Abstract
Chromatin modifying and remodeling enzymes play critical roles in many aspects of chromosome biology including transcription, replication, recombination, and repair. Our laboratory recently identified and characterized two multisubunit human chromatin remodeling enzymes designated the INO80 and SRCAP complexes. Mechanistic studies revealed that the human INO80 complex catalyzes nucleosome sliding and the SRCAP complex catalyzes ATP-dependent exchange of histone H2A/H2B dimers containing the histone variant H2A.Z into nucleosomes. Here we describe methods for purification and assay of the INO80 and SRCAP chromatin remodeling complexes.
Keywords: Nucleosome, chromatin, histone, histone exchange, chromatin remodeler
1. Introduction
In eukaryotes, chromosomal DNA is packaged into chromatin. The nucleosome is the basic repeating unit of chromatin. The canonical nucleosome is composed of approximately 140 base pairs of chromosomal DNA wrapped around a histone octamer containing two copies each of the histones H2A, H2B, H3, and H4. Though the nucleosome performs an essential role in packaging chromosomal DNA into compact higher order structures in the nucleus of eukaryotic cells, it also acts as an impediment to many critical DNA transactions including transcription, replication, recombination, and repair. As a consequence, eukaryotes have evolved a sophisticated machinery for regulating the organization of nucleosomes on chromosomal DNA .This machinery comprises a growing collection of chromatin modifying and remodeling enzymes that regulate chromatin structure by diverse mechanisms that include catalyzing post-translational modification of nucleosomal histones, relocalizing nucleosomes on DNA, incorporating histone variants into nucleosomes, and, in some cases, completely removing nucleosomes from the DNA [1].
Our laboratory recently identified two multisubunit human chromatin remodeling complexes designated the SRCAP [2,3]and INO80 [4]complexes. The SRCAP and INO80 complexes share some structural and functional properties with the S. cerevisiae SWR1 [5,6,7]and INO80 [8,9] chromatin remodeling complexes, respectively. Purification and characterization of the subunit composition of the human SRCAP complex revealed that it includes apparent human orthologs of most of the subunits of the S. cerevisiae SWR1 chromatin remodeling complex. The human SRCAP complex includes the SNF2 family ATPase SRCAP (SWI2/SNF2-related CBP activator protein), which is closely related in amino acid sequence to the S. cerevisiae SWR1 ATPase. SRCAP was originally identified as a CBP-interacting protein that functions as a coactivator for CREB and the glucocorticoid and androgen receptors [10,11,12]. In addition to the SRCAP protein, the human SRCAP complex includes the actin-related proteins Arp4 and Arp6, the Ruvb-like AAA+ ATPases Tip49a and Tip49b, and the DMAP1, Gas41, ZHIT1, and YL1 proteins, which are likely human orthologs of the Eaf2, Yaf9, Vps71, and Vps72 subunits of the S. cerevisiae SWR1 chromatin remodeling complex. Like the SWR1 complex [7], the SRCAP complex catalyzes ATP-dependent exchange of histone H2A/H2B dimers containing the histone variant H2A.Z into nucleosomes in vitro [3].
Purification and characterization of the subunit composition of the human INO80 complex revealed that it includes apparent human orthologs of a subset of the subunits of the S. cerevisiae INO80 chromatin remodeling complex [4]. The human INO80 complex includes a SNF2 family ATPase encoded by KIAA1259 ORF, which shares sequence similarity with the Ino80 subunit of the S. cerevisiae INO80 chromatin remodeling complex. In addition to the KIAA1259 protein, the human INO80 complex includes the actin-related proteins Arp4, Arp5, and Arp8, the AAA+ATPases Tip49a and Tip49b, and the PAPA-1 and C18orf37 proteins, which are likely human orthologs of the Ies2 and Ies6 subunits of the S. cerevisiae INO80 chromatin remodeling complex. Notably, the human INO80 complex appears to lack human orthologs of the S. cerevisiae INO80 subunits Ies1, Ies3, Ies5, Taf14, and Nhp10, but includes an interesting collection of apparently metazoan-specific subunits, among which are NFRKB, Amida, forkhead associated domain-containing MCRS1, and proteins encoded by the previously uncharacterized FLJ90652 and FLJ20309 ORFs. Like the S. cerevisiae INO80 chromatin remodeling complex [8], the human INO80 complex catalyzes ATP-dependent nucleosome sliding in vitro [4].
2. Preparation of the human SRCAP and INO80 chromatin remodeling complexes
Multiprotein complexes such as the human SRCAP and INO80 chromatin remodeling complexes can be readily prepared by immunoaffinity purification from cultured cell lines that stably express epitope-tagged versions of their subunits. Of the commonly used epitope tags, including FLAG, HA, or c-Myc, we have found the FLAG epitope (DYKDDDDK) to be the most useful for this purpose. Commercially available anti-FLAG monoclonal antibodies (M2) bind the FLAG epitope with high specificity and affinity. Accordingly, single step purifications using anti-FLAG agarose immunoaffinity chromatography are often sufficient to yield highly enriched preparations of the desired complex. Importantly, FLAG epitope-tagged proteins can be eluted in good yield from anti-FLAG antibodies by incubation with an epitope peptide at 4°C, while in our hands both HA and c-Myc tagged proteins must be eluted at or above room temperature. For these reasons, we typically prepare the SRCAP, INO80, and other multiprotein complexes by anti-FLAG chromatography of lysates from cell lines stably expressing N- or C-terminally FLAG-tagged subunits of the each complex.
2.1 Generation of stable cell lines expressing subunits of the human SRCAP and INO80 complexes
Full-length cDNAs encoding the human Tip49a, Tip49b, Arp5, Arp8, PAPA-1 (hIes2), C18orf37 (hIes6), Amida, FLJ90652, FLJ20309 (106-523), YL1, ARP6, ZHIT1, and H2A.Z proteins were obtained from the American Type Culture Collection (ATCC). The cDNAs were subcloned into pcDNA3.1 for introduction into HeLa S3 cells or into the pcDNA5/FRT Flp-In™ expression vector (Invitrogen) for introduction into HEK293/FRT cells (Invitrogen). HeLa cells can be grown in suspension and are therefore particularly useful when it is desirable to prepare extracts from large numbers of cells; however, generation of suitable HeLa cell lines can be time-consuming, since it is often necessary to screen a large number of clones to identify one that expresses appropriate amounts of the desired protein. Although it is more difficult to grow large quantities of HEK293/FRT cells, they offer a distinct advantage for rapid generation of many cell lines, because they have been engineered to contain a stably integrated FRT (FLP recombinase target) site at a single transcriptionally active locus. Cotransfection of the pcDNA5/FRT expression vector with a plasmid encoding FLP recombinase allows highly efficient, targeted integration of the pcDNA5/FRT expression vector at the genomic FRT locus. Importantly, there is little clonal variation in protein expression levels; as a consequence, it is usually not necessary to screen more than three to five clones to identify cell lines that stably express the desired proteins.
HEK293/FRT cells in a 10 cm dish were cotransfected with 0.5 μg of pcDNA5/FRT expression vector DNA and 9.5 μg of pOG44 (which encodes FLP recombinase) using FuGENE6 transfection reagent (Roche Diagnostics) at a 1:4 ratio of plasmid DNA: FuGENE6. After 48 hrs, the cells in one 10 cm dish were split into ten 10 cm dishes and grown in the presence of 100 μg/ml of hygromycin B for at least 3 to 4 weeks. The cell culture medium should be changed every 3-5 days depending on growth rate. To recover hygromycin B-resistant foci, a sterile cloning disc was placed around each colony. Colonies were incubated for ~5 min at room temperature in PBS containing 1× trypsin (Invitrogen Corporation), and cells were resuspended and transferred to a single well of a 24 well plate. Cells were harvested using 1X Trypsin in PBS (Gibco/INVITROGEN) once cells had reached 60-70% confluency. 5 % of the cells were transferred to a new single well of a 24 well plate. To identify positive clones, the remaining cells were pelleted by centrifugation at 1,000 rpm for 5 min in a GH3.8A rotor in an Allegra 6R centrifuge (Beckman-Coulter), lysed with 4× SDS sample buffer, and subjected to SDS polyacrylamide gel eletrophoresis and Western blotting with anti-FLAG (M2) antibody (Sigma).
HeLa S3 cells expressing FLAG epitope-tagged subunits of the human INO80 or SRCAP complexes were generated using essentially the same procedures. HeLa S3 cells in a 10 cm dish were transfected with 0.5 μg of pcDNA3.1 expression vector DNA using FuGENE6. After 48 hrs, cells were split into ten cm dishes and selected with 300 μg/ml of hygromysin B. Hygromysin B- resistant clones were selected and screened as described above.
Parental and stably transformed HEK293/FRT and HeLa S3 cells were maintained in DMEM with 5% glucose and 10% fetal bovine serum. For large-scale cultures, HeLa cells were grown in spinner culture in a custom-modified Joklik medium (JRH 67721-10L 4456) with 5% calf serum. HeLa S3-derived cell lines need to be maintained at a density between 2 × 105 and 6 × 105 cells/ml. Large-scale cultures of 293/FRT cells were grown in roller bottles in DMEM with 5% glucose and 10% calf serum and were not allowed to exceed ~70 % confluency. Cells were grown without hygromycin B selection when in large scale culture.
2.2 Immunoaffinity purification of the human SRCAP and INO80 chromatin remodeling complexes
Characterization of the human SRCAP and INO80 chromatin remodeling complexes revealed that they could be efficiently purified free of contaminating remodeling activities by anti-FLAG agarose immunoaffinity chromatography from cell lysates prepared from HeLa or 293 cells stably expressing one of several FLAG-tagged subunits unique to each complex. Preparation of cell lysates and anti-FLAG agarose chromatography were carried out as follows and should be scaled proportionately depending on the number of cells used as starting material.
We typically prepare the SRCAP and INO80 complexes by anti-FLAG agarose chromatography from ~6 ml nuclear extract, which is prepared according to the method of Dignam et al [13] from approximately 1.8 × 109 HeLa S3 cells (~3.5 L cells) or HEK293/FRT cells (~6-7 roller bottles of cells grown to ~70% confluency). 6 ml of frozen nuclear extract were thawed quickly and spun for 20-30 min at 40,000 rpm (4°C) in a type 70.1 Ti ultracentrifuge rotor (Beckman-Coulter). The supernatants were then diluted to 0.3 M NaCl with a buffer containing 40 mM Hepes-NaOH (pH 7.9), 0.2% Triton X-100, and 10% glycerol, mixed with anti-FLAG (M2) agarose beads (Sigma) in a ratio of 100 μl of packed beads per 6 ml of starting nuclear extract, and gently rocked for 4 hr at 4°C. The beads are washed 3 times with a 50-fold excess of buffer containing 0.25 M NaCl in 40 mM Hepes-NaOH (pH 7.9), 0.2% Triton X-100, and 10% glycerol and once with the same buffer containing 0.1 M NaCl. Proteins are eluted from the beads by two or three sequential elutions with 100μl the same buffer containing 0.1 M NaCl and 0.2 mg/ml FLAG peptide (DYKDDDDK). The peptide can be custom-synthesized or obtained from Sigma (Cat No: F4799). Following FLAG-immunopurification, SRCAP complexes were concentrated 5-fold using an Amicon® Ultra Centrifugal Filter Device (50,000 molecular weight cutoff).
3. Assay of ATP-dependent exchange of H2A/H2B dimers containing the histone variant H2A.Z into nucleosomes
3.1 Preparation of the H2A.Z/H2B dimer or histone octamer
To generate recombinant human histone octamers and H2A.Z/H2B dimers, human histone cDNAs encoding H2A, H2B, H3.3, H4, and H2A.Z were subcloned into pET-21a for expression in bacteria. H2A.Z/H2B dimers were prepared with C-terminally FLAG-tagged H2A.Z and C-terminally c-Myc-tagged H2B so they could be easily differentiated from H2A and H2B incorporated into substrate nucleosomes. BL21(DE3) cells freshly transformed with pET-21a vectors containing each histone gene were grown overnight at 37°C on LB plates containing ampicillin (100μg/ml). The next day, 2 ml LB medium containing 50 μg/ml ampicillin was inoculated with a single colony from each plate, and cells were grown for 6-8 hours at 37°C. 1 ml of each culture was transferred into a 250 ml flask containing 50 ml of LB medium with 50 μg/ml ampicillin and incubated overnight at 37°C without shaking. The next day, the 50 ml culture was transferred into 500 ml LB medium containing ampicillin, and the culture was grown at 37°C until the OD600 was approximately 0.6. Expression of histones was induced by adding 0.2 mM IPTG. After 3 hours, the cells were collected by centrifugation for 15 min at 5000 × g in a JA10 rotor (Beckman-Coulter). Cell pellets were washed with buffer A containing 50 mM Tris-HCl, pH 7.5, 0.1 M NaCl, 1 mM EDTA, 1 mM benzamidine, and 5 mM β-mercaptoethanol. Cells were then resuspended in 20 ml of buffer A and disrupted using a French® Pressure Cell Press (Thermo Spectronic). Inclusion bodies, which contained the insoluble histone fraction, were collected by centrifugation at 15,000 rpm for 20 min in a JA20 rotor (Beckman-Coulter). The pellets were then washed twice with buffer A containing 1% (v/v) Triton X-100 and twice more with buffer A. Inclusion bodies were solubilized in unfolding buffer (7 M guanidine-HCl, 20 mM Tris-HCl, pH 7.5, and 10 mM DTT). After centrifugation for 10 min at 25,000 × g, supernatants were loaded onto a 5 ml Hi-Trap SP-Sepharose column (Amersham). Bound proteins were eluted with a 10 column volume linear gradient from 0.2 M to 0.6 M NaCl in buffer containing 7 M urea, 20 mM NaC2H3O2, pH 5.2, 1 mM EDTA, and 5 mM β- mercaptoethanol. Peak protein fractions were pooled and dialyzed against deionized water containing 5 mM β-mercaptoethanol and 0.5 mM PMSF. The proteins were lyophilized and dissolved in unfolding buffer. Mixtures containing equimolar amounts of histones H2A, H2B, H3.3 and H4 for preparation of octamers or H2A.Z-FLAG and H2B-c-Myc for preparation of dimers were dialyzed overnight at 4°C against a refolding buffer containing 10 mM Tris-HCl, pH 7.5, 2 M NaCl, 1 mM EDTA, and 5 mM β-mercaptoethanol. Dialyzed octamers or dimers were centrifuged for 5 min at 25,000 × g and applied to a 16/60 Superdex 200™ column equilibrated in refolding buffer. 10 μl of every other fraction were analyzed by SDS-PAGE and Coomassie Blue staining to identify octamer and dimer containing fractions. Fractions containing octamers or dimmers were pooled and concentrations were determined by measuring absorbance at 276 nm (A276 ≅ 0.45 for a 1 mg/ml solution of either octamers or dimers).
3.2 Preparation of mononucleosomes
A 216 bp DNA fragment (dSH-A) was generated by PCR using biotinylated oligonucleotides as primers (forward: 5’-ACA TTA ACC TAT AAA AAT AGG CGT ATC ACG-3’; reverse: 5’-ATC TAG ACG GCC ACG TGG TTC-3’) and the plasmid pGUB-dSH [14] as template. Purified recombinant histone octamers (3 μg) and DNA (150 ng) were used to reconstitute mononucleosomes according to the protocol described by Owen-Hughes et. al. [15]. Briefly, histones and DNA were brought to a total volume of 10 μl in buffer containing 10 mM Hepes-NaOH, pH 7.9, 1 mM EDTA, 0.5 mM PMSF. The assembly reaction was brought to 2 M NaCl by addition of 3.4 μl of 5 M NaCl and incubated at 37°C for 15 min. The mixture was transferred to 30°C and serially diluted with 3.3, 6.7, 5, 3.6, 4.7, 6.7, 10, 30, and 20 μl of 10 mM Hepes-NaOH, pH 7.9, 1 mM EDTA, 0.5 mM PMSF, with a 15 min incubation after each dilution. The assembly reaction was then diluted with 100 μl of a final dilution buffer containing 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.1 % NP-40, 5 mM EGTA, 5 mM DTT, 0.5 mM PMSF, 20 % glycerol, and 0.1 mg/ml BSA and incubated at 30°C for 15 min. The entire 200 μl reaction was mixed with an equal volume of avidin-coupled Dynabeads (Dynal Biotech) and incubated at room temperature for 3 hrs. The beads were washed with a buffer containing 0.6 M NaCl in 20 mM Hepes-NaOH, pH 7.5, 1 mM EDTA, 10 % glycerol, 0.5 mM DTT, and 0.5 mM PMSF and finally resuspended in 200 μl of the same buffer. The bead-bound mononucleosomes can be stored at 4°C for a maximum of ~2-3 weeks.
3.2 Histone exchange assay
Exchange assays typically included ~400 ng SRCAP complex (~2 μl of anti-FLAG agarose fraction); however, it is necessary to determine the optimal amount of complex to use in assays by titration. Reactions in which SRCAP complex or ATP are omitted should be included to control for non-specific binding of H2A.Z/H2B dimers to immobilized nucleosomes or beads, while reactions containing immobilized free DNA instead of nucleosomes should be included to ensure that H2A.Z/H2B dimers are being exchanged into nucleosomes and not just binding DNA.
In our standard assays, 7.5 ng DNA equivalents of mononucleosomes immobilized on avidin-coupled Dynabeads were pre-incubated with varying amounts of SRCAP complex in 50 μl of exchange buffer (25 mM Hepes-KOH, pH 7.6, 0.1 mM EDTA, 5 mM MgCl2, 10 % glycerol, 0.02% NP-40, 1 mM DTT, 0.1 mg/ml BSA, and 70 mM KCl) for 30 min at 37°C. The beads were washed twice with 200 μl of exchange buffer and resuspended in the same buffer with 75 ng recombinant H2A.Z-FLAG/H2B-c-Myc dimmer to a total volume of 100 μl. The reactions were incubated for 60 min at 37°C in the presence of 0.1 mM ATP. After incubation, the beads were washed three times with 500 μl of the exchange buffer. Bound proteins were eluted using SDS-PAGE loading buffer and fractionated on 18% SDS-PAGE gels (29:1 acrylamide:bisacrylamide). The FLAG-tagged H2A.Z was detected by Western blotting using anti-FLAG (M2) antibody (Sigma). Alternatively, bound histones can be detected by silver staining if reactions are scaled up ~3-5 fold. A representative example of an exchange assay is shown in Fig. 1.
Figure 1.
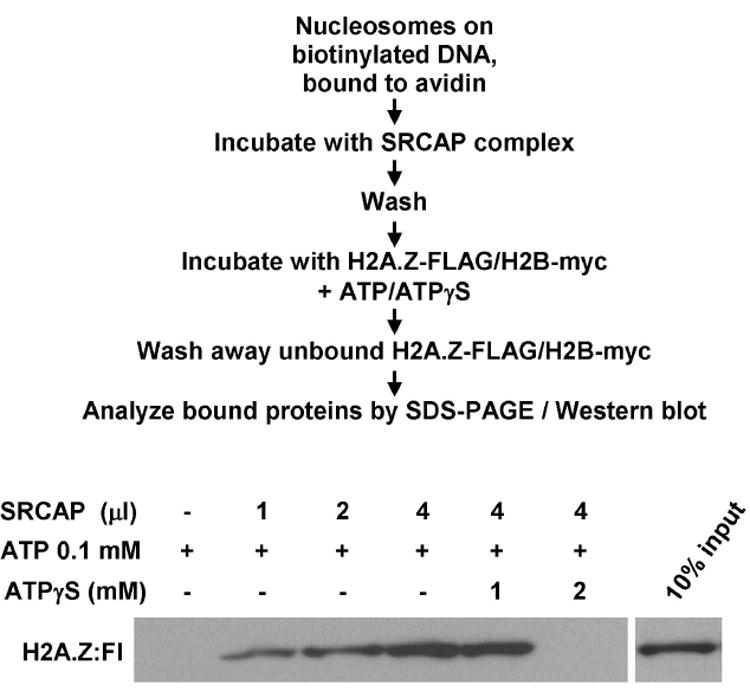
Assay for SRCAP-dependent exchange of H2A.Z/H2B histone dimers into mononucleosomes. Assays were performed as described in the text and outlined in the figure with the indicated amounts of immunopurified SRCAP complex prepared from cells expressing FLAG-ZHIT1.
4. Assay of ATP-dependent nucleosome sliding by the human INO80 complex
4.1 Preparation of mononucleosomes
Mononucleosomes were reconstituted on 32P-labeled DNA fragments by dilution transfer from HeLa long oligonucleosomes. In some experiments, we used the 216 bp dSH-A fragment [14] generated by PCR in the presence of [α-32P]dCTP. This DNA fragment does not include a nucleosome positioning sequence; hence, the starting mononuclosomes will be located at multiple positions and will give rise to multiple species with different electrophoretic mobilities when analyzed by native gel electrophoresis (Fig. 2A, lane 1). To obtain more homogeneous populations of starting mononucleosomes, we have also used mononucleosomes reconstituted on DNA fragments containing derivatives of the 601.2 [16] or 5S [17] nucleosome positioning sequences (Fig. 2B, lane 1).
Figure 2.
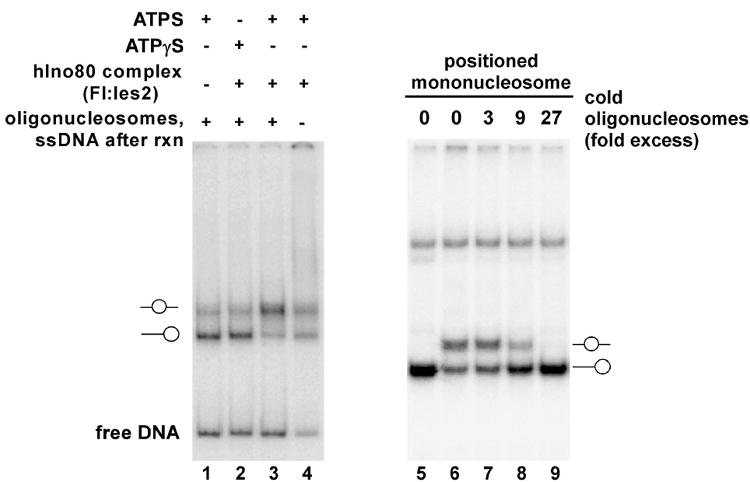
Assays for nucleosome sliding activity of the human INO80 complex. Human INO80 complex was prepared as described in the text from cells expressing FLAG-hIes2 (PAPA-1). Panel A. Reactions contained ~500 ng INO80 complex, 10 fmol mononucleosomes reconstituted on the 216 bp dSH-A fragment, which does not contain a nucleosome positioning sequence, and ~270 fmol of long oligonucleosomes, and either 1 mM ATP or 1 mM ATPγS. The starting mononucleosomes are a mixture of laterally positioned (lower band) and centrally positioned (upper band) mononucleosomes. At the conclusion of the reactions shown in lanes 1-3, a mixture of long oligonucleosomes and salmon sperm DNA was added to compete bound INO80 complex off the labeled mononucleosomes and free DNA. Panel B. Reactions contained ~500 ng INO80 complex, 10 fmol mononucleosomes reconstituted on a 212 bp DNA fragment with a 601.2 nucleosome positioning sequence at one end, and the indicated amounts of short oligonucleosome competitor, prepared as described [15].
To reconstitute mononucleosomes, 1 pmol of 32P-labeled DNA fragment was mixed with 3 ug of long oligonucleosomes from Hela cells, prepared as described by Owen-Hughes et al [15], in 25 ul of a buffer containing 1.0 M NaCl, 10 mM Tris-HCl, pH 8, 1 mM EDTA (pH 8.0), 0.5 mM PMSF, and 5 mM DTT. After 30 min at 30°C, the mixture was sequentially adjusted to 0.8, 0.6, and 0.4 M NaCl by dilution with 6.25 μl, and 20.8 μl respectively, of 10 mM Tris-HCl, pH 8, 1 mM EDTA, 0.5 mM PMSF, and 5 mM DTT, with a 30 min incubation at 30°C between each dilution. Finally, the mixture was sequentially diluted to 0.2 and 0.1 M NaCl by addition of 62.5 μl and then 125 μl of the same buffer containing 0.1% Nonidet P-40, 20% glycerol, and 200 ug/ml BSA. After reconstitution, the mononucleosomes can be stored in small aliquots at -20°C. We note that nucleosomes are not stable to repeated cycles of freeze-thawing and thus should be stored at 4°C once they have been thawed.
In cases where it was desirable to obtain reconstituted mononucleosomes that are free of the long oligonucleosomes used in the nucleosome transfer reaction, we have prepared mononucleosomes using a 32P-labeled DNA fragment that is biotinylated at one end and has a restriction site near the biotinylated end. Following nucleosome assembly, mononucleosomes are immobilized on magnetic beads coupled to avidin as described above, washed with a buffer that is compatible with the relevant restriction enzyme, and removed from the beads by restriction digestion.
4.2 Assay of ATP-dependent nucleosome sliding by the human INO80 complex
The optimal amount of the human INO80 complex to be used in assays needs to be determined by titration and will vary depending on the amount of competing unlabeled nucleosomes and on whether the mononucleosomes were reconstituted on DNA fragments with or without a positioning sequence. Typically, we use 400-800 ng of anti-FLAG agarose purified INO80 complex in buffer containing 20mM Hepes-NaOH,pH 7.9, 50 mM NaCl, 4.5 mM MgCl2, 2 mM DTT, 0.5 mM PMSF, 45 ug/ml BSA, 10% glycerol, 0.02% Triton X-100, 0.02% Nonidet P-40, and 1 mM ATP in assays containing ~10 fmol of mononucleosomes reconstituted on a DNA fragment without a positioning sequence and about 250 fmol of unlabeled oligonucleosomes that remain from the transfer reaction. Reactions mixtures are typically incubated at 37 °C for 30 minutes; however, it is often useful to vary reaction time in order to optimize the reaction.
At the conclusion of the remodeling reaction, a mixture containing of 0.5 ug of HeLa cell long oligonucleosomes and 0.75 μg of salmon sperm or calf thymus DNA (which had been sonicated, boiled, and quick-chilled) in 1.5μl of buffer containing 0.6 M NaCl, 20 mM Hepes pH 7.5, 1 mM EDTA, 1 mM β-mercaptoethanol, and 10% glycerol was added to the reaction and incubated for an additional 30 min at 37 °C to compete off DNA- or nucleosome-binding proteins that would alter mononucleosome electrophoretic mobility. Reaction products are then analyzed by 5% native PAGEs (37.5:1 acrylamide:bis-acrylamide) gels in 0.5X TBE (45 mM Tris borate, 1 mM EDTA) at 4 °C for 4.5 h at 200V. The gels were then dried and exposed to a storage phosphor screen overnight. Results of typical assays performed with mononucleosomes assembled on DNA fragments without or with nucleosome positioning sequences are shown in Fig. 2A and 2B, respectively.
Acknowledgments
This work was supported in part by National Institutes of Health Grant R37GM041628.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Becker PB, Horz W. Annu Rev Biochem. 2005;71:247–273. doi: 10.1146/annurev.biochem.71.110601.135400. [DOI] [PubMed] [Google Scholar]
- 2.Cai Y, Jin J, Florence L, Swanson SK, Kusch T, Li B, Workman JL, Washburn MP, Conaway RC, Conaway JW. J Biol Chem. 2005;280:13665–13670. doi: 10.1074/jbc.M500001200. [DOI] [PubMed] [Google Scholar]
- 3.Ruhl DD, Jin J, Cai Y, Swanson S, Florens L, Washburn MP, Conaway RC, Conaway JW, Chrivia JC. Biochem. 2006;45:5671–5677. doi: 10.1021/bi060043d. [DOI] [PubMed] [Google Scholar]
- 4.Jin J, Cai Y, Yao T, Gottschalk AJ, Florens L, Swanson SK, Gutierrez JL, Coleman MK, Workman JL, Mushegian A, Washburn MP, Conaway RC, Conaway JW. J Biol Chem. 2005;280:41207–41212. doi: 10.1074/jbc.M509128200. [DOI] [PubMed] [Google Scholar]
- 5.Kobor MS, Venkatasubrahmanyam S, Meneghini MD, Gin JW, Jennings JL, Link AJ, Madhani HD, Rine J. PLoS Biol. 2004;2:E131. doi: 10.1371/journal.pbio.0020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krogan NJ, Baetz K, Keogh MC, Datta N, Sawa C, Kwok TC, Thompson NJ, Davey MG, Pootoolal J, Hughes TR, Emili A, Buratowski S, Hieter P, Greenblatt JF. Proc Natl Acad Sci U S A. 2004;101:13513–13518. doi: 10.1073/pnas.0405753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. Science. 2004;303:343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- 8.Shen X, Mizuguchi G, Hamiche A, Wu C. Nature. 2000;406:541–544. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 9.Shen X, Ranallo R, Choi E, Wu C. Mol Cell. 2003;12:147–155. doi: 10.1016/s1097-2765(03)00264-8. [DOI] [PubMed] [Google Scholar]
- 10.Johnston H, Kneer J, Chackalaparampil I, Yaciuk P, Chrivia J. J Biol Chem. 1999;274:16370–16376. doi: 10.1074/jbc.274.23.16370. [DOI] [PubMed] [Google Scholar]
- 11.Monroy MA, Ruhl DD, Xu X, Granner DK, Yaciuk P, Chrivia JC. J Biol Chem. 2001;276:40721–40726. doi: 10.1074/jbc.M103615200. [DOI] [PubMed] [Google Scholar]
- 12.Monroy MA, Schott NM, Cox L, Chen JD, Ruh M, Chrivia JC. Mol Endocrinol. 2003;17:2519–2528. doi: 10.1210/me.2003-0208. [DOI] [PubMed] [Google Scholar]
- 13.Dignam JD, Lebovitz RM, Roeder RG. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juan LJ, Utley RT, Vignali M, Bohm L, Workman JL. J Biol Chem. 1997;272:3635–3640. doi: 10.1074/jbc.272.6.3635. [DOI] [PubMed] [Google Scholar]
- 15.Owen-Hughes T, Utley RT, Steger DJ, West JM, John S, Cote J, Havas KM, Workman JL. Methods Mol Biol. 1999;119:319–331. doi: 10.1385/1-59259-681-9:319. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JD, Widom J. J Mol Biol. 2006;296:979–987. doi: 10.1006/jmbi.2000.3531. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes D. EMBO J. 1985;4:3473–3482. doi: 10.1002/j.1460-2075.1985.tb04106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


