics of North America. 2002;25:271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, et al. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. Journal of Neuroscience. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research – past, present, and future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, et al. CRF(1) and CRF(2) receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;36:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology. 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. Journal of Neuroscience. 2004;24:6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Hormones and Behavior. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, et al. Corticotropin-releasing factor in posttraurnatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biological Psychiatry. 2003;54:1382–1388. doi: 10.1016/s0006-3223(03)00571-7. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Silver RC, Holman EA, McIntosh DN, Poulin M, Gil-Rivas V. Nationwide longitudinal study of psychological responses to September 11. Journal of the American Medical Association. 2002;288:1235–1244. doi: 10.1001/jama.288.10.1235. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Stam R. PTSD and stress sensitisation: a tale of brain and body. Part 1: human studies. Neuroscience and Biobehavioral Reviews. 2007;31:530–557. doi: 10.1016/j.neubiorev.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Steckler T, Dautzenberg FM. Corticotropin-releasing factor receptor antagonists in affective disorders and drug dependence – an update. CNS and Neurological Disorders – Drug Targets. 2006;5:147–165. doi: 10.2174/187152706776359619. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. Journal of Neuroscience. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nature Genetics. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Todorovic C, Radulovic J, Jahn O, Radulovic M, et al. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. European Journal of Neuroscience. 2007;25:3385–3397. doi: 10.1111/j.1460-9568.2007.05592.x. [DOI] [PubMed] [Google Scholar]
- Ugolini A, Sokal DM, Arban R, Large CH. CRF1 receptor activation increases the response of neurons in the basolateral nucleus of the amygdala to afferent stimulation. Frontiers in Behavioral Neuroscience. 2008;2:2. doi: 10.3389/neuro.08.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. New England Journal of Medicine. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Conrad CD, Fleshner M, Diamond DM. Acute episodes of predator exposure in conjunction with chronic social instability as an animal model of post-traumatic stress disorder. Stress. 2008;11:259–281. doi: 10.1080/10253890701768613. [DOI] [PMC free article] [PubMed] [Google Scholar]