Abstract
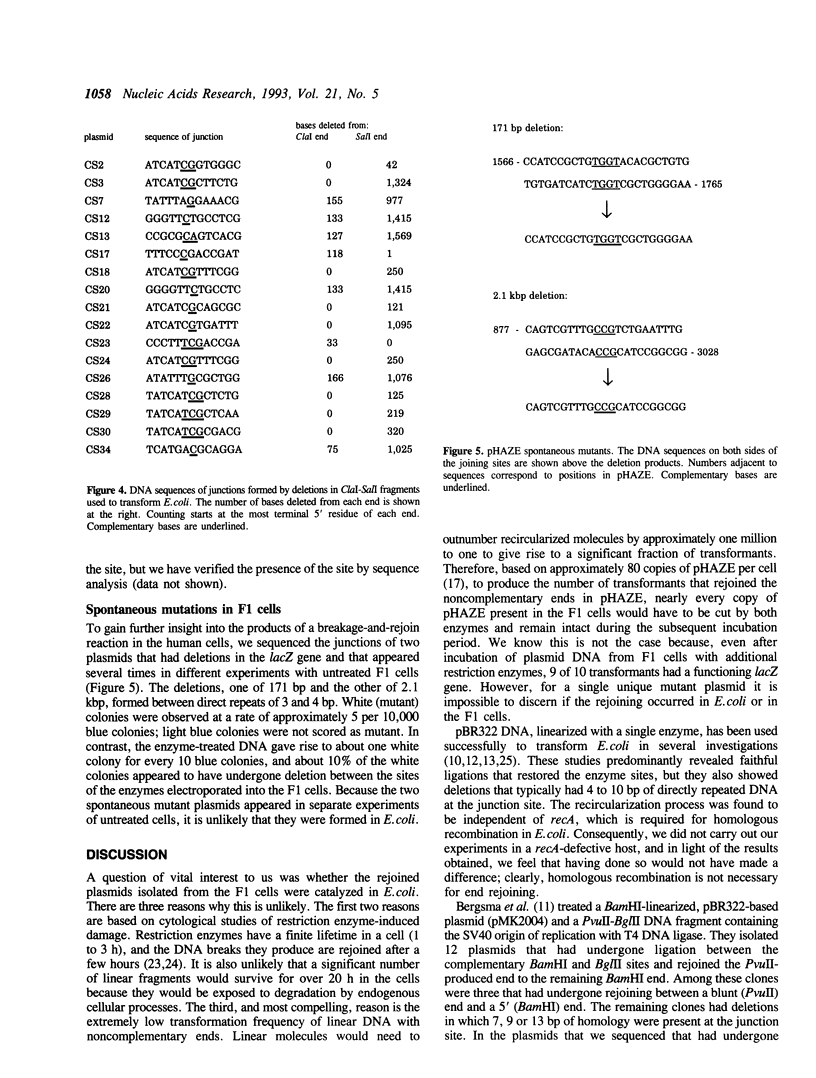
We examined the rejoining of noncomplementary restriction enzyme-produced DNA double-strand breaks in Escherichia coli and in cultured human cells. The enzymes used in this study, ClaI, BamHI and SalI, produce double-strand breaks with 5 protruding single strands. The joining of a ClaI-produced DNA end to a BamHI-produced end or to a SalI-produced end was examined at the DNA sequence level. End rejoining in E.coli was studied by transforming cultures with linear plasmid DNA that was gel purified from restriction digests, and end rejoining in cultured human cells was studied by introducing enzymes into the cells by electroporation. The human cells used contain an Epstein-Barr virus (EBV)-based shuttle vector, pHAZE, that was recovered and introduced into E.coli for further analysis. The major products of DNA end-joining processes observed in linear plasmid-transformed E.coli and in the human cells exposed to restriction enzymes were identical. Furthermore, the deletions observed in both systems and in the spontaneous mutant plasmids in untreated human cells had a common underlying feature: short stretches of directly repeated DNA at the junction sites.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager D. D., Phillips J. W., Columna E. A., Winegar R. A., Morgan W. F. Analysis of restriction enzyme-induced DNA double-strand breaks in Chinese hamster ovary cells by pulsed-field gel electrophoresis: implications for chromosome damage. Radiat Res. 1991 Nov;128(2):150–156. [PubMed] [Google Scholar]
- Bergsma D. J., Olive D. M., Hartzell S. W., Byrne B. J., Subramanian K. N. Cyclization of linear chimeric plasmids in vivo by a novel end-to-end joining reaction or by intramolecular recombination: one of the products contains a 147-bp perfect palindrome stable in Escherichia coli. Gene. 1982 Dec;20(2):157–167. doi: 10.1016/0378-1119(82)90034-8. [DOI] [PubMed] [Google Scholar]
- Bryant P. E. Enzymatic restriction of mammalian cell DNA using Pvu II and Bam H1: evidence for the double-strand break origin of chromosomal aberrations. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Jul;46(1):57–65. doi: 10.1080/09553008414551061. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Conley E. C., Saunders J. R. Recombination-dependent recircularization of linearized pBR322 plasmid DNA following transformation of Escherichia coli. Mol Gen Genet. 1984;194(1-2):211–218. doi: 10.1007/BF00383519. [DOI] [PubMed] [Google Scholar]
- Conley E. C., Saunders V. A., Jackson V., Saunders J. R. Mechanism of intramolecular recyclization and deletion formation following transformation of Escherichia coli with linearized plasmid DNA. Nucleic Acids Res. 1986 Nov 25;14(22):8919–8932. doi: 10.1093/nar/14.22.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley E. C., Saunders V. A., Saunders J. R. Deletion and rearrangement of plasmid DNA during transformation of Escherichia coli with linear plasmid molecules. Nucleic Acids Res. 1986 Nov 25;14(22):8905–8917. doi: 10.1093/nar/14.22.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaev M. M., Bobkov A. F., Bobkova A. F., Kalinin V. N., Smirnov V. D., Khudakov YuE, Tikchonenko T. I. The site-specific deletion in plasmid pBR322. Gene. 1982 Apr;18(1):21–28. doi: 10.1016/0378-1119(82)90052-x. [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Cory S., Adams J. M. Translocation of the myc cellular oncogene to the immunoglobulin heavy chain locus in murine plasmacytomas is an imprecise reciprocal exchange. Cell. 1984 Apr;36(4):973–982. doi: 10.1016/0092-8674(84)90047-3. [DOI] [PubMed] [Google Scholar]
- Ho K. S., Mortimer R. K. Induction of dominant lethality by x-rays in radiosensitive strain of yeast. Mutat Res. 1973 Oct;20(1):45–51. doi: 10.1016/0027-5107(73)90096-1. [DOI] [PubMed] [Google Scholar]
- Hoekstra W. P., Bergmans J. E., Zuidweg E. M. Role of recBC nuclease in Escherichia coli transformation. J Bacteriol. 1980 Aug;143(2):1031–1032. doi: 10.1128/jb.143.2.1031-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutze L. H., Winegar R. A., Jostes R., Cross F. T., Cleaver J. E. Radon-induced deletions in human cells: role of nonhomologous strand rejoining. Cancer Res. 1992 Sep 15;52(18):5126–5129. [PubMed] [Google Scholar]
- Lutze L. H., Winegar R. A. pHAZE: a shuttle vector system for the detection and analysis of ionizing radiation-induced mutations. Mutat Res. 1990 Dec;245(4):305–310. doi: 10.1016/0165-7992(90)90161-c. [DOI] [PubMed] [Google Scholar]
- Morgan W. F., Valcarcel E. R., Abella Columna E., Winegar R. A., Yates B. L. Induction of chromosome damage by restriction enzymes during mitosis. Radiat Res. 1991 Jul;127(1):101–106. [PubMed] [Google Scholar]
- Natarajan A. T., Obe G. Molecular mechanisms involved in the production of chromosomal aberrations. III. Restriction endonucleases. Chromosoma. 1984;90(2):120–127. doi: 10.1007/BF00292448. [DOI] [PubMed] [Google Scholar]
- Pfeiffer P., Vielmetter W. Joining of nonhomologous DNA double strand breaks in vitro. Nucleic Acids Res. 1988 Feb 11;16(3):907–924. doi: 10.1093/nar/16.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh E. A., Murray N. E., Revel H., Blumenthal R. M., Westaway D., Reith A. D., Rigby P. W., Elhai J., Hanahan D. McrA and McrB restriction phenotypes of some E. coli strains and implications for gene cloning. Nucleic Acids Res. 1988 Feb 25;16(4):1563–1575. doi: 10.1093/nar/16.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Porter T. N., Wilson J. H. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985 Oct;5(10):2599–2607. doi: 10.1128/mcb.5.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufer J. T., Morgan W. F. Potentiation of DNA damage by inhibition of poly(ADP-ribosyl)ation: a test of the hypothesis for random nuclease action. Exp Cell Res. 1992 Jun;200(2):506–512. doi: 10.1016/0014-4827(92)90202-j. [DOI] [PubMed] [Google Scholar]
- Thode S., Schäfer A., Pfeiffer P., Vielmetter W. A novel pathway of DNA end-to-end joining. Cell. 1990 Mar 23;60(6):921–928. doi: 10.1016/0092-8674(90)90340-k. [DOI] [PubMed] [Google Scholar]
- Wake C. T., Gudewicz T., Porter T., White A., Wilson J. H. How damaged is the biologically active subpopulation of transfected DNA? Mol Cell Biol. 1984 Mar;4(3):387–398. doi: 10.1128/mcb.4.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle B., Draper B. W., Yin Y. X., O'Gorman S., Wahl G. M. A central role for chromosome breakage in gene amplification, deletion formation, and amplicon integration. Genes Dev. 1991 Feb;5(2):160–174. doi: 10.1101/gad.5.2.160. [DOI] [PubMed] [Google Scholar]
- Winegar R. A., Lutze L. H., Rufer J. T., Morgan W. F. Spectrum of mutations produced by specific types of restriction enzyme-induced double-strand breaks. Mutagenesis. 1992 Nov;7(6):439–445. doi: 10.1093/mutage/7.6.439. [DOI] [PubMed] [Google Scholar]



