Abstract
Opitz and Kaveggia [1974] reported on a family of five affected males with distinctive facial appearance, mental retardation, macrocephaly, imperforate anus and hypotonia. Risheg et al. [2007] identified an identical mutation (p.R961W) in MED12 in six families with Opitz-Kaveggia syndrome, including a surviving affected man from the family reported in 1974. The previously defined behavior phenotype of hyperactivity, affability, and excessive talkativeness is very frequent in young boys with this mutation, along with socially oriented, attention-seeking behaviors. We present case studies of two older males with FG syndrome and the p.R961W mutation to illustrate how their behavior changes with age. We also characterize the behavior of eight additional individuals with FG syndrome and this recurrent mutation in MED12 using the Vineland Adaptive Behavior Scales 2nd ed., the Reiss Profile of Fundamental Goals and Motivation Sensitivities, and the Achenbach Child Behavior Checklist. Males with this MED12 mutation had deficits in communication skills compared to their socialization and daily living skills. In addition, they were at increased risk for maladaptive behavior, with a propensity towards aggression, anxiety, and inattention. Based on the behavior phenotype in 10 males with this recurrent MED12 mutation, we offer specific recommendations and interventional strategies. Our findings reinforce the importance of testing for the p.R961W MED12 mutation in males who are suspected of having developmental and behavioral problems with a clinical phenotype that is consistent with FG syndrome.
Keywords: FG Syndrome, Opitz-Kaveggia Syndrome, FGS1, Behavior Phenotype, X-Linked Mental Retardation, Cognitive Disability
INTRODUCTION
Distinctive behavior has become a useful delineating manifestation of many clinical syndromes. FG syndrome (Opitz-Kaveggia syndrome, OMIM 305450) was delineated by Opitz and Kaveggia in 1974 based on the clinical findings in three brothers and two of their male first cousins. In this first family, FG syndrome was defined as a multiple congenital anomaly syndrome characterized by relatively large head, broad and flat thumbs, imperforate anus, hypotonia and moderately severe mental retardation [Opitz and Kaveggia, 1974]. Facial characteristics included a prominent forehead, upswept frontal hairline, down-slanting palpebral fissures, ocular hypertelorism, and small prominent ears with a simplified helical pattern. The corpus callosum was incompletely developed or absent altogether, and there were occasional EEG abnormalities. Associated defects included anal stenosis or other anomalies of the intestinal tract, heart defects, craniosynostosis, and hernias, and this combination of defects was lethal during early childhood in some affected males. Skeletal manifestations included stature in the lower range of normal, broad and flat thumbs and halluces, partial syndactyly, pectus excavatum, joint contractures, and spinal curvature. Surviving males had congenital hypotonia with constipation, and during early childhood they were friendly, inquisitive, and hyperactive with a very short attention span. One older male was noted to have temper tantrums with attacks of screaming and aggressive or self-abusive behaviors requiring medication with tranquilizers [Opitz and Kaveggia, 1974].
A second family with several affected males was reported by Keller et al. [1976], who commented on the distinctive affable,outgoing personality in the surviving males. Since these initial reports, there occurred a remarkable expansion of the range of clinical manifestations associated with this X-linked mental retardation condition, which included core manifestations of hypotonia, constipation, and short stature with relative macrocephaly, which are relatively common manifestations among persons with cognitive disability.
In 2007, Risheg et al. identified an identical nucleotide substitution (c.2881C>T) in exon 21 of MED12 (p.R961W) in six families with Opitz-Kaveggia syndrome, including a surviving affected male from the original Opitz-Kaveggia family. It was difficult to confirm this diagnosis in individuals with the more generalized phenotype prior to the discovery of the p.R961W mutation in MED12 [Lyons et al., 2008]., and many previously reported patients with FG syndrome lack the specific syndrome of features associated with the p.R961W mutation in MED12. Only the following reports can be confirmed to have the p.R961W mutation and clinical signs consistent with FG (Opitz-Kaveggia) syndrome: Opitz and Kaveggia [1974]; Keller et al., [1976]; Riccardi et al., Family 1 [1977]; McCardle and Wilson [1993]; and Graham et al., Family 1 and Family 3 [1998]. In the report by Graham et al. [1998], Family 2 had a mutation in the X-linked gene, UPF3B [Tarpey et al., 2007].
Since both previous reports of the behavior phenotype in FG syndrome included patients without the p.R961W mutation in exon 21 of MED12 [Graham et al., 1999; Ozonoff et al., 2000], this study further delineates the specific behavior phenotype in eight previously reported males with FG syndrome and this mutation in MED12 [Risheg et al., 2007; Lyons et al., 2008], plus the two additional cases reported herein. Based on our results, specific recommendations are provided for anticipatory guidance and treatment strategies.
CLINICAL STUDIES
Patient 1 was noted to have decreased fetal movement, and he was delivered at term by cesarean because of breech presentation weighing 3430 g and measuring 51 cm in length. He was born with severe anal stenosis, which was repaired shortly after birth, followed by anal dilatation and repeat rectal surgery at age 5 years. He had generalized hypotonia, poor suck, feeding problems, tracheomalacia, congenital heart defects (ventricular septal defect with anomalous right ventricular muscle bundle, infundibular pulmonary stenosis, and right bundle branch block), relative macrocephaly, small ears, broad high forehead, flat nasal bridge, down-slanted palpebral fissures, ocular hypertelorism, ptosis (Fig. 1), broad thumbs and halluces, and sacral dimple. A maternal uncle of his mother died at age 6 years with a similar facial appearance, generalized hypotonia, anal and cardiac defects, and an outgoing personality with a quick temper. A second affected brother died shortly after birth. For this reason, an X-linked recessive disorder was suspected.
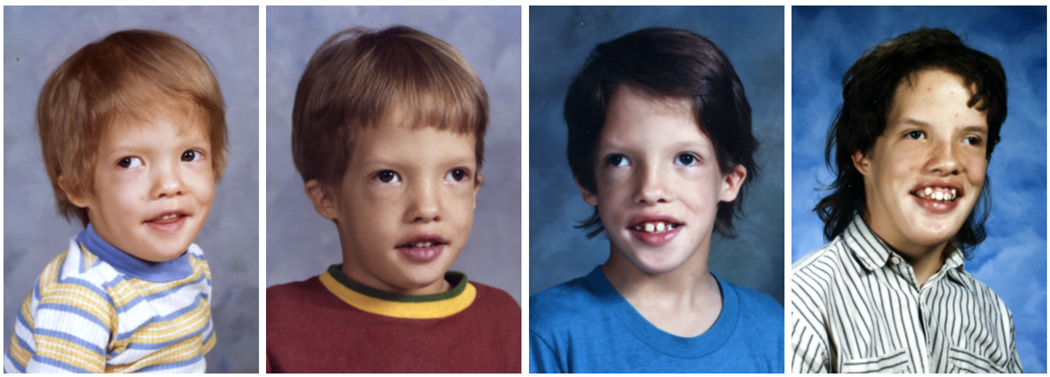
Figure 1.
Patient 1 at ages 3 years, 5 years, 13 years and 15 years demonstrating characteristic facial features of relative macrocephaly, small ears, broad high forehead, flat nasal bridge, downslanted palpebral fissures, ocular hypertelorism, and ptosis.
He had chronic constipation and recurrent impaction treated with laxatives and manual disimpactions. As an adult, he developed rectal prolapse and hemorroids. Persistent respiratory infections required frequent hospitalizations due to tracheomalacia, and small ear canals had frequent wax obstruction. He had persistent hypotonia and drooling, and global developmental delay with better receptive language skills than expressive skills. A right optic and retinal coloboma was diagnosed at 18 months during an evaluation for alternating esotropia. His cardiac defects were treated initially with digoxin and then surgically repaired at age 3 years. Subluxing patellae resulted in repeated falls, treated initially with braces and finally by surgery at age 17.
He had moderate cognitive disability (full-scale IQ 49, verbal IQ 55, performance IQ 51) and required special education. Brain MRI showed agenesis of corpus callosum. He was diagnosed with attention deficit hyperactivity disorder (ADHD) in early childhood and with Tourette syndrome at age 10 years. He was easily frustrated and had extensive temper outbursts that lasted for hours until he exhausted himself. He manifested verbal and motor tics, was extremely impulsive, and had to be watched constantly for his own protection. He also developed unusual habits and rituals (e.g. licking his hands and rubbing saliva on his face, his stomach and the bottoms of his feet). He was treated with methylphenidate hydrochloride for ADHD, which did not stop his tics, impulsiveness or ritualistic behaviors at home, but teachers felt he was more attentive with fewer outbursts at school.
Methylphenidate hydrochloride was subsequently discontinued, and although he was still impulsive and hyperactive, he became calmer and happier. He had persistent problems with drooling and constipation, and at age 11 years, his behavior deteriorated, and he became extremely obsessive and compulsive, with almost constant verbal and motor tics and severe temper outbursts. He was treated with haloperidol, which reduced his tics and resulted in calmer behavior until early adolescence, but he remained extremely impulsive. When he reached maximal doses of haloperidol, fluoxetine hydrochloride was added, but his behaviors worsened, and he became suicidal and obsessed with knives, requiring an inpatient psychiatric admission to wean him off all medications.
He was placed in various group home settings, where he was closely supervised over the next few years, meeting regularly with his team of teachers, group home staff and specialists in behavior management. Success was attributed to consistency, understanding, and mutual trust between the patient and his care-providers. At age 16, shortly after the death of his father, he had a serious incident when he threatened the staff at his group home with a knife, resulting in his discharge and admission to an adult psychiatric ward for three weeks. He returned home to live with his mother. As he became more able to self-monitor his behaviors and learned some coping skills through counseling, his temper outburst became less frequent. His most difficult issues were with obsessive compulsive disorder (OCD), which was treated with pimozide. Propranolol was also prescribed for a time, but he began to gain weight and to eat obsessively, so this was discontinued.
He graduated from high school at age 20, and went on to work with Good Will Industries, where he began the transition from school to work in a supported living environment in one side of a duplex he shared with his mother. Even though he continued to have seasonal challenging and difficult behaviors with temper outbursts, they became less frequent, and he seemed to thrive and mature in this new setting. He had difficulty with uncontrollable drooling his entire life, and at age 20 he began taking benztropene, which was effective in controlling his constant drooling. His tics resolved, but he continued to manifest OCD and remained on pimozide. He was generally self-controlled with use of medications and a one-on-one staffing program that met his needs through a very strict daily routine.
He was diagnosed with FG syndrome by Dr. James Hanson of the University of Iowa at age 16, and Dr. John M. Opitz confirmed this diagnosis at age 22, when he was 164 cm tall (3rd–5th centile), weighed 81 kg (75th centile), and had a head circumference (OFC) of 63 cm (>97th centile). As an adult, his most difficult issue continued to be OCD, so paroxetine hydrochloride was added to his treatment with pimozide. Within a few weeks of starting paroxetine hydrochloride, he lost the ability to control his behavior and developed more tics, so paroxetine hydrochloride was increased. He was weaned off pimozide, which was replaced with risperidone, but he reverted back to his previous behavioral problems while he was on haloperidol and fluoxetine hydrochloride. His tics became constant, so paroxetine hydrochloride was discontinued and risperidone was increased, which resulted in an excellent behavioral response. He tolerated a move to a new city, where he was able to make friends and participate in community programs. He has continued to prosper under the guidance of a psychologist at age 32, and he remains on risperidone as a mood stabilizer.
Patient 2 was born at term weighing 3289 g, measuring 51 cm in length with OFC of 34 cm. Apgar scores were 4 and 8. After birth, he was intubated and a tracheal plug was removed. He was initially diagnosed with severe anal stenosis, which was corrected with dilatation and anoplasty. As a neonate, he required surgery for pyloric stenosis. His heart was dextropositioned with a patent ductus arteriosus, ventricular septal defect, and atrial septal defect, which all resolved spontaneously. His thumbs were broad with duplication of the metacarpal and the proximal phalanx on the left side, and radiographs documented 13 ribs on the right side with a vertebral segmentation abnormality at T9 and T10. He had a high, broad forehead, large head size, upsweep of the frontal hair; high, narrow palate, and absent tear ducts, which required surgical correction at age 3 years.
During infancy, he developed seizures, beginning as absence or petit mal seizures, with development of grand mal seizures around age 2 years. These occurred primarily during sleep, with EEG abnormalities recorded on multiple occasions. An MRI showed cavum septi pellucidi and cavum vergae with a hypoplastic corpus callosum, dilated lateral and fourth ventricles, and mild cerebellar vermis hypoplasia. At times he had periodic absence episodes, and his seizures appeared to resolve in later childhood. Throughout his life, he has had persistent sleep abnormalities consisting of not sleeping through the night and getting up during the night. Exotropia was repaired at age 6 years.
He manifested global developmental delay and IQ testing documented a full-scale IQ of 49, with performance IQ 53 and verbal IQ 54, suggesting moderate cognitive disability at age 19 years, with communication skills at an age equivalent of 3 years 10 months, daily living skills at age equivalent 3–10, community and domestic skills at age equivalent 4–1, socialization skills at age equivalent 7–11, and interpersonal relationship skills at age equivalent 6–8. It was felt that some of his limitations in daily living skills were due to having been placed in a structured residential school setting at age 12 years, where many daily living tasks were completed by staff. He had mild sensorineural hearing loss bilaterally, which did not require hearing aids, and his hearing was adequate for daily functioning. He has had a life-long problem with chronic constipation, requiring manual disimpaction and laxatives.
His frequent urination has been attributed to a possible side effect of lithium. As a teen-ager he had a major psychiatric episode during which time he had excessive tantrums or manic behavior with aggression. He was hospitalized, and quite a number of medications were tried to help control his manic behavior at that time. Behavioral measures documented poor self-esteem and excessive anxiety, impulsivity and depression, especially during times of stress. Currently he is on citalopram, desmopressin, valproate, lithium, and lorazepam. These medications seem to have provided distinct improvement in his mood.
His adult height is at the 10th–25th centile with head circumference at the 90th centile. His ears are small (less than 3rd centile) with deficient lower helices, a frontal upsweep, a tall, narrow frontal forehead, fullness in the malar areas and prognathism (Fig, 2). He manifests a constant tongue-thrust with speech, persistent drooling, high palate and external strabismus of the left eye. He has pectus excavatum with broad, flat thumbs, particularly distally, and the nails tend to adhere distally to the soft tissues of the thumbs. The great toes are wide, and the nails are flat and adhere distally. Neurologically, his muscle tone in the limbs appears good, but he has some contracture at the elbows with out-toeing on ambulation. Reflexes are normal (2+ in the lower limbs and 1+ in the upper limbs). The diagnosis of FG syndrome was made by Dr. Elaine Zackai of Children’s Hospital of Philadelphia at age 7 and confirmed by Dr. John M. Opitz at age 15.
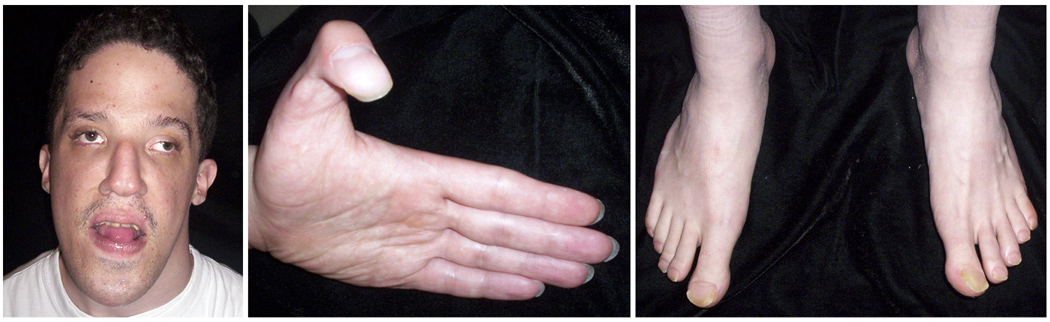
Figure 2.
Patient 2 at age 24 years demonstrating relative macrocephaly (90th centile) with small ears (<3rd centile), tall narrow forehead, down-slanted palpebral fissures, ocular hypertelorism, ptosis, and broad flat thumbs and halluces.
METHODS
Subjects with FG syndrome were ascertained through our previous research study [Risheg et al., 2007] and the national FG Syndrome Family Alliance support group. After review and approval of our study protocol by the Greenwood Genetic Center Institutional Review Board, interested families were recruited through support group mailings, as well as from referrals through university-based geneticists. Participant clinical histories and molecular test results were reviewed to confirm their diagnosis. Mean age of 10 males with the p.R961W MED12 mutation was 28.58 years (range 7.33–62.08).
Parents and/or caretakers were invited to participate in a study on the behavioral and personality aspects of individuals with FG syndrome. Parents and/or caretakers completed the Vineland Adaptive Behavior Scales 2nd Edition-Parent/Caregiver Rating Form [Sparrow et al., 2005]. This measure assesses three domains of adaptive functioning: communication, daily living skills, and socialization.
We also used the Child Behavior Checklist [CBCL; Achenbach, 1991], which asks parents or caretakers to rate 112 problem behaviors on a three-point scale: (0) not true; (1) sometime true; (2) often true. The CBCL consists of an internalizing domain (withdrawn, anxious/depressed and somatic complaints), externalizing domain (aggressive behavior and delinquent behavior), and three other sub domains (social problems, thought problems, and attention problems) that sum for a total score. In this study, total CBCL T-scores were examined to assess clinical severity. The reliability and validity of this measure have been well established in persons with mental retardation.
The Reiss Personality Profiles of Fundamental Goals and Motivation Sensitivities for Persons with Mental Retardation [Reiss and Havercamp, 1998] consist of 100 statements that are rated by an informant on a 5-point scale (strongly disagree to strongly agree). This instrument assesses personality-motivation using normative groups of people with or without mental retardation. The Reiss Profiles differ from other available instruments for people with mental retardation since they do not measure maladaptive behavior or psychopathology but instead assess motivational strengths and styles.
RESULTS
Results from the Vineland Adaptive Behavior Scales, 2nd Edition (Vineland-II) are shown in Table I. On the Vineland-II, males with the recurrent MED12 mutation had great deficits in areas of communication, daily living skills, and socialization, with communication being the most impaired.
Table I.
Vineland Adaptive Behavior Scales, 2nd edition, in males with FG syndrome and the p.R961W MED12 mutation: Mean and Range
| FG Syndrome (n = 10) |
||
|---|---|---|
| Mean | Range | |
| Communication | 33.3 | 20.0–80.0 |
| Daily Living Skills | 37.6 | 20.0–65.0 |
| Socialization | 37.9 | 20.0–61.0 |
Results from the CBCL are shown in Table II. The CBCL total domain raw scores are converted to T-scores. Clinically significant T-scores are those above 64, as established by Achenbach [1991] using large epidemiological samples of children with and without identified problems. Males with the p.R961W MED12 mutation had higher scores for social, thought, attention, aggression, and anxiety domains, with the total CBCL scores exceeding the clinical cutoff score of 64.
Table II.
Child Behavior Checklist T-scores in males with FG syndrome and the p.R961W MED12 mutation; Mean and Range
| FG Syndrome (n = 10) |
||
|---|---|---|
| Domains | Mean | Range |
| Internalizing | 62 | 50–75 |
| Externalizing | 59 | 40–73 |
| Total | 66* | 60–75 |
| Subdomains | ||
| Anxious/depressed | 64* | 54–81 |
| Withdrawn | 57 | 50–68 |
| Social problems | 68* | 58–77 |
| Thought problems | 66* | 51–74 |
| Attention problems | 65* | 57–71 |
| Rule-breaking behavior | 56 | 50–68 |
| Aggressive behavior | 64* | 50–86 |
Domains at or exceeding the clinical cut-point of 64
On the Reiss Personality Profiles (Table III), males with the p.R961W MED12 mutation demonstrated high scores in the following domains: frustration, attention, social, and rejection.
Table III.
Reiss Personality Profile Domains; Mean and Range
| FG Syndrome (n = 10) |
||
|---|---|---|
| Mean | Range | |
| Activity | 15.7 | 7.0–24.0 |
| Anxiety | 14.7 | 7.0–22.0 |
| Attention | 33.3 | 11.0–82.0 |
| Curiosity | 25.2 | 17.0–34.0 |
| Food | 19.1 | 14.0–34.0 |
| Frustration | 27.2 | 17.0–32.0 |
| Help Others | 24.9 | 14.0–32.0 |
| Independence | 15.6 | 9.0–25.0 |
| Order | 19.0 | 9.0–22.0 |
| Pain | 13.1 | 6.0–20.0 |
| Rejection | 28.4 | 19.0–38.0 |
| Sex | 8.6 | 5.0–13.0 |
| Social | 27.6 | 17.0–34.0 |
| Vengeance | 18.1 | 13.0–29.0 |
| Morality | 12.3 | 5.0–19.0 |
Of note, this is a cross-sectional study examining adaptive and maladaptive functioning of males with the MED12 mutation, and it does not assess how their functioning changes across age groups.
DISCUSSION
We have documented a consistent behavior phenotype in 10 males with FG syndrome and the p.R961W in MED12. It is important to consider testing for FG syndrome in males who present with developmental delay and/or a behavioral phenotype of hyperactivity, affability, and excessive talkativeness along with the clinical features of FG syndrome [Graham et al., 1999; Risheg et al., 2007; Lyons et al., 2008]. Males with this recurrent p.R961W mutation in MED12 have relative strengths in socialization and daily living skills, despite their communicative deficits (Table I). Indeed, the strengths in the socialization skills of males with the p.R961W MED12 mutation may mask their communicative deficits. Although parents may report that boys with FG syndrome are social and talk excessively, they may have problems in articulation, pragmatic use, syntax, and intonation, which may be further complicated by their low facial muscle tone and oral-motor discoordination. These language deficits may lead to secondary behavioral problems and inability to perform routine daily living skills, such as, toilet training, feeding, or dressing. Furthermore, language deficit is the greatest barrier to independent living and integration with the community as indicated in a previous study of individuals with Down syndrome [Chapman and Hesketh, 2000].
Furthermore, based on the Reiss Personality Profiles, males with the p.R961W MED12 mutation have increased frustration and attention problems, which may also be attributed to their poor expressive language skills and multiple medical issues (e.g. chronic constipation as indicated in Cases 1 and 2). Thus, early and ongoing language interventional therapy is essential in all males with FG syndrome. Language therapy should also include oral-motor exercises to improve tone, strength, and coordination. Total communication strategies combining non-verbal (e.g., sign language and augmented communication devices) and verbal skills should be considered.
Males with the MED12 mutation are vulnerable to internalizing problem behaviors, especially in the anxiety and inattention domains (Table II). We hypothesize that males with the MED12 mutation are at increased risk for attention and anxiety problems because they have major deficits in communication skills, similar to what is seen in other syndromes with these difficulties [Graham et al., 2005; Visootsak et al., 2007]. Deficits in communication skills, specifically within the expressive communication domain, often lead to frustration, tantrums, and isolation, as documented in Patient 1. Inattention and social deficits appear to be heightened by changes in routines and transitions.
Based on these findings, it is important to provide a highly structured environment for males with the p.R961W MED12 mutation, since they prefer routines. Providing adequate warnings of transitions and changes in their routines is important to lessen anxiety and maladaptive behavior. Use of a calendar system may be of benefit to help them anticipate changes in the future. Positive behavioral attributes include an interest in helping others and in socializing with their peers; however, their socialization may be inappropriate and immature for their age. For these reasons, it is important to provide prompting to encourage interaction with peers, including opportunities to imitate and model appropriate behavior. Exposure to typically developing peers may be especially helpful in achieving these goals.
Behavioral assessment and intervention should be offered for males with the MED12 mutation. Because there are currently no studies on the effectiveness of behavioral treatments in these males, behavior therapy should be individualized and designed to meet the individual’s specific needs. Behavior challenges may also wax and wane with increasing age; thus, therapy should be adapted to these changes. For example, hyperactivity may subside during adulthood with withdrawal and anxiety becoming more prominent. Parents should also be instructed on how to implement behavior therapy at home. Principles of functional behavior analysis and the Antecedent-Behavior-Consequence model may be useful, since they are based on how maladaptive behaviors are maintained and rewarded, and how they can be replaced by positive reinforcement of appropriate behaviors.
Psychopharmacologic intervention should be combined with other interventional strategies, including speech therapy, sensory integration therapy, an individualized educational plan, and tailored behavioral treatments to enhance developmental outcome and minimize maladaptive behaviors. Presently, there is no psychopharmacologic research specific for individuals with FG syndrome. Based on the results of our data, internalizing maladaptive behavior problems, especially in the attention and anxiety domains, are typically noted in males with the p.R961W MED12 mutation. For these reasons, an evaluation of Attention Deficit Hyperactivity Disorder (ADHD) may be needed. Stimulants are helpful in targeting symptoms of hyperactivity, impulsivity, and distractibility. Their efficacy and side effects vary for each individual, and the response rate to stimulants may be lowered in adult males with the p.R961W MED12 mutation because of their decreased activity level (Case 1). Selective serotonin reuptake inhibitors (SSRIs) should be considered to treat mood disorder and anxiety. Atypical antipsychotics (e.g., respiridone) have been used to treat self-injury, aggressive behaviors, and autism. Adverse side effects associated with antipsychotics include weight gain, sedation, nausea, constipation, diabetes, and tardive dyskinesia. Individuals with hyperarousal to sensory stimuli can be treated with α2-adrenergic agonists (e.g. clonidine), which are thought to dampen sensory input to the brain and have shown good efficacy in decreasing these behaviors in boys with Fragile X syndrome [Berry-Kravis, 2004].
Overall, males with the p.R961W MED12 mutation are lower functioning in their communication domain compared to their socialization and daily living skills, and they are at higher risk for attentional and anxiety problems. These deficits appear to persist into adulthood based on our results. Hence, it is important to offer anticipatory guidance throughout childhood and adulthood with sufficient parental, educational, and social support. Therapeutic interventions should include physical therapy, occupational therapy, language therapy and behavioral modification. An individualized educational plan with appropriate resources (e.g., behavioral therapy, adaptive physical education) is essential in ensuring that the individual with FG syndrome continues to make progress. The FG Syndrome Family Alliance (www.fg-syndrome.org) is also an excellent resource and support for families.
ACKNOWLEDGMENTS
We appreciate support from SHARE's Childhood Disability Center, the Steven Spielberg Pediatric Research Center, the NIH/NICHD Program Project Grant (HD22657), and the Medical Genetics NIH/NIGMS Training Program Grant (5-T32-GM08243). We also appreciate assistance from the Cedars-Sinai General Clinical Research Center Grant (M01-RR00425) for samples collected under CSMC IRB Protocols 0463 and 4232. Supported by a NIH grant (HD26202) to CES and in part by a grant from the South Carolina Department of Disabilities and Special Needs (SCDDSN). NIH/NCRR 1KL2RR025009, National Down Syndrome Society, Emory Egleston Children’s Research Center, NIH LRP(JV).
REFERENCES
- Achenbach TM. Manual for the child behavior checklist/4–18 and 1991 profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Berry-Kravis E, Potanos K. Psychopharmacology in fragile X syndrome--present and future. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:42–48. doi: 10.1002/mrdd.20007. [DOI] [PubMed] [Google Scholar]
- Briault S, Hill R, Shrimpton A, Zhu D, Till M, Ronce N, Margaritte-Jeannin P, Baraitser M, Middleton-Price H, Malcolm S, Thompson E, Hoo J, Wilson G, Romano C, Guichet A, Pembrey M, Fontes M, Poustka A, Moraine C. A gene for FG syndrome maps in the Xq12-q21.31 region. Am J Med Genet. 1997;73:87–90. [PubMed] [Google Scholar]
- Briault S, Odent S, Lucas J, Le Merrer M, Turleau C, Munnich A, Moraine C. Paracentric inversion of the X chromosome [inv(X)(q12q28)] in familial FG syndrome. A. J Med Genet. 1999;86:112–114. doi: 10.1002/(sici)1096-8628(19990910)86:2<112::aid-ajmg4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Briault S, Villard L, Rogner U, Coy J, Odent S, Lucas J, Passage E, Zhu D, Shrimpton A, Pembrey M, Till M, Guichet A, Dessay S, Fontes M, Poustka A, Moraine C. Mapping of X chromosome inversion breakpoints [inv(X)(q11q28)] associated with FG syndrome: a second FG locus [FGS2]? Am J Med Genet. 2000;95:178–181. doi: 10.1002/1096-8628(20001113)95:2<178::aid-ajmg17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Chapman R, Hesketh L. Behavioral phenotype of individuals with DS. Ment Retard Dev Dis Res Rev. 2000;6:84–95. doi: 10.1002/1098-2779(2000)6:2<84::AID-MRDD2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Dessay S, Moizard MP, Gilardi JL, Opitz JM, Middleton-Price H, Pembrey M, Moraine C, Briault S. FG syndrome: linkage analysis in two families supporting a new gene localization at Xp22.3 (FGS3) Am J Med Genet. 2002;112:6–11. doi: 10.1002/ajmg.10546. [DOI] [PubMed] [Google Scholar]
- Graham JM, Jr, Rosner B, Dykens E, Visootsak J. Behavioral features of CHARGE syndrome (Hall-Hittner syndrome): comparison with Down syndrome, Prader-Willi syndrome and Williams syndrome. Am J Med Genet Part A. 2005;133A:240–247. doi: 10.1002/ajmg.a.30543. [DOI] [PubMed] [Google Scholar]
- Graham JM, Jr, Superneau D, Rogers RC, Corning K, Schwartz CE, Dykens EM. Clinical and behavioral characteristics in FG syndrome. Am J Med Genet. 1999;85:470–475. [PubMed] [Google Scholar]
- Graham JM, Jr, Tackels D, Dibbern K, Superneau D, Rogers C, Corning K, Schwartz CE. FG syndrome: report of three new families with linkage to Xq12-q22.1. Am J Med Genet. 1998;80:145–156. [PubMed] [Google Scholar]
- Keller MA, Jones KL, Nyhan W, Francke U, Dixson B. A new syndrome of mental deficiency with craniofacial, limb, and anal abnormalities. J Pediat. 1976;88:589–591. doi: 10.1016/s0022-3476(76)80012-1. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Graham JM, Jr, Neri G, Hunter AGW, Clark RD, Rogers RC, Moscada M, Simensen R, Dodd J, Dupont B, Friez MJ, Schwartz CE, Stevenson RE. Clinical experience in the evaluation of 30 patients with a prior diagnosis of FG syndrome. J Med Genet. 2008 doi: 10.1136/jmg.2008.060509. in press. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Williams BJ, Rauch AM, Opitz JM. Behavior phenotype of FG syndrome: cognition, personality, and behavior in eleven affected boys. Am J Med Genet (Semin Med Genet) 2000;97:112–118. doi: 10.1002/1096-8628(200022)97:2<112::aid-ajmg2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Opitz JM, Kaveggia EG. The FG syndrome: an X-linked recessive syndrome of multiple congenital anomalies and mental retardation. Z Kinderheilk. 1974;117:1–18. doi: 10.1007/BF00439020. [DOI] [PubMed] [Google Scholar]
- Opitz JM, Kaveggia EG, Adkins WN, Jr, Gilbert EF, Viseskul C, Pettersen JC, Blumberg B. Studies of malformation syndromes of humans. XXXIIIC: The FG syndrome--further studies on three affected individuals from the FG family. Am J Med Genet. 1982;12:147–154. doi: 10.1002/ajmg.1320120205. [DOI] [PubMed] [Google Scholar]
- Reiss S, Havercamp SM. Toward a comprehensive assessment of fundamental motivation: factor structure of the Reiss profiles. Psychol Assess. 1998;10:97–106. [Google Scholar]
- Riccardi VM, Hassler E, Lubinsky MS. The FG syndrome: further characterization, report of a third family, and of a sporadic case. Am J Med Genet. 1977;1:47–58. doi: 10.1002/ajmg.1320010106. [DOI] [PubMed] [Google Scholar]
- Risheg H, Graham JM, Jr, Clark RD, Rodgers RC, Opitz JM, Moeschler JB, Pfeiffer AP, May M, Joseph SM, Jones JR, Stevenson RE, Schwartz CE, Friez MJ. A recurrent mutation in MED12 leading to R961W causes Opitz-Kaveggia syndrome. Nature Genetics. 2007;39:451–453. doi: 10.1038/ng1992. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. Vineland adaptive behavior scales- 2nd Edition. Survey Interview Form/Caretaker Rating Form. Livonia, MN: Pearson Assessments; 2005. [Google Scholar]
- Tarpey PS, Lucy Raymond F, Nguyen LS, Rodriguez J, Hackett A, Vandeleur L, Smith R, Shoubridge C, Edkins S, Stevens C, O'meara S, Tofts C, Barthorpe S, Buck G, Cole J, Halliday K, Hills K, Jones D, Mironenko T, Perry J, Varian J, West S, Widaa S, Teague J, Dicks E, Butler A, Menzies A, Richardson D, Jenkinson A, Shepherd R, Raine K, Moon J, Luo Y, Parnau J, Bhat SS, Gardner A, Corbett M, Brooks D, Thomas P, Parkinson-Lawrence E, Porteous ME, Warner JP, Sanderson T, Pearson P, Simensen RJ, Skinner C, Hoganson G, Superneau D, Wooster R, Bobrow M, Turner G, Stevenson RE, Schwartz CE, Andrew Futreal P, Srivastava AK, Stratton MR, Gecz J. Mutations in UPF3B, a member of the nonsense-mediated mRNA decay complex, cause syndromic and nonsyndromic mental retardation. Nat Genet. 2007;39:1127–1133. doi: 10.1038/ng2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visootsak J, Rosner B, Dykens E, Tartaglia N, Graham JM., Jr Behavioral phenotype of sex chromosome anomalies: 48,XXYY, compared to 48,XXXY and 49, XXXXY. Am J Med Genet Part A. 2007;143A:1198–1203. doi: 10.1002/ajmg.a.31746. [DOI] [PubMed] [Google Scholar]