Abstract
Corticotropin-releasing factor (CRF) and CRF-related neuropeptides are involved in the regulation of stress-related physiology and behavior. Members of the CRF family of neuropeptides bind to two known receptors, the CRF type 1 (CRF1) receptor, and the CRF type 2 (CRF2) receptor. Although the distribution of CRF2 receptor mRNA expression has been extensively studied, the distribution of CRF2 receptor protein has not been characterized. An area of the brain known to contain high levels of CRF2 receptor mRNA expression and CRF2 receptor binding is the dorsal raphe nucleus (DR). In the present study we investigated in detail the distribution of CRF2 receptor immunoreactivity throughout the rostrocaudal extent of the DR. CRF2 receptor-immunoreactive perikarya were observed throughout the DR, with the highest number and density in the mid-rostrocaudal DR. Dual immunofluorescence revealed that CRF2 receptor immunoreactivity was frequently co-localized with tryptophan hydroxylase, a marker of serotonergic neurons. This study provides evidence that CRF2 receptor protein is expressed in the DR, and that CRF2 receptors are expressed in topographically organized subpopulations of cells in the DR, including serotonergic neurons. Furthermore, these data are consistent with the hypothesis that CRF2 receptors play an important role in the regulation of stress-related physiology and behavior through actions on serotonergic and non-serotonergic neurons within the DR.
Keywords: CRF2 receptor, immunofluorescence, immunohistochemistry, raphe, serotonin, tryptophan hydroxylase
1. Introduction
Corticotropin-releasing factor (CRF) is a 41-amino acid neuropeptide that has been linked to the regulation of stress-related physiology and behavior, including regulation of the hypothalamic-pituitary-adrenal (HPA) axis (Vale et al., 1981; Vale et al., 1983). The CRF family of neuropeptides also includes urocortin (Ucn) 1, Ucn 2, and Ucn 3; the role of these CRF-related neuropeptides in the HPA axis and stress-related physiological and behavioral responses is less clear, although evidence suggests that they also play an important role in modulating these responses (Reul and Holsboer, 2002). CRF and its family of neuropeptides are known to bind to two distinct G protein-coupled receptors, the CRF type 1 (CRF1) receptor, and the CRF type 2 (CRF2) receptor (Perrin et al., 1993; Lovenberg et al., 1995b), for which they have different binding affinities. CRF preferentially binds to CRF1 receptors, Ucn 1 binds with high affinity to both receptors, and Ucn 2 and Ucn 3 preferentially bind to CRF2 receptors (Lewis et al., 2001; Reyes et al., 2001). Whereas CRF1 receptors have a widespread distribution in the central nervous system (CNS), including subcortical and cortical regions, CRF2 receptor distribution is mainly limited to subcortical structures (Chalmers et al., 1995; Radulovic et al., 1998; Sanchez et al., 1999; Van Pett et al., 2000; Chen et al., 2000; Korosi et al., 2006).
Multiple isoforms of the CRF2 receptor are known to be expressed including the alpha, beta, and gamma (found only in humans) isoforms, a soluble isoform, and a truncated isoform (currently described only in rats). These isoforms have distinct expression patterns and binding affinities for CRF and its family of peptides (Lovenberg et al., 1995a; Kostich et al., 1998; Miyata et al., 1999; Miyata et al., 2001; Chen et al., 2005b; Evans and Seasholtz, 2009).
A subcortical area of the rat CNS that contains a high density of cells expressing CRF2 receptor mRNA expression is the dorsal raphe nucleus (DR). The DR is a brainstem region that, together with the median raphe nucleus (MnR), contains the majority of serotonergic neurons with ascending projections to forebrain structures (Steinbusch et al., 1978; Steinbusch, 1981). In situ hybridization studies have shown that the DR is one of a few brain regions that contains more CRF2 receptor mRNA expression than CRF1 receptor mRNA expression in rats (Van Pett et al., 2000; Day et al., 2004; Korosi et al., 2006). Co-localization studies in rat brain have shown that CRF2 receptor mRNA expression within the DR is expressed predominantly in serotonergic neurons, although, in caudal parts of the DR, CRF2 receptor mRNA expression is commonly found in non-serotonergic neurons, including γ-aminobutyric acid-synthesizing (GABA)ergic neurons (Day et al., 2004). Consistent with these findings, and consistent with expression of functional receptor protein in the DR, dense CRF2 receptor binding has been described in the DR of several vole species (Lim et al., 2005). Although the distribution of CRF2 receptor mRNA expression has been extensively studied, the distribution of CRF2 receptor protein has not been characterized. This could be due to 1) difficulties generating CRF2 receptor-specific antibodies, 2) antibodies used in many previous studies, such as the CRF1/2 receptor antibody sc-1757 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), recognize both CRF1 and CRF2 receptors (Campbell et al, 2003; Chen et al, 2000; Hinkle et al, 2003, also see Figure 4), and 3) some CRF2 receptor-specific antibodies used in previous studies (Waselus et al., 2009; Wang et al. 2007) are no longer commercially available. Recent studies using immunoelectron microscopy have demonstrated that CRF2 receptor immunoreactivity is predominantly intracellular in DR neurons under basal unstressed conditions, and shifts toward a greater expression at the plasma membrane following stress exposure (Waselus et al., 2009). The shift in intracellular versus plasma membrane localization of CRF2 receptors is associated with a shift from inhibitory to excitatory neuronal firing rate responses to CRF administration (Waselus et al., 2009). The hypothesis that functional CRF2 receptors are expressed in the DR is supported by studies using immediate-early gene expression (e.g., nuclear c-Fos induction representing increased cellular responses) and electrophysiology to investigate the effects of CRF2 receptor ligands on responses of serotonergic neurons in the DR. Intracerebroventricular injections of the CRF2 receptor ligand mouse Ucn 2 (mUcn 2) (Staub et al., 2005) or microinjections of mUcn 2 directly into the DR (Amat et al., 2004) increase c-Fos expression in DR serotonergic neurons and increase serotonin release in DR projection sites, while pretreatment with the CRF2 receptor antagonist [DPhe11, His12]sauvagine(11–40) (antisauvagine-30; ASV-30) blocks these effects (Amat et al., 2004; Staub et al., 2006). Likewise, electrophysiological studies in anesthetized rats have shown that injections of mUcn 2 directly into the DR can increase the firing rates of serotonergic neurons and these effects can be prevented by pretreatment with ASV-30 (Pernar et al., 2004). Together, these studies are consistent with the finding that activation of CRF2 receptors in the DR increases extracellular serotonin concentrations within the basolateral amygdaloid nucleus (Amat et al., 2004) and nucleus accumbens (Lukkes et al., 2008), forebrain targets of serotonergic neurons arising from the DR (Abrams et al., 2005; Van Bockstaele et al., 1993).
Fig. 4.
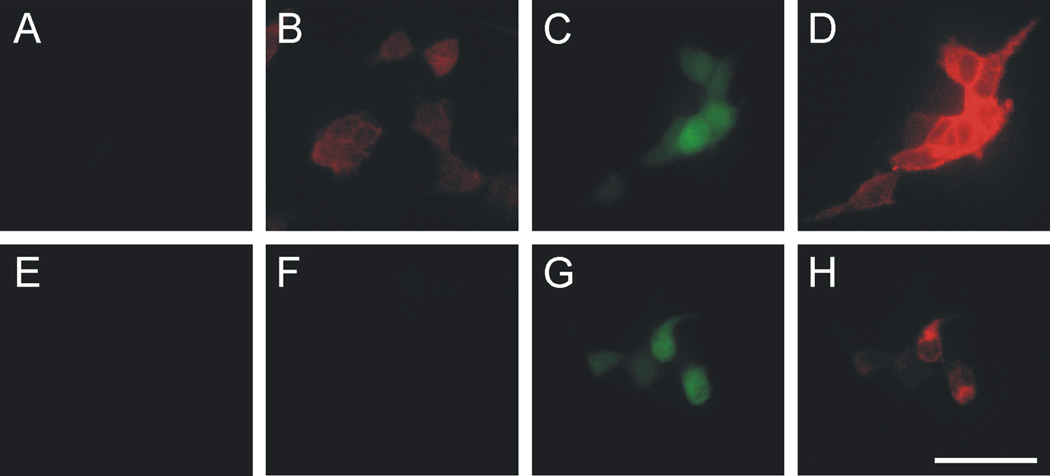
Photomicrographs illustrate immunofluorescence of HEK-293 wild type cells (A, B, E, and F) or HEK-293 cells stably expressing enhanced green fluorescent protein (EGFP; C, G) and mCRF2α receptor (D, H). The green fluorescence (C and G) results from EGFP expressed in conjunction with the mCRF2α receptor. Note that only light green autofluorescence is present in HEK-293 wild type cells (A and E). (B, D) photomicrographs illustrating immunofluorescence using the goat anti-CRF1/2 receptor antibody (sc-1757). (E–H) photomicrographs illustrating immunofluorescence using the goat anti-CRF2 receptor antibody (sc-1826) used in this study, replicating results from the experiment illustrated in Figure 3. Scale bar = 50 µm.
Understanding the distribution of CRF2 receptors within the DR is important because there is increasing evidence that the DR may include anatomically and functionally distinct subpopulations of serotonergic neurons (Imai et al., 1986; Abrams et al., 2004; Lowry et al., 2005; Lowry et al., 2008; Lowry and Hale, 2010). Indeed, serotonergic neurons can be divided into several different types, some of which are restricted to specific regions of the DR, based on their physiological properties and behavioral correlates (Rasmussen et al., 1984; Fornal et al., 1996; Jacobs and Fornal, 1999; Sakai and Crochet, 2001; Kocsis et al., 2006).
Several studies suggest that interactions between CRF or CRF-related ligands and serotonergic systems may play an important role in the regulation of anxiety-related behaviors in rats (Stout et al., 2001; Maier and Watkins, 2005; Lowry et al., 2005) and anxiety and affective disorders in humans (Arborelius et al., 1999; Austin et al., 2003). These data, together with 1) the potential for mismatch between CRF2 receptor mRNA and protein expression in neurons within the DR, 2) the potential for expression of multiple isoforms of the CRF2 receptor, and 3) the evidence for anatomical and functional heterogeneity within the DR, led us to characterize the detailed topographical organization of CRF2 receptor protein expression within this brainstem region and the extent of its co-localization with cytoplasmic tryptophan hydroxylase (TrpOH), a marker of serotonergic neurons. These studies used an antibody directed against the N-terminus of the CRF2 receptor, including an epitope that is highly conserved among CRF2α and CRF2β receptor isoforms, and therefore is likely to detect all known CRF2 receptor isoforms in rat brain, including truncated isoforms of the receptor.
2. Experimental procedures
2.1. DR immunohistochemistry
2.1.1. Animals
Adult male Sprague Dawley rats (n = 8; 250–300 g; Harlan Laboratories, Indianapolis, IN, USA) were used. The rats were group housed, 2 per cage, in wire cages (17 cm × 35 cm × 45 cm; Alternative Design, Siloam Springs, AR, USA) and were maintained with free access to food and water under a 12 h light/dark cycle with lights on at 0700 h in a room with standard temperature (21 °C) and humidity (22%). Rats were acclimated for at least 1 week before experimental manipulation. All procedures were approved by the University of Colorado at Boulder Institutional Animal Care and Use Committee. In addition, all studies were consistent with the NIH Guide for the Care and Use of Laboratory Animals (N.I.H. Publication No. 85-23). All possible efforts were made to minimize the number of animals used and their suffering.
Mice lacking functional CRF1 receptors (n = 3) and wild type littermates (n = 3) (Smith et al., 1998) were maintained with free access to food and water under a 12 h light/dark cycle. All experimental protocols were approved by The Weizmann Institute of Science Institutional Animal Care and Use Committee. Mice lacking functional CRF2 receptors were not used as methods used to develop the existing CRF2 mutants did not delete the 5’region of the gene encoding the N-terminus of the CRF2 receptor, the region that is targeted by the anti-CRF2 receptor antibody used in this study.
2.1.2. Tissue processing
Rats or mice were anesthetized with 0.5 ml sodium pentobarbital (200 mg/ml; Vortech Pharmaceuticals, Dearborn, MI, USA). Animals were transcardially perfused with 150 ml of 0.1 M phosphate buffered saline (PBS; pH 7.4) at room temperature (RT), followed by 200 ml of cold (4 °C) 4% paraformaldehyde (prepared using 40 g paraformaldehyde, 404 ml 0.2 M Na2HPO4, 96 ml 0.2 M NaH2PO4, and 500 ml dH2O, brought to pH 7.4 with sodium hydroxide pellets). Brains were removed from the cranium, post-fixed for 1 h in the same fixative at 4 °C, then immersed in 0.1 M PBS with 25% sucrose and stored for 2 days at 4 °C. Following cryopreservation with sucrose, brains were then blocked in a coronal plane using a rat (RBM-4000C, ASI Instruments, Warren, MI, USA) or mouse (RBM-2000C, ASI Instruments) brain matrix; brains were cut in the coronal plane at the caudal border of the mammillary bodies (approximately −5.30 mm bregma in rat brains or −2.80 mm bregma in mouse brains) and rapidly frozen in liquid isopentane chilled on dry ice. The brains were stored at −80 °C until sectioning. Brain sections (30 µm) were made using a cryostat and stored as 6 alternate sets of sections at −20 °C in cryoprotectant (prepared using 270 ml ethylene glycol, 160 ml glycerol, 202 ml 0.2 M Na2HPO4•7H2O, 48 ml 0.2 M NaH2PO4•H2O, and 320 ml dH2O) until immunostaining was performed.
2.1.3. Immunohistochemistry
For immunostaining, every sixth section of the midbrain containing the DR (from approximately −5.8 to −10.3 mm bregma in rat brains; approximately −3.5 to − 5.7 mm bregma in mouse brains) was removed from the cryoprotectant and washed in 0.01 M PBS for 15 min. The tissue was then placed in 0.01 M PBS containing 1% H2O2 for 15 min and then sections were washed twice in 0.01 M PBS for 15 min each. Sections were then pre-incubated in blocking buffer (0.01 M PBS, pH 7.4, containing 1% normal rabbit serum, Cat. No.011-000-120, Jackson ImmunoResearch, West Grove, PA, USA) at RT for 2 h to reduce non-specific staining. Sections were then incubated in goat anti-CRF2 receptor-selective polyclonal antibody (1:300; Cat. No. sc-1826; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in 0.01 M PBS containing 0.04% normal rabbit serum and 0.2 % Triton X-100 for 20 hours at 4 °C with gentle agitation (Lukkes et al., 2009). Sections were washed in 0.01 M PBS containing 0.04% normal rabbit serum 2 times for 15 min, and incubated for 2 h at RT in biotin-conjugated donkey anti-goat secondary antibody (1:200; Cat. No.705-066-147, Jackson ImmunoResearch) in 0.01 M PBS containing 0.04% normal rabbit serum and 0.03% Triton X-100 to visualize CRF2 receptor-like immunoreactivity. After incubation with secondary antibody, sections were washed in 0.01 M PBS containing 0.04% normal rabbit serum 2 times for 15 min, and then placed in an avidin-biotin-peroxidase complex (ABC) reagent (Cat. No. PK-6106; Vector Laboratories, Burlingame, CA, USA) at 1:200 in 0.01 M PBS for 90 min. The tissue was then rinsed in 0.01 M PBS two times for 15 min each. The tissue was then placed in a peroxidase-based substrate reaction (Vector SG chromogen kit; Cat. No. SK-4700; Vector Laboratories) for 6 min. The reaction was stopped by rinsing the tissue in 0.01 M PBS for 15 min. After this the tissue was briefly rinsed in 0.1 M sodium phosphate buffer (PB) and mounted onto clean glass microscope slides. Once mounted the sections were dehydrated, cleared with xylene and mounted with cover slips.
2.1.4. Immunocytochemistry
Human embryonic kidney (HEK-293) cells that stably express the mouse CRF2α receptor and enhanced green fluorescent protein (EGFP) and wild type (WT) HEK-293 cells were used to determine the specificity of the anti-CRF2 receptor antibody (Chen et al., 2005a). Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM; Cat. No. 10938025; Invitrogen, Paisley, UK) solution supplemented with 10% fetal calf serum. For immunocytochemistry cells were grown on glass cover slips in a 90 cm2 petri dish. After reaching the desired confluency the cover slips were placed in 0.05 M PBS, and then placed in 4% paraformaldehyde in 0.05 M PBS for 30 min. The cover slips were then rinsed in 0.05 M PBS and then placed in 0.05 M PBS containing 0.3% Triton X-100 (PBST) for 5 min. Cover slips were then placed in 1% bovine serum albumin (BSA) in 0.05 M PBS for 30 min. Some cover slips were then placed in either goat anti-CRF2 receptor antibody (1:100; Cat. No. sc-1826; Santa Cruz Biotechnology) in 1% BSA, goat anti-CRF1/2 receptor antibody (1:100; Cat. No. sc-1757; Santa Cruz Biotechnology) in 1% BSA, or in 1% BSA overnight at 4 °C. Cover slips were then placed in 0.05 M PBS and then incubated in either a rabbit anti-goat antibody conjugated to Alexa Fluor 555 (1:500; Cat. No. A21431; Invitrogen, Paisley, UK) in 1% BSA, or 1% BSA for 2 h. The cover slips were then placed in 0.05 M PBS and mounted onto slides using a fluorescent mounting medium (Cat. No. S3023; DAKO, Ely, UK).
2.2. Western blot, microdissected brain regions
Adult male Sprague Dawley rats (n = 2; 250–300 g; Harlan Laboratories) were maintained as described above for immunohistochemical procedures and used for western blots. Western blots were performed to determine the relative molecular weight of the protein(s) recognized by the CRF2 receptor antibody used, and to determine the relative amounts of CRF2 receptor protein in different brain regions. Rats were decapitated, brains were then removed, frozen and stored at −80 °C, and then sectioned frozen (300 µm) using a cryostat (Leica CM 1900; North Central Instruments, Plymouth, MN, USA) at −12 °C. The motor cortex (M1), entorhinal cortex (En), lateral septum (LS), ventromedial hypothalamus (VMH), median raphe nucleus (MnR), and DR were dissected at −10 °C using the Palkovits punch technique (Palkovits and Brownstein, 1988) and a 22 gauge cannula (Cat. No. 18036-22; 0.41 mm i.d.; Fine Science Tools, Foster City, CA, USA). Tissue was then homogenized in 60 µl of HEPES buffer (Cat. No. H3375; Sigma-Aldrich, St. Louis, MO, USA) containing 2.4 µl protease inhibitor stock “complete” (1:25 dilution; Roche Diagnostics Ltd, Indianapolis, IN, USA). Total protein concentrations were determined within 5 µl sample duplicates using a Pierce protein assay (Cat. No. 1856210; Thermoscientific, Rockford, IL, USA) and a microplate reader (MultiSkan EX 355; Thermo Electron Corporation, Waltham, MA, USA). Samples (30 µg total protein) were processed for western blotting, and CRF2 receptor and actin levels were detected using methods described previously (Lukkes et al., 2009). Briefly, protein was mixed in 1 x sodium dodecyl sulfate (SDS)/β-mercaptoethanol loading buffer, vortexed and boiled for 3 min prior to separation by 8% SDS-polyacrylamide gel electrophoresis. Following electrophoresis (Mini-PROTEAN 3 cell, Bio-Rad Laboratories, Hercules, CA, USA), proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Immuno-Blot; 0.2 µm, Bio-Rad Laboratories). The membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) for 20 min at RT and incubated with goat anti-CRF2 receptor polyclonal antibody (1:100; Cat. No. sc-1826; Santa Cruz Biotechnology) in 5% non-fat dry milk in TBS-T overnight at 4 °C. The membranes were rinsed 3 times for 10 min each time at RT in TBS-T. After the rinsing procedure, the membranes were incubated for 2 h at RT in affinity-purified rabbit anti-goat IgG (whole molecule)-peroxidase conjugated secondary antibody (1:30,000; Cat. No. A5420; Sigma-Aldrich) in 5% non-fat dry milk in TBS-T. Control for protein loading was achieved by using mouse anti-actin primary antibody (1:2000; Cat. No. MAB1501R; Chemicon International, Billerica, MA, USA) and goat anti-mouse IgG (Fab specific)-peroxidase conjugated secondary antibody (1:5000; Cat. No. A3682, Sigma-Aldrich) in 5% non-fat dry milk in TBS-T. Proteins were detected using a ChemiDoc-IT imaging system with cooled CCD camera (UVP Bioimaging Systems, Upland, CA, USA).
2.3. Antibodies
The goat anti-CRF2 receptor antibody used (N-20, Cat. No. sc-1826, Santa Cruz Biotechnology) is an affinity-purified polyclonal antibody raised against a peptide corresponding to amino acid sequence 47–66 mapping at the N-terminus of the CRF2β receptor of mouse origin (RTTIGNFSGPYTYCNTTLDQ) (Figure 1). There is no similarity with the amino acid sequence of the CRF1 receptor (only 4 of 20 amino acids are conserved; Figure 1). The sequence used to generate the antibody is unique based on a search of the BLAST database; this does not preclude the possibility that there are peptides that have not been characterized that have similar sequences.
Fig. 1.
Amino acid sequences of the N-termini, up to the first predicted transmembrane domain, of mouse and rat CRF2α and CRF2β isoforms of the CRF2 receptor, as well as a soluble form of the mouse and rat CRF2α receptor, a truncated form of the rat CRF2α receptor, and the rat CRF1 receptor. Black line indicates the amino acid sequence corresponding to the 20 amino acid peptide from the mouse CRF2β receptor isoform that was used as the antigen for development of the polyclonal CRF2β receptor antibody (sc-1826) used in this study. Amino acid sequences were obtained from the following sources: mCRF2β (Perrin et al., 1995) (NCBI Protein database accession no.: Q60748), rCRF2β (NCBI Protein database accession no.: EDL88098), mCRF2α (Chen et al., 2005a) (NCBI Protein database accession no.: AAS07021), mCRF2αsol (Chen et al., 2005b; Evans and Seasholtz, 2009), (NCBI Protein database accession no.: AAU94301), rCRF2α-tr receptor (Miyata et al., 1999), rCRF2α (Lovenberg et al., 1995b) (NCBI Protein database accession no.: NP_073205), rat CRF2αsol (Evans and Seasholtz, 2009), rCRF1 receptor (Perrin et al., 1993) (NCBI Protein database accession no.: NP_112261). Abbreviations: mCRF2α, mouse CRF2α receptor; mCRF2β, mouse CRF2β receptor; mCRF2αsol, mouse soluble CRF2α receptor; rCRF2α, rat CRF2α receptor; rCRF2β, rat CRF2β receptor; rCRF2αsol, rat CRF2αsol receptor; rCRF2αtr, rat truncated CRF2α receptor; rCRF1, rat CRF1 receptor. Black shading indicates conserved amino acids; light gray shading indicates conservative amino acid substitutions. Alignments were obtained using: www.ebi.ac.uk/Tools/clustalw2/index.html.
The goat anti-CRF1/2 receptor antibody used (C-20, Cat. No. sc-1757, Santa Cruz Biotechnology) is a polyclonal antibody raised against a peptide corresponding to amino acid sequence 396–415 mapping at the C-terminus of the CRF1 receptor of human origin (SIPTSPTRVSFHSIKQSTAV). There is a 3 amino acid difference between the rat CRF1 and rat CRF2 receptor at the region that corresponds to this sequence. Western blot analysis indicates that this antibody recognizes a band corresponding to the predicted molecular weight of the CRF1 receptor in mice 77–80 kDa (Radulovic et al., 1998; Chen et al., 2000).
2.4. Analysis of the distribution of CRF2 receptor immunoreactivity
For analysis, the DR was divided into rostral (−6.92 to −7.64 mm bregma), mid-rostrocaudal (−7.73 to −8.45 mm bregma), and caudal (−8.54 to −9.26 mm bregma) regions (Abrams et al., 2004). Analysis of CRF2 receptor immunostaining in the DR was performed at 12 anatomical levels at 180 µm intervals (rostral DR; mid-rostrocaudal DR; and caudal DR; 1 section / level / rat). At each level the DR was further divided into different regions. These regions were the dorsal part of the DR (DRD), the ventral part of the DR (DRV), the ventrolateral part of the DR (DRVL), the caudal part of the DR (DRC; subdivided into dorsal (dDRC) and ventral (vDRC) parts at −9.08 mm bregma), and the interfascicular part of the DR (DRI). The rostrocaudal levels and subdivisions were defined based on a standard rat brain stereotaxic atlas (Paxinos and Watson, 1998).
2.5. CRF2 receptor/TrpOH double immunofluorescence
Adult male Sprague Dawley rats (n=6; 250–300 g; Harlan Laboratories) were maintained as described above for immunohistochemical procedures and used for CRF2 receptor/TrpOH double immunofluorescence.
Immunofluorescence was used to identify CRF2 receptor-expressing and TrpOH-expressing cells because this method provides the cellular resolution required to determine co-localization of CRF2 and TrpOH immunoreactivity. Perfusion and postfixation methods were as described above for CRF2 receptor immunohistochemistry.
For immunofluorescence, every sixth section of the midbrain containing the DR (from approximately −7.64 to −8.54 mm bregma) was removed from the cryoprotectant and washed in 0.05 M PBS twice, for 15 min each time. Sections were then incubated in 0.05 M PBS containing 0.3% Triton X-100 (PBST) and 0.01% sodium azide for 60 min at RT and then placed in mouse anti-TrpOH monoclonal antibody (1:2000; Cat. No. T-0678, Sigma-Aldrich) in PBST containing 0.01% sodium azide overnight at RT. Following incubation with primary antibody, sections were rinsed 2 times for 15 min each time in 0.05 M PBS and then placed in Cy5-conjugated donkey anti-mouse IgG secondary antibody (1:200; Cat. No. 115-175-205, peak emission 670 nm, red fluorescence; Jackson ImmunoResearch) in 0.05 M PBS for 1 h at RT. Sections were then washed in 0.05 M PBS 2 times for 15 min each time at RT and then incubated in goat anti-CRF2 receptor polyclonal antibody (1:300; Cat. No. sc-1826, Santa Cruz Biotechnology) in 0.01 M PBS containing 0.04% normal rabbit serum (Cat. No. 011-000-120, Jackson ImmunoResearch) and 0.2% Triton X-100 for 20 h at 4 °C with gentle agitation (Lukkes et al., 2009). Sections were then washed in 0.01 M PBS containing 0.04% normal rabbit serum 2 times for 15 min each time, and incubated for 2 h at RT in FITC-conjugated donkey anti-goat IgG secondary antibody (1:200; Cat. No. 705-095-147, peak emission 520 nm, green fluorescence; Jackson ImmunoResearch) in 0.01 M PBS containing 0.04% normal rabbit serum to visualize CRF2 receptor immunofluorescence. After incubation in secondary antibody, sections were washed in 0.01 M PBS containing 0.04% normal rabbit serum 2 times for 15 min each time and mounted onto glass slides, then cover-slipped with 4',6-diamidino-2-phenylindole DAPI Vectashield (Cat. No. H-1200, Vector Labs), which fluorescently stains cell nuclei.
2.6. Quantification of CRF2 receptor/TrpOH double immunofluorescence
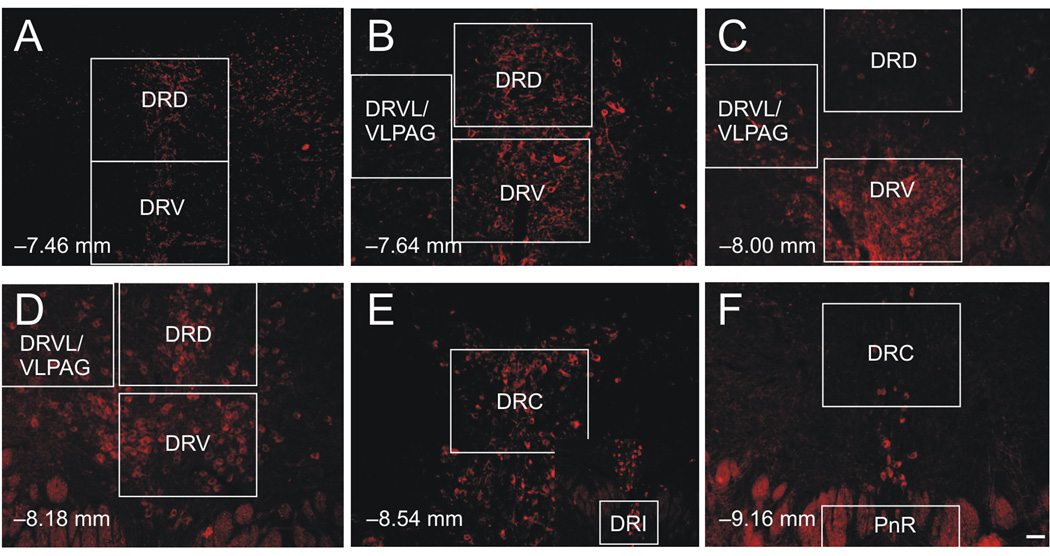
For each subject (n = 6; Sprague Dawley rats; Harlan Laboratories, Indianapolis, IN, USA), 100X and 200X magnification photomicrographs were generated for regions of interest using a Nikon 90i microscope and a Roper Scientific CoolSNAP ES digital camera linked to a computer with NIS Elements imaging software (A.G. Heinze Inc., Lake Forest, CA, USA). The 100X photomicrographs were used to identify and document the rostrocaudal level of the sample; 200X photomicrographs were used for quantification with each subdivision. For each photomicrograph, we placed the region of interest in the center of the field of view using the 20X objective lens and then took photographs using FITC, Cy5, and DAPI filters. Analysis of fluorescence in the DR and pontine raphe nucleus (PnR) was performed at 6 anatomical levels (−7.46, −7.64, −8.00, −8.18, −8.54, and −9.16 mm bregma; 1 section / level / rat; Figure 2), within the dorsal part of the DR (DRD), the ventral part of the DR (DRV), the ventrolateral part of the DR and ventrolateral part of the periaqueductal gray (DRVL/VLPAG), the interfascicular part of the DR (DRI), the caudal part of the DR (DRC), and PnR. The anatomical levels and subdivisions were defined based on a rat brain atlas (Abrams et al, 2004; Paxinos and Watson, 1998). Cell counts collected from photomicrographs were probably not biased by differences in cell number or density, as photomicrographs were obtained at a plane of focus in the middle of the tissue sections, which were 30 µm thick. Separate layers for CRF2 receptor-immunoreactive (ir) and TrpOH-ir photomicrographs were created using Adobe Photoshope CS (Adobe Systems Incorporated, San Jose, CA, USA). The numbers of CRF2 receptor-ir and TrpOH-ir neurons were quantified by placing dots over each CRF2 receptor-ir and TrpOH-ir profile in additional cell counting layers. The cell counting layers were superimposed and the numbers of single-labeled CRF2 receptors, single-labeled TrpOH, and double-labeled neurons were counted. Double-labeled (CRF2 receptor-ir/TrpOH-ir) neurons were confirmed with the slides themselves using 400X magnification.
Fig. 2.
Photomicrographs of tryptophan hydroxylase (TrpOH) immunofluorescence illustrating the subdivisions analyzed in the dorsal raphe nucleus (DR) at 6 different rostrocaudal levels (A–F). White boxes indicate regions photographed at higher magnification (200X) and used for analysis. The entire fields of view of the boxes indicating the regions sampled at 200X magnification for the dorsal raphe nucleus, ventrolateral part/ventrolateral part of the periaqueductal gray (DRVL/VLPAG; B–D) and pontine raphe nucleus (PnR; F) are not visible in the figure. Abbreviations: DRD, dorsal raphe nucleus, dorsal part; DRV, dorsal raphe nulceus, ventral part; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral part of the periaqueductal gray; DRI, dorsal raphe nucleus, interfascicular part; DRC, dorsal raphe nucleus, caudal part; and PnR, pontine raphe nucleus. Scale bar: 50 µm; inset scale bar: 100 µm.
2.7. Image capture
Brightfield photomicrographs were taken using a Nikon 90i microscope and a Nikon DS-Fi1 digital camera linked to a computer with NIS Elements 3.00 imaging software (A.G. Heinze Inc., Lake Forest, CA, USA). Low magnification images of immunofluorescence in cell culture studies were generated using a Leica DMRB fluorescence microscope, a Leica DC500 camera and image capture software (Leica Microsystems, Heidelberg, Germany). Confocal images of immunfluorescence in cell culture studies were generated using a Leica TCS SP2 AOBS laser scanning confocal microscope using FITC, Cy5, and DAPI filter cubes and Leica Confocal Software (v. 2.00, Leica Microsystems), presented as 8-µm-thick z-stack projections. Contrast and brightness of the photographs were adjusted using Adobe Photoshop CS (Adobe Systems Incorporated, San Jose, CA, USA). Photographic plates were prepared in CorelDraw for Windows 12.0 (Viglen Ltd., Wembley, UK).
3. Results
3.1. Immunocytochemistry
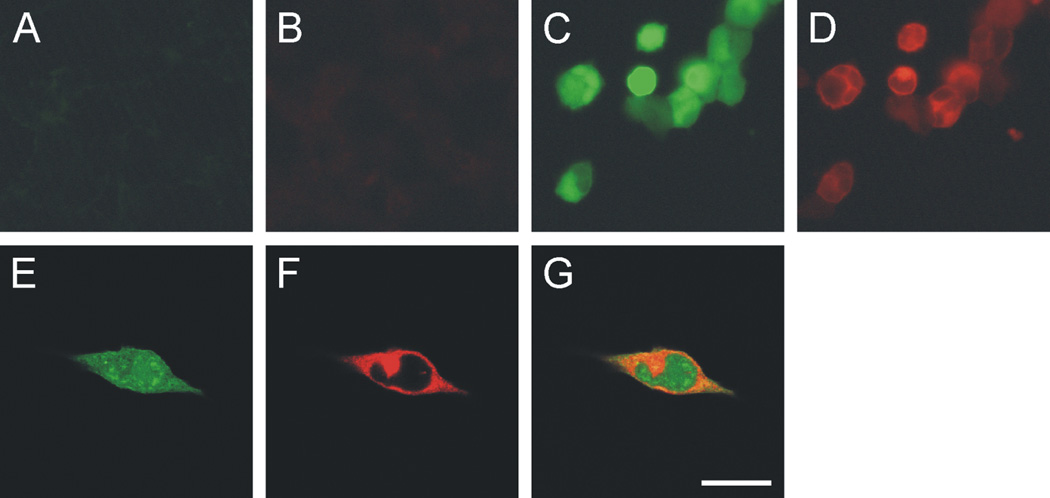
In order to determine the specificity of the anti-CRF2 receptor antibody used in these studies, we conducted immunocytochemical staining of wild type HEK-293 cells that are known to lack CRF2 receptor expression (Dautzenberg et al., 2000), and HEK-293 cells stably expressing the mouse CRF2α receptor and enhanced green fluorescent protein (EGFP). The anti-CRF2 receptor antibody did not immunostain wild type HEK-293 cells (Figure 3A,B) but did immunostain HEK-293 cells stably expressing the mouse CRF2α (mCRF2α) receptor (Figure 3D) and EGFP (Figure 3C; for additional controls, see Figure 4). High magnification confocal images revealed that the CRF2 receptor immunostaining was restricted to the plasma membrane and cytoplasmic compartments of the cell, while EGFP was expressed throughout the cell including the nuclear compartment (Figure 3E–G).
Fig. 3.
Photomicrographs illustrate immunocytochemical staining of HEK-293 wild type cells (A and B) or HEK-293 cells stably expressing enhanced green fluorescent protein (EGFP) and mCRF2α receptor (C–G). The green fluorescence (A, C, E, G) results from EGFP expressed in conjunction with the mCRF2α receptor. Note that only light green autofluorescence is present in HEK-293 wild type cells (A). B, D, F and G illustrate results of immunofluorescence using the goat anti-mCRF2β receptor antibody (sc-1826) used in this study. B, D, low magnification photomicrographs illustrating immunofluorescence with the goat anti-mCRF2β receptor antibody (sc-1826) used in this study. (E–G) high magnification confocal photomicrographs of immunofluorescence of HEK-293 cells stably expressing EGFP and mCRF2α receptor: (E) EGFP expression in the HEK-293 cells, (F) mCRF2α receptor immunofluorescence, (G) photomicrograph illustrating the cellular distribution of EGFP and mCRF2α receptor expression in HEK-293 cells. Scale bar = A–D, 60 µm; E–G, 20 µm.
As opposed to CRF2 receptors, wild type HEK-293 cells are known to express CRF1 receptors (Dautzenberg et al., 2000). The anti-CRF1/2 receptor antibody immunostained both the wild type HEK-293 cells and the HEK-293 cells that stably express the mCRF2α receptor and EGFP (Figure 4). The intensity of staining in the HEK-293 cells stably expressing the mCRF2α receptor was greater than in the wild type HEK-293 cells, suggesting that this antibody detected both endogenous CRF1 and CRF2 receptors in cells stably expressing the mCRF2α receptor (Figure 4).
CRF2 receptor immunostaining in CRF1 receptor knockout mice
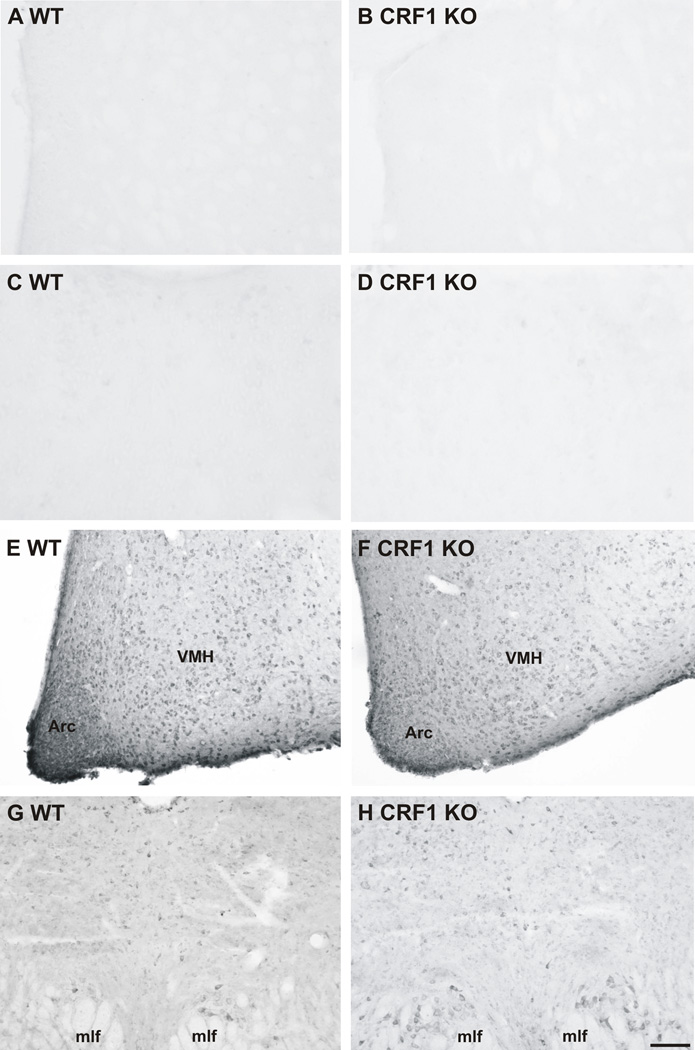
Very few, faintly stained, CRF2 receptor-ir cells were found in regions of the brain known to contain little to no CRF2 receptor mRNA expression, including the caudate putamen (CP; Figure 5A,B) and paraventricular nucleus of the thalamus (PV; Figure 4C,D (Van Pett et al., 2000)) in either wild type (Figure 5A,C) or CRF1 receptor knockout mice (CRF1 KO; Figure 5B,D). In contrast, large numbers of CRF2 receptor-ir cells were found in regions known to contain high amounts of CRF2 receptor mRNA expression, such as the arcuate nucleus (Arc; Figure 5E,F), ventromedial hypothalamus (VMH; Figure 5E,F), and dorsal raphe nucleus (DR; Figure 5G,H; (Van Pett et al., 2000)), in both wild type (Figure 5E,G) and CRF1 receptor KO mice (Figure 5F,H), indicating that the immunostaining was not due to cross-reactivity with CRF1 receptors.
Fig. 5.
Photomicrographs illustrating the distribution of CRF2 receptor-immunoreactive (ir) cells in specific brain regions of wild type (A, C, E, G) and CRF1 receptor knockout (B, D, F, H) mouse brains. (A–B) Very few, faintly immunostained, CRF2 receptor-ir cells were found in the caudate putamen of either wild type (A) or CRF1 receptor knockout mice (B). (C–D) Very few, faintly immunostained, CRF2 receptor-ir cells were found in the paraventricular nucleus of the thalamus of either wild type (C) or CRF1 receptor knockout mice (D). (E–F) Numerous and densely packed CRF2 receptor-ir cells were found throughout the arcuate nucleus (Arc) and ventromedial hypothalamus (VMH) of both wild-type (E) and CRF1 receptor knockout (F) mice. (G–H) CRF2 receptor-ir cells were found throughout the dorsal raphe nucleus (DR) of both wild type (G) and CRF1 receptor knockout (H) mice. Abbreviations: Arc, arcuate nucleus; DR, dorsal raphe nucleus; mlf, medial longitudinal fasciculus; VMH, ventromedial hypothalamus. Scale bar = A–B, E–H, 100 µm; C–D, 50 µm.
3.2. Western blot
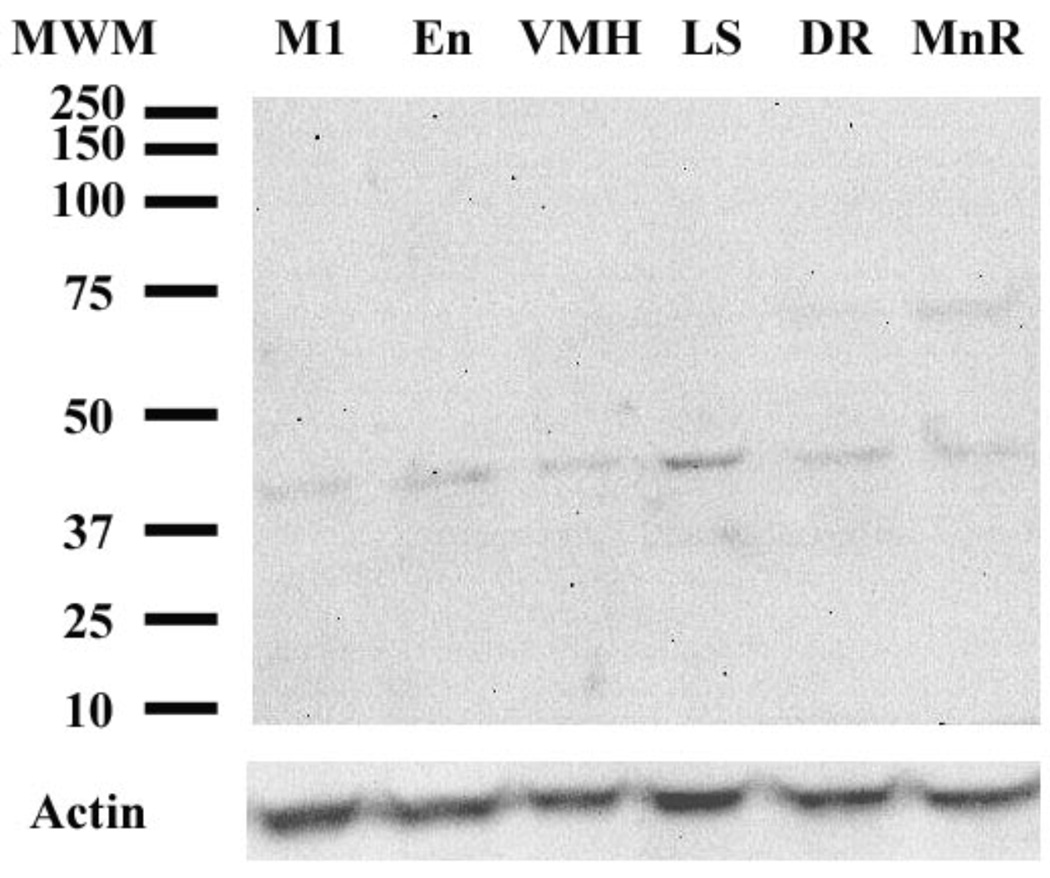
The anti-CRF2 receptor antibody recognized a band between 37 and 50 kDa (the predicted molecular weight of the full-length CRF2α receptor is about 40–45 kDa) in western blot analysis using homogenized microdissected brain regions (Figure 6). All brain regions examined are known to contain CRF2 receptor mRNA expression (Van Pett et al., 2000), and the density of the bands in different brain regions corresponded with the density of CRF2 receptor mRNA expression in the brain (Van Pett et al., 2000). For example, band density was highest in the LS, whereas it was lowest in cortical regions such as the En and M1 (Figure 6). There were no differences in the actin levels in different brain regions (Figure 6). There was no band at the predicted molecular weight for the CRF1 receptor (76–80 kDa; (Radulovic et al., 1998)) in any region examined (Figure 6).
Fig. 6.
Western blot analysis using the anti-CRF2 receptor antibody (sc-1826) in microdissected rat brain tissue. In all brain regions analyzed, the anti-CRF2 receptor antibody recognized one major band at between 37 and 50 kDA, which corresponds to the predicted molecular weight of the full-length CRF2 receptor. The density of the bands in different brain regions corresponded with the density of CRF2 receptor mRNA expression previously described in rat brain. Band density was highest in the lateral septum (LS), whereas it was lower in cortical regions such as the entorhinal cortex (En) and motor cortex (M1). There were no differences in actin levels among the different regions. Results are representative of three replicate western blots. Abbreviations: DR, dorsal raphe nucleus; En, entorhinal cortex; LS, ventral part of the lateral septum; M1, motor cortex; MnR, median raphe nucleus; MWM, molecular weight markers; VMH, ventromedial hypothalamus.
3.3. CRF2 receptor immunostaining in the rostral DR
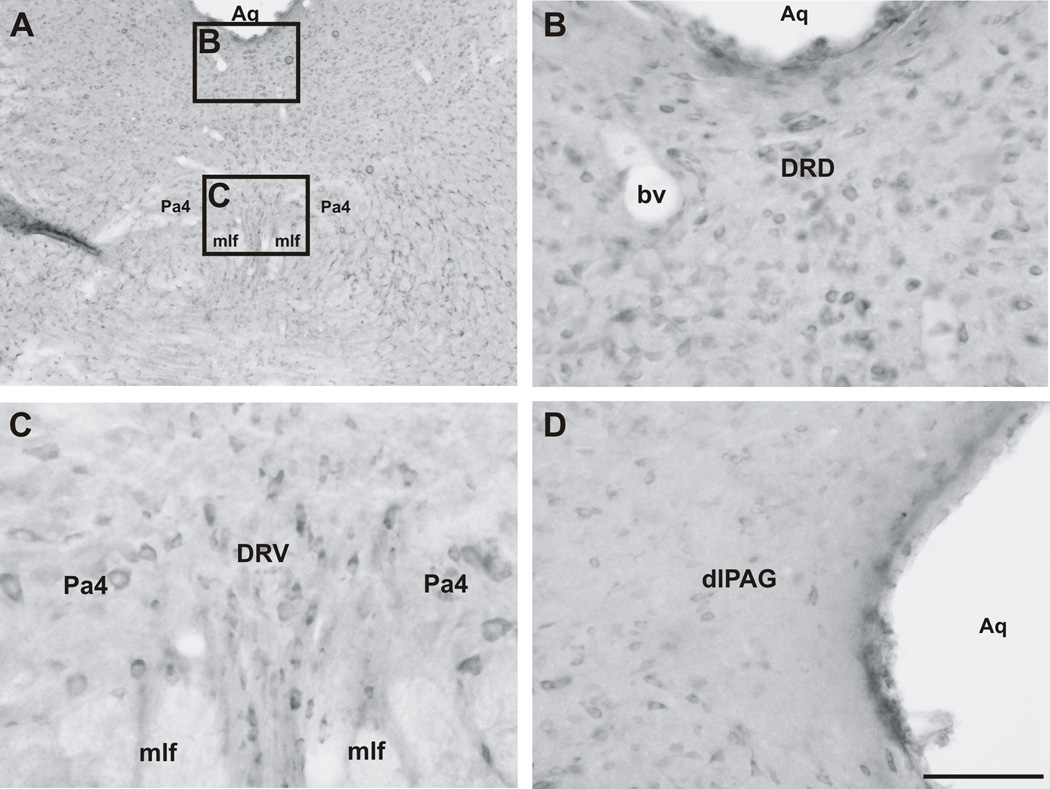
Rostral regions of the rat DR (−6.92 mm to −7.64 mm bregma) contained a low (in the most rostral sections of the rostral DR) to moderate (in the most caudal sections of the rostral DR; Figure 7A–C; Table 1) number of CRF2 receptor-ir cells. Although some CRF2 receptor-ir cells were apparent in structures adjacent to the DR, such as the periaqueductal gray region (Figure 7D) both the density and intensity of staining of cells were lower compared to regions in the DR. In the rostral DRD, CRF2 receptor-ir cells were dense near the cerebral aqueduct (Aq) at the midline, although some were located outside the boundaries of the DRD laterally (Figure 7A,B). In the most rostral part of the rostral DR, the majority of CRF2 receptor-ir cells were located along the midline within the DR (with some cells scattered outside the DR, in the adjacent oculomotor complex and periaqueductal gray; Figure 7A,B). Large, densely stained CRF2 receptor-ir cells were observed within the paratrochlear nucleus (Pa4; Figure 7A,C).
Fig. 7.
Photomicrographs illustrating the distribution of CRF2 receptor-ir cells in the rostral DR. (A) Low magnification image of CRF2 receptor immunostaining in the rostral DR (−7.10 mm bregma) and surrounding regions. The black boxes indicate regions photographed at higher magnification and shown in panels B and C. (B) Scattered CRF2 receptor-ir cells were found throughout the rostral dorsal part of the dorsal raphe nucleus (DRD). (C) Scattered CRF2 receptor-ir cells were found within the midline ventral part of the dorsal raphe nucleus (DRV) and the adjacent paratrochlear nucleus (Pa4). (D) Few, faintly-stained CRF2 receptor-ir cells were found within the dorsolateral periaqueductal gray (dlPAG) dorsal to the DR. Abbreviations: Aq, cerebral aqueduct; bv, blood vessel; DR, dorsal raphe nucleus; DRD, dorsal raphe nucleus, dorsal part; DRV, dorsal raphe nucleus, ventral part; mlf, medial longitudinal fasciculus; Pa4, paratrochlear nucleus; dlPAG, dorsolateral periaqueductal gray. Scale bar = A, 500 µm; B–D, 100 µm.
Table 1.
Expression of CRF, receptors in serotonergic cells of the rat DR, determined by dual immunofluorescence
| Region | Rostrocaudal level (mm bregma) |
# CRF2R+ neurons |
#TrpOH+ neurons |
# double-labeled neurons |
% TrpOH+ neurons that are CRF2R+ |
% CRF2R+ neurons that are 5-HT+ |
|---|---|---|---|---|---|---|
| DRD | −7.46 | 63.3 ± 8.2 | 42.7 ± 5.4 | 13.8 ± 1.8 | 35.2 ± 6.7 | 23.1 ± 3.6 |
| DRV | −7.46 | 51.8 ± 8.4 | 40.8 ± 5.9 | 16.3 ± 2.9 | 40.0 ± 6.6 | 31.7 ± 4.8 |
| DRD | −7.64 | 75.0 ± 9.6 | 41.3 ± 3.0 | 13.8 ± 2.5 | 32.6 ± 5.4 | 20.3 ± 3.8 |
| DRV | −7.64 | 98.4 ± 4.8 | 61.0 ± 7.5 | 32.5 ± 6.7 | 50.5 ± 7.9 | 38.0 ± 3.2 |
| DRVL/VLPAG | −7.64 | 83.9 ± 2.9 | 29.8 ± 4.6 | 10.8 ± 3.2 | 32.9 ± 5.2 | 14.5 ± 4.2 |
| DRD | −8.00 | 91.8 ± 4.6 | 64.2 ± 7.7 | 29.5 ± 5.0 | 44.6 ± 3.1 | 31.6 ± 4.8 |
| DRV | −8.00 | 104.2 ± 3.9 | 87.4 ± 1.8 | 44.3 ± 3.2 | 47.9 ± 2.5 | 42.0 ± 3.3 |
| DRVL/VLPAG | −8.00 | 78.8 ± 7.7 | 59.4 ± 5.7 | 18.8 ± 3.5 | 32.5 ± 6.7 | 26.7 ± 6.4 |
| DRD | −8.18 | 89.5 ± 10.8 | 64.0 ± 3.0 | 34.8 ± 0.9 | 54.4 ± 2.3 | 36.5 ± 2.4 |
| DRV | −8.18 | 86.2 ± 7.6 | 62.0 ± 6.9 | 36.0 ± 6.2 | 58.1 ± 8.3 | 41.2 ± 5.0 |
| DRVL/VLPAG | −8.18 | 68.8 ± 7.5 | 39.3 ± 3.7 | 17.8 ± 2.4 | 46.3 ± 6.3 | 26.0 ± 2.1 |
| DRC | −8.54 | 78.3 ± 8.5 | 54.7 ± 7.2 | 23.3 ± 4.4 | 43.3 ± 1.6 | 30.4 ± 6.0 |
| DRI | −8.54 | 28.2 ± 4.9 | 29.4 ± 3.8 | 12.4 ± 3.0 | 39.7 ± 7.6 | 44.9 ± 9.1 |
| DRC | −9.16 | 42.4 ± 5.5 | 40.5 ± 4.7 | 15.0 ± 1.8 | 38.5 ± 4.9+ | 35.6 ± 5.4 |
| PnR | −9.16 | 29.2 ± 5.7 | 24.8 ± 0.8 | 10.7 ± 1.7 | 39.3 ± 6.3 | 38.0 ± 4.1 |
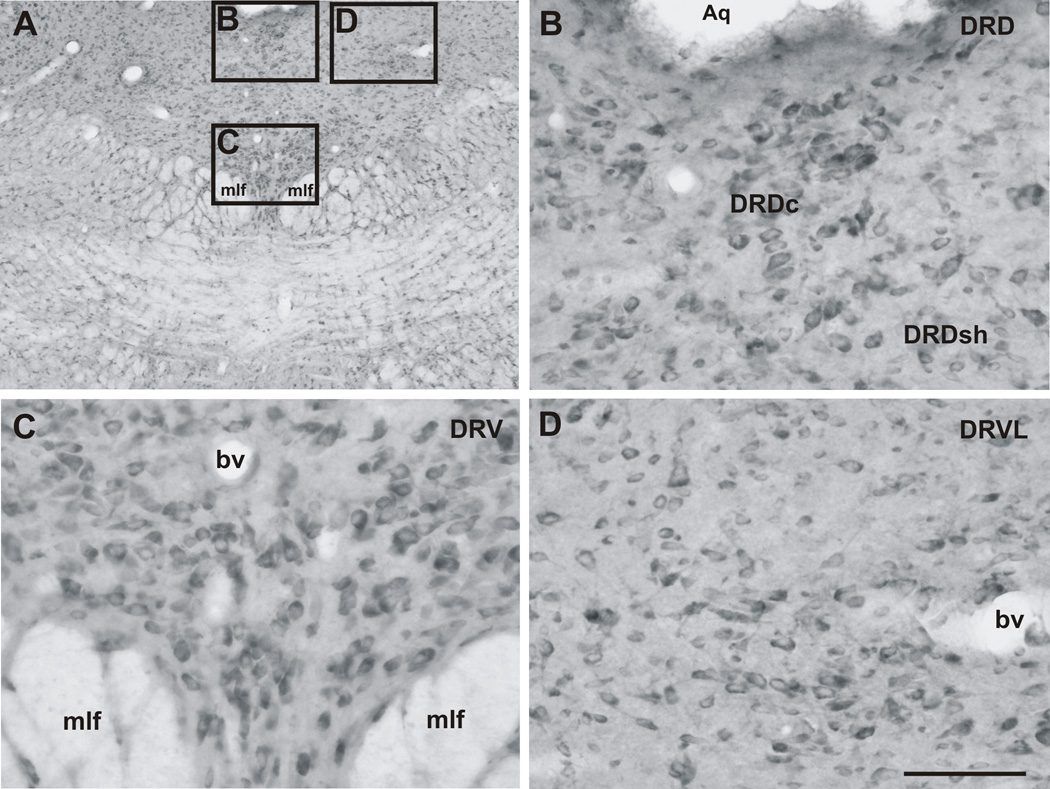
3.4. CRF2 receptor immunostaining in the mid-rostrocaudal DR
Mid-rostrocaudal regions of the DR (−7.73 mm to −8.45 mm bregma) contained the greatest density and numbers of CRF2 receptor-ir cells in the DR (Figure 8; Table 1). The largest numbers of CRF2 receptor-ir cells were located in the DRV, particularly at and around −8.00 mm bregma (104.2 ± 3.9 cells). At this level, although there were also many CRF2 receptor-ir cells scattered around the midline of the DRV, there were bilateral clusters of intensely stained CRF2 receptor-ir cells above the medial longitudinal fasciculus (mlf) on either side of the midline (Figure 8A,C). The majority of CRF2 receptor-ir cells were dorsal to the mlf, but some were located between the fiber bundles of the mlf with a few cells in the region lateral to the superior cerebellar peduncle (Figure 8A, C). A dense central cluster of CRF2 receptor-ir cells in the midline was located in the DRD throughout the mid-rostrocaudal DR (Figure 8A, B), a region referred to as the DRD core region by Abrams et al. (2005). There also were some CRF2 receptor-ir cells scattered outside this dense cluster (Figure 8A,B), within a region referred to as the DRD shell region by Abrams et al. (2005). Scattered CRF2 receptor-ir cells were present in the DRVL region (Figure 8A,D).
Fig. 8.
Photomicrographs illustrating the distribution of CRF2 receptor-ir cells in the mid-rostrocaudal DR. (A) Low magnification image of CRF2 receptor immunostaining in the mid-rostrocaudal DR (−8.00 mm bregma) and surrounding regions. The black boxes indicate regions photographed at higher magnification and shown in panels B–D. (B) CRF2 receptor-ir cells were differentially distributed in the dorsal part of the dorsal raphe nucleus (DRD) core (DRDc) and DRD shell (DRDSh) regions, with dense clusters of CRF2 receptor-ir cells in the DRDc and scattered CRF2 receptor-ir cells in the DRDSh. (C) Numerous and densely packed CRF2 receptor-ir cells were found throughout the mid-rostrocaudal dorsal raphe nucleus, ventral part (DRV). (D) Numerous CRF2 receptor-ir cells were found in the dorsal raphe nucleus, ventrolateral part (DRVL). Abbreviations: Aq, cerebral aqueduct; bv, blood vessel; DR, dorsal raphe nucleus; DRD, dorsal raphe nucleus, dorsal part; DRDc, dorsal raphe nucleus, dorsal part, core region; DRDSh, dorsal raphe nucleus, dorsal part, shell region; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; mlf, medial longitudinal fasciculus. Scale bar = A, 500 µm; B–D, 100 µm.
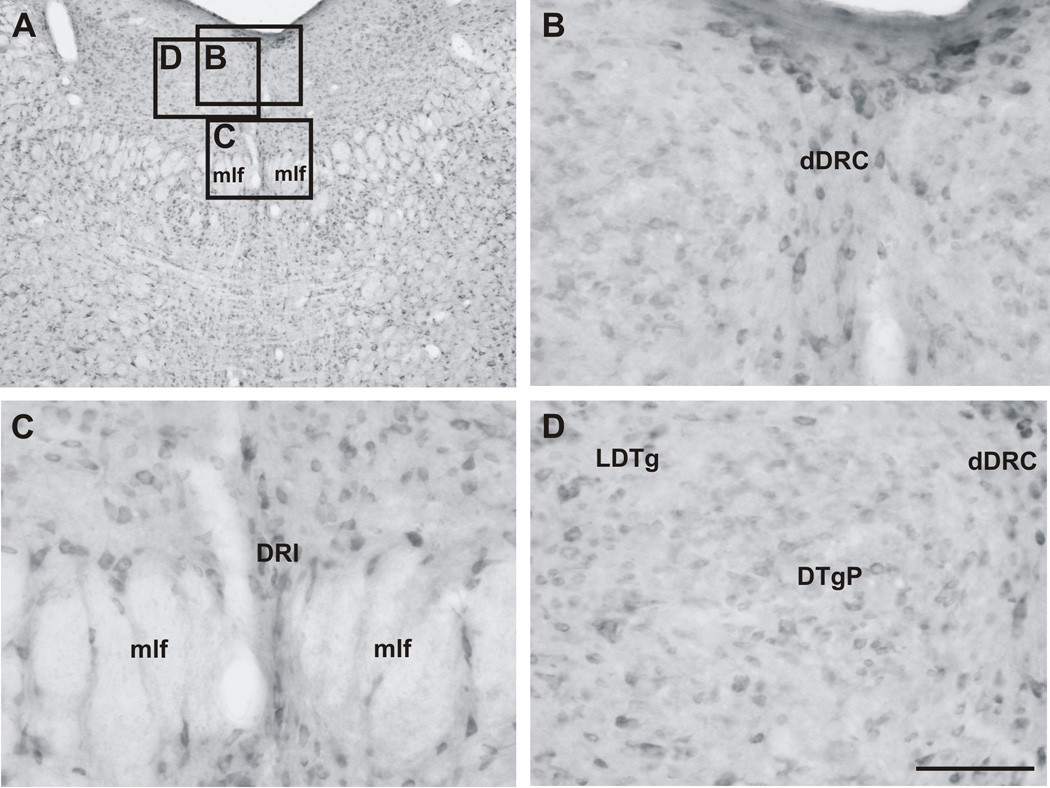
3.5. CRF2 receptor immunostaining in the caudal DR
Caudal regions of the DR (−8.54 mm to −9.26 mm bregma) contained fewer numbers of CRF2 receptor-ir cells (Figure 9A–C; Table 1), relative to the mid-rostrocaudal DR. CRF2 receptor-ir cells within the caudal DR were observed in the DRI where a small number of cells, oriented in a vertical plane between the mlf, were arranged in bilateral columns (Figure 9A, C). There were, however, a few scattered, intensely stained CRF2 receptor-ir cells dorsal to the DRI, in the DRC (Figure 9A, B). These were located in the midline ventrally, extending laterally in clusters of cells located just below the cerebral aqueduct in the dorsal part of the DRC (Figure 9A, B). Lateral to the DRC, scattered CRF2 receptor-ir cells were observed within the lateral and pericentral part of the dorsal tegmental nucleus (LTDg, DTgP; Figure 9A, D).
Fig. 9.
Photomicrographs illustrating the distribution of CRF2 receptor-ir cells in the caudal dorsal raphe nucleus (DR). (A) Low magnification image of CRF2 receptor immunostaining in the caudal DR (−8.90 mm bregma) and surrounding regions. The black boxes indicate regions photographed at higher magnification and shown in panels B–D. (B) Densely immunostained CRF2 receptor-ir cells were evident in the midline of the dorsal raphe nucleus, caudal part, dorsal part (dDRC) and in bilateral clusters ventral to the cerebral aqueduct. (C) CRF2 receptor-ir cells were found within the interfascicular part of the dorsal raphe nucleus (DRI), showing a vertical orientation and bipolar, fusiform shape, features that are consistent with the phenotype of serotonergic neurons in the region. (D) Scattered CRF2 receptor-ir cells were found within the adjacent dorsal tegmental nucleus, both the lateral (LDTg) and pericentral parts (DTgP). Abbreviations: Aq, cerebral aqueduct; bv, blood vessel; DR, dorsal raphe nucleus; dDRC, dorsal raphe nucleus, caudal part, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DTgP, dorsal tegmental nucleus, pericentral part; LDTg, dorsal tegmental nucleus, lateral part; mlf, medial longitudinal fasciculus. Scale bar = A, 500 µm; B–D, 100 µm.
3.6. CRF2 receptor/TrpOH double immunofluorescence
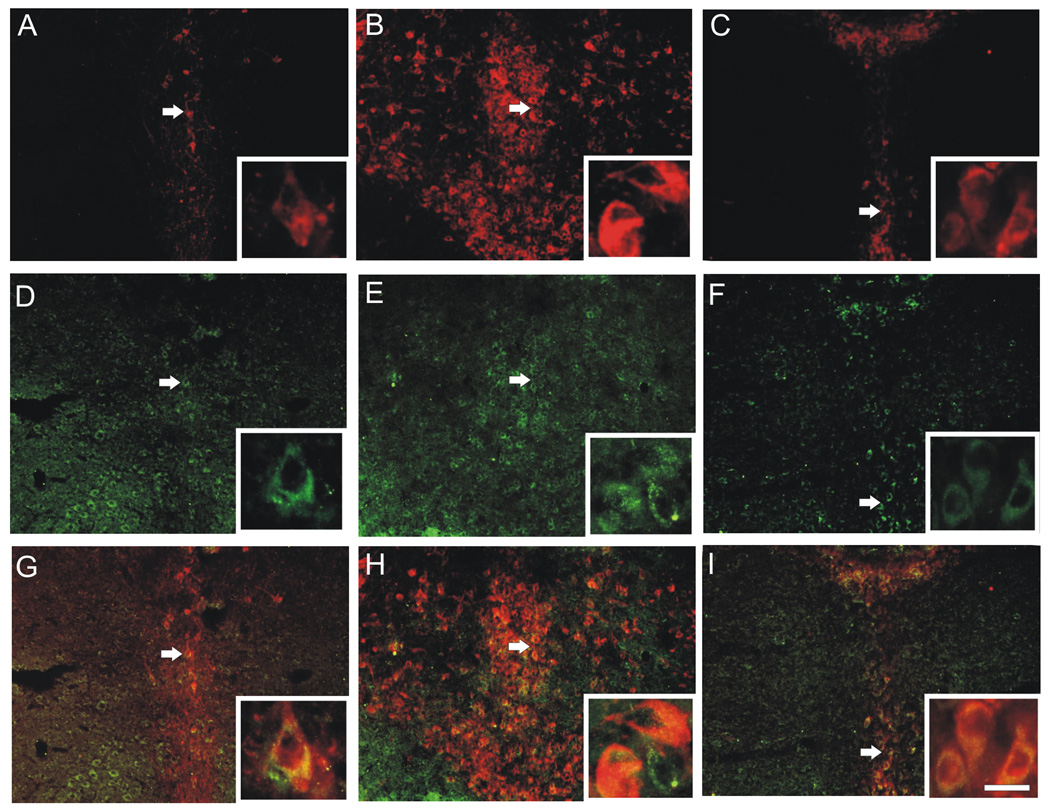
CRF2 receptor and TrpOH immunofluorescence were found throughout the rostrocaudal extent of the DR (Figure 10 and 11). CRF2 receptor immunofluorescence was found outside of the DR, but it was most prominent in the DR (Figure 10D–F). In addition, a significant proportion of TrpOH-positive neurons also expressed CRF2 receptors, (32.5–58.1%, depending on the subdivision) and caudal parts of the DR (Figure 10G–I; Table 1), which is consistent with previous studies describing co-localization of CRF2 receptor and serotonin transporter mRNA expression (Day et al., 2004).
Fig. 10.
Dual CRF2 receptor and tryptophan hydroxylase (TrpOH) immunofluorescence in the dorsal raphe nucleus (DR). Photomicrographs of TrpOH (Panels A–C), CRF2 receptor (Panels D–F), and CRF2 receptor/TrpOH double immunofluorescence (G–I) at the rostral (A, D, G), mid-rostrocaudal (B, E, H), and caudal (C, F, I) levels of the DR. Arrows indicate double-labeled cells shown in insets at the lower right corner of each photomicrograph. Scale bar, 100 µm, insets, 15 µm.
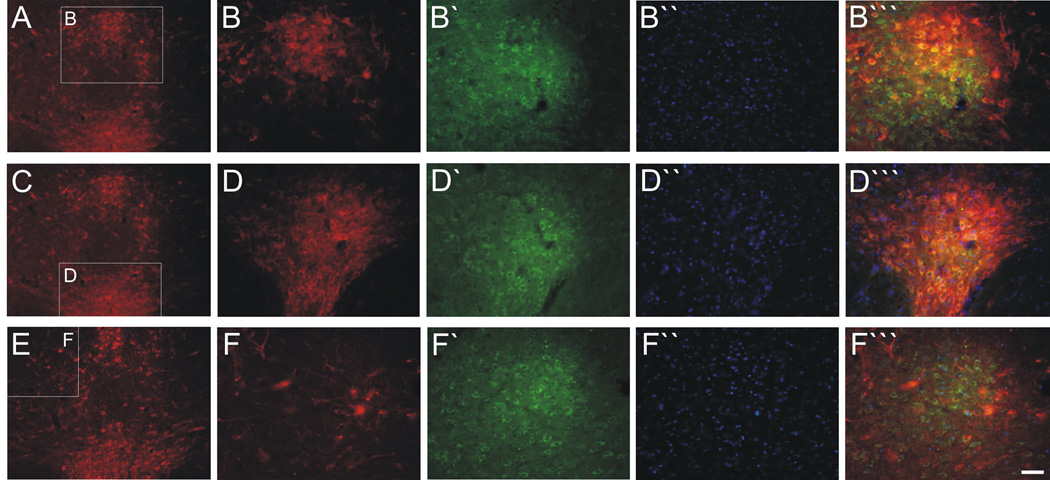
Fig. 11.
Photomicrographs of CRF2 receptor immunofluorescence and tryptophan hydroxylase (TrpOH) immunofluorescence in the rat DR that were used for quantification. Photomicrographs of TrpOH immunofluorescence (Panels A–F), CRF2 receptor immunofluorescence (Panels B`, D`, F`), DAPI (B``, D``, F``), and CRF2 receptor/TrpOH/DAPI triple immunofluorescence (B```, D```, F```) at a rostral anatomical level (−7.64). Row A–B``` represents the dorsal raphe nucleus, dorsal part (DRD), row C–D``` represents the dorsal raphe nucleus, ventral part (DRV) and row E–F``` represents the dorsal raphe nucleus, ventrolateral part (DRVL). Scale bar for Panels A, C, E: 50 µm, scale bar for rest of panels: 100 µm).
4. Discussion
CRF2 receptor-ir cells were distributed throughout the rostrocaudal extent of the DR, with clear regional differences in the number and density of cells. There were low to moderate numbers of CRF2 receptor-ir cells within the rostral and caudal DR. The highest densities and the greatest numbers of CRF2 receptor-ir cells were observed in the mid-rostrocaudal DR. At this anatomical level, CRF2 receptor-ir cells were observed in the dorsal, ventral, and, to a lesser extent, the ventrolateral parts of the DR. The highest densities of CRF2 receptor-ir cells within the DRD, DRV, and DRI corresponded to the location of the highest densities of serotonergic neurons in these regions (Steinbusch, 1981). In agreement with previous studies of CRF2 and serotonin transporter mRNA expression (Day et al., 2004), CRF2 receptor immunofluorescence was co-localized with TrpOH-ir neurons. These data show that CRF2 receptor-ir cells are expressed throughout the DR, but the highest concentrations seem to be restricted to specific subdivisions of the mid-rostrocaudal DR and in some cases specific parts of these subdivisions. These data indicate that CRF2 receptor mRNA expression previously described in the DR is associated with CRF2 receptor immunostaining of perikarya, and that the topographical distribution of the CRF2 receptor mRNA and protein expression are similar, including expression in serotonergic neurons.
4.1. Technical considerations
Immunostaining resulted in clear CRF2 receptor immunostaining of perikarya within the DR and other brain regions with high levels of CRF2 receptor mRNA expression. Recent studies by Waselus et al (2009) using electron microscopy and immunogold labeling have demonstrated that CRF2 receptor immunoreactivity is predominantly cytoplasmic in DR neurons under basal unstressed conditions (with a ratio of cytoplasmic to total immunogold particles of 0.85 ± 0.01), and shifts toward a greater expression at the plasma membrane following stress exposure (with a ratio of cytoplasmic to total immunogold particles of 0.56 ± 0.03). Although we cannot distinguish plasma membrane-associated from cytoplasmic CRF2 receptor in our studies, our data are suggestive of expression of CRF2 receptor in both cellular compartments in unstressed rats, consistent with findings by Waselus et al (2009). Interestingly, the anti-CRF2 receptor antibodies used in the studies by Waselus et al (2009) and in our studies were both directed at the N-terminus of the CRF2 receptor (human CRF2 receptor; Novus Biologicals, personal communication), and both identified a single major band between 37–50 kDa, as determined using western blot (Wang et al., 2007)
4.2. CRF2 receptor isoforms
It is possible that the CRF2 receptor immunostaining described here represents immunostaining of CRF2α, CRF2β, CRF2α-tr, and/or soluble (s)CRF2α receptor isoforms in the rat DR. The antibody that was used is likely to recognize all known isoforms of the CRF2 receptor because the antibody recognizes a 12-amino acid sequence of the N-terminus that is nearly identical in all CRF2 receptor isoforms that have been characterized (Figure 1). A recent study (Evans and Seasholtz, 2009) has shown that the sCRF2α receptor is found in the rat brain, and that the mRNA is efficiently translated. In addition Evans and Seasholtz (2009) showed that the sCRF2α receptor is not trafficked to the membrane, which may account in part for the apparent abundance of cytoplasmic staining seen in this study and previous studies (Waselus et al., 2009). Consistent with these findings, recent studies by Schulz and colleagues (2010) showed that CRF2α receptor contains an N-terminal pseudo signal peptide that is unable to target the peptide to the endoplasmic reticulum membrane, resulting in very low cell surface expression (Schulz et al., 2010). Furthermore, Tian and colleagues (Tian et al., 2006) showed that the sc-1826 antibody recognizes the CRF2α-tr receptor isoform using western blot analysis (band at 16–32 kDa) in the forebrain, olfactory bulb, and cerebellum of rats and mice. The sc-1826 antibody was also shown to recognize the CRF2α-tr receptor in HEK-293 cells expressing the CRF2α-tr receptor isoform, using both immunocytochemistry and western blot analysis (Tian et al., 2006). Based on the immunohistochemical and immunofluorescence results in our study, together with in situ hybridization studies, receptor autoradiography studies, and functional studies using direct microinjections of selective CRF2 receptor agonists and antagonists within the DR, it seems likely that at least one high-affinity receptor for CRF2 receptor ligands is present in the DR, and perhaps more. Future work using specific antibodies or probes will be required to determine which isoform(s) of the CRF2 receptor are expressed in the DR, their relative abundance, and functional properties.
4.3. Topography of the distribution of CRF2 receptor-ir cells in the DR
The dorsal and ventral portions of the mid-rostrocaudal DR, where we observed the greatest concentrations of CRF2 receptor-ir cells, have unique patterns of anatomical projections to and from forebrain limbic structures. The mid-rostrocaudal DRD (relative to other subdivisions of the DR) is known to receive strong projections from specific forebrain structures including the prelimbic cortex, central amygdaloid nucleus, bed nucleus of the stria terminalis, medial and lateral preoptic area, paraventricular nucleus of the hypothalamus, and dorsal hypothalamic area (Peyron et al., 1998; Vertes, 2004). The mid-rostrocaudal DRD is known to project to a number of forebrain regions involved in regulation of emotional behavior, including the medial prefrontal cortex, basolateral amygdaloid nucleus, central amygdaloid nucleus, nucleus accumbens, dorsal hypothalamic nucleus, and bed nucleus of the stria terminalis (Imai et al., 1986; Van Bockstaele et al., 1993; Petrov et al., 1994; Petit et al., 1995; Commons et al., 2003; Abrams et al., 2005). This pattern of projections suggests that this region provides a major contribution to the dorsal raphe forebrain tract, one of six major serotonergic tracts innervating the forebrain (Azmitia and Segal, 1978; Azmitia, 1981; Lowry et al., 2008). The mid-rostrocaudal DRV also contains large numbers of neurons projecting to the basolateral amygdaloid nucleus (Abrams et al., 2005) as well as large numbers of neurons projecting to the caudate putamen and horizontal limb of the diagonal band of Broca (Steinbusch et al., 1980; Steinbusch, 1981; Semba et al., 1988). The mid-rostrocaudal DRV (relative to other subdivisions of the DR) receives strong projections from the lateral orbital cortex and medial and lateral preoptic areas (Peyron et al., 1998). Some of the forebrain regions that project to the mid-rostrocaudal DR contain neurons that express Ucn 2 and Ucn 3, both of which have high affinity for CRF2 receptors and therefore could be sources of endogenous ligand(s) acting on full-length CRF2 receptors within the DR (reviewed by (Reul and Holsboer, 2002)). For example, neurons within the paraventricular nucleus of the hypothalamus express Ucn 2 mRNA while neurons within the bed nucleus of the stria terminalis and medial and lateral preoptic areas express Ucn 3 mRNA (Peyron et al., 1998; Lewis et al., 2001; Reyes et al., 2001; Li et al., 2002). Consistent with the hypothesis that urocortins play an important role in regulation of serotonergic systems and behavior, Ucn 1/Ucn 2 knockout mice (Neufeld-Cohen et al., 2009) as well as Ucn 1/Ucn 2/Ucn 3 triple knockout mice have altered baseline serotonergic activity in neural systems regulating anxiety states and altered anxiety-like behavior (Neufeld-Cohen et al., 2010). The pattern of CRF2 receptor immunostaining in the DR lends support to the proposal that the antibody used in the current study recognizes one or more protein products of the CRF2 receptor gene in the rat brain. It is known that Ucn 1-ir neurons from the Edinger-Westphal nucleus also project to the DR (Bittencourt et al., 1999), but it is currently unclear if the DR is also innervated by Ucn 2-, or Ucn 3-containing fibers. As the DR is one of the few brain structures where CRF2 receptor mRNA expression is higher than CRF1 receptor mRNA expression, future studies should examine the innervation of the DR by high-affinity CRF2 receptor ligands including Ucn 2 and Ucn 3 and the sources of fibers containing these CRF-related neuropeptides.
4.5. Electrophysiological responses to CRF2 receptor ligands in the DR
The current study, describing high numbers of CRF2 receptor-ir cells in the mid-rostrocaudal DR, is in agreement with a number of electrophysiological studies investigating the effects of CRF and CRF-related neuropeptides on serotonergic neuronal firing rates. Electrophysiological studies provide strong evidence that functional CRF2 receptors are expressed within the mid-rostrocaudal DR. Low concentrations of ovine CRF, concentrations that are likely to bind to CRF1 receptors (Primus et al., 1997), microinjected into the DR decrease the in vivo neuronal firing rates of DR serotonergic neurons (Kirby et al., 2000), while high concentrations of ovine CRF, doses that are likely to bind to CRF1 and CRF2 receptors (Primus et al., 1997), increase both in vivo and in vitro firing rates of DR serotonergic neurons (Lowry et al., 2000; Kirby et al., 2000). In vitro extracellular recording studies by Lowry and colleagues (Lowry et al., 2000) revealed that the majority of presumed serotonergic neurons activated by CRF were located in the mid-rostrocaudal DRV relative to the mid-rostrocaudal DRD. These in vitro extracellular recordings are consistent with our current findings, as the highest density of CRF2 receptor-ir cells were found in the mid-rostrocaudal DRV region. Microinjections of low doses of Ucn 2 (mUcn 2; Valentino, personal communication) into the mid-rostrocaudal DR of anesthetized rats inhibits the firing rates of serotonergic neurons, while injections of high doses of mUcn 2 into the same region increase the firing rates of serotonergic neurons (Pernar et al., 2004). Thus, the concentrations of both CRF and mUcn 2 appear to be critical for their effects on the neuronal activity of serotonergic neurons in the DR, with a possibility of both inhibitory and excitatory responses depending on the local concentration of ligand. Future studies should take into account the rostrocaudal and dorsoventral differences in the distribution of CRF2 receptors as well as the possibility of the presence of multiple isoforms of CRF2 receptors in the DR.
4.6. Functional implications—anxiety-related physiological and behavioral effects of CRF2 receptor ligands in the DR
Our current findings of high numbers of CRF2 receptor-ir cells in the mid-rostrocaudal DR are in agreement with a number of studies investigating neurochemical and behavioral responses to intra-DR injections of CRF2 receptor ligands. Direct microinjections of mUcn 2 into the DR induce potentiation of fear conditioning and escape deficits measured 24 hours later in a model of learned helplessness (Hammack et al., 2003a). This potentiation of fear conditioning and escape deficits is prevented by prior injections of ASV-30 but not the CRF1 receptor antagonist 2-methyl-4-(N-propyl-N-cycloproanemethylamino)-5-chloro-6-(2,4,6-trichloranilino)pyrimidine (NBI27914) (Hammack et al., 2003b). Furthermore, the potentiation of fear conditioning and escape deficits was observed following injections of high doses of CRF (doses likely to bind to CRF2 receptors (Primus et al., 1997)) into the caudal, but not the rostral, DR (Hammack et al., 2002). These functional studies suggest that activation of CRF2 receptors within the DR, particularly within the mid-rostrocaudal and caudal DR may play an important role in the regulation of emotional behavior.
Intra-DR microinjections of Ucn 1, which binds with high affinity to both CRF1 and CRF2 receptors, have also been shown to induce physiological and behavioral responses. Intra-DR injections of Ucn 1 induce hypothermia and decrease food and fluid consumption in mice (Turek and Ryabinin, 2005; Weitemier and Ryabinin, 2006). Furthermore, intra-DR injections of the CRF2 receptor antagonist ASV-30 prevent ethanol-induced hyperthermia (Turek and Ryabinin, 2005). Together, these functional studies support the hypothesis that CRF2 receptors within the DR are involved in the regulation of multiple physiological and behavioral responses, consistent with their widespread distribution in the DR.
In conclusion, this study describes for the first time the distribution of CRF2 receptor-ir cells throughout the DR. The highest numbers and the highest densities of CRF2 receptor-ir cells were found in the mid-rostrocaudal DR, particularly in the DRD and DRV subdivisions. Together with previous studies, these findings are consistent with the hypothesis that CRF2 receptor activation within the mid-rostrocaudal and caudal DR is an important component of neural mechanisms regulating anxiety- or stress-related physiological and behavioral responses. Based on the widespread distribution of CRF2 receptors in the DR and the functional heterogeneity of the DR, CRF2 receptors in this region are likely to modulate diverse physiological and behavioral responses.
Functionally integrated CRF and mesolimbocortical serotonergic systems arising from the mid-rostrocaudal and caudal DR are likely to play an important role in stress-related psychopathology including anxiety and mood disorders, as well as stress-induced relapse to drug abuse (Arborelius et al., 1999; Sarnyai et al., 2001; Heinrichs and Koob, 2004). CRF innervation of the human DR is greatest in the mid-to caudal levels (Austin et al., 1997), and depressed patients have increased CRF immunoreactivity in the caudal subnucleus of the DR (Austin et al., 2003). In addition, recent studies of human depressed suicide patients have revealed that the widely reported increases in tryptophan hydroxylase 2 (tph2) mRNA and protein expression in the DR (Underwood et al., 1999; Bonkale et al., 2006; Bach-Mizrachi et al., 2006) may be restricted to the caudal subnucleus (Bach-Mizrachi et al., 2008) and its projection sites (Perroud et al., 2010). Consequently, dysregulation of CRF2 receptor signaling within the caudal DR may have important implications for affective disorder and other stress-related neuropsychiatric disorders.
Acknowledgements
C.A. Lowry was supported by a Wellcome Trust Research Career Development Fellowship (RCDF 068558/Z/02/Z), and a 2007 NARSAD Young Investigator Award and is currently supported by an NSF CAREER Award (NSF-IOS #0845550). The project described was supported by Award Numbers R01MH065702 (AS/CAL), R01MH086539 (CAL) and 1F32MH084463 (JLL) from the NIMH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Index of Abbreviations
- 4V
4th ventricle
- Aq
cerebral aqueduct
- Arc
arcuate nucleus
- ASV-30
antisauvagine-30
- bv
blood vessel
- CP
caudate putamen
- CNS
central nervous system
- CRF
corticotropin-releasing factor
- CRF1 receptor
CRF type 1 receptor
- CRF2 receptor
CRF type 2 receptor
- dlPAG
dorsolateral periaqueductal gray
- dDRC
dorsal raphe nucleus, caudal part, dorsal part
- DR
dorsal raphe nucleus
- DRD
dorsal raphe nucleus, dorsal part
- DRI
dorsal raphe nucleus, interfascicular part
- DRV
dorsal raphe nucleus, ventral part
- DRVL
dorsal raphe nucleus, ventrolateral part
- DTgP
dorsal tegmental nucleus, pericentral part
- En
entorhinal cortex
- EGFP
enhanced green fluorescent protein
- KO
knockout
- LDTg
laterodorsal tegmental nucleus
- LV
lateral ventricle
- LS
lateral septum
- M1
motor cortex
- mlf
medial longitudinal fasciculus
- MnR
median raphe nucleus
- Pa4
paratrochlear nucleus
- PV
paraventricular nucleus of the thalamus
- TrpOH
tryptophan hydroxylase
- Ucn1 2, 3
urocortin 1, 2, 3
- mUcn 2
urocortin 2 (mouse)
- TrpOH
tryptophan hydroxylase
- vDRC
dorsal raphe nucleus, caudal part, ventral part
- VMH
ventromedial hypothalamus
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Austin MC, Janosky JE, Murphy HA. Increased corticotropin-releasing hormone immunoreactivity in monoamine-containing pontine nuclei of depressed suicide men. Mol Psychiatry. 2003;8:324–332. doi: 10.1038/sj.mp.4001250. [DOI] [PubMed] [Google Scholar]
- Austin MC, Rhodes JL, Lewis DA. Differential distribution of corticotropin-releasing hormone immunoreactive axons in monoaminergic nuclei of the human brainstem. Neuropsychopharmacology. 1997;17:326–341. doi: 10.1016/S0893-133X(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Azmitia EC. The serotonin-producing neurons of the midbrain median and dorsal raphe nuclei. In: Iversen LL, Iversen SH, Snyder SH, editors. Chemical Pathways in the Brain. New York: Plenum; 1981. pp. 233–314. [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- Bonkale WL, Turecki G, Austin MC. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse. 2006;60:81–85. doi: 10.1002/syn.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RE, Grove KL, Smith MS. Distribution of corticotropin releasing hormone receptor immunoreactivity in the rat hypothalamus: coexpression in neuropeptide Y and dopamine neurons in the arcuate nucleus. Brain Res. 2003;973:223–232. doi: 10.1016/s0006-8993(03)02487-9. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Perrin M, Brar B, Li C, Jamieson P, DiGruccio M, Lewis K, Vale W. Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol. 2005a;19:441–458. doi: 10.1210/me.2004-0300. [DOI] [PubMed] [Google Scholar]
- Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, Lewis KA, Rivier JE, Sawchenko PE, Vale WW. A soluble mouse brain splice variant of type 2{alpha} corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci U S A. 2005b;102:2620–2625. doi: 10.1073/pnas.0409583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc Natl Acad Sci U S A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Higelin J, Teichert U. Functional characterization of corticotropin-releasing factor type 1 receptor endogenously expressed in human embryonic kidney 293 cells. Eur J Pharmacol. 2000;390:51–59. doi: 10.1016/s0014-2999(99)00915-2. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RT, Seasholtz AF. Soluble Corticotropin-Releasing Hormone Receptor 2{alpha} Splice Variant is Efficiently Translated, but not Trafficked for Secretion. Endocrinology. 2009;150:4191–4202. doi: 10.1210/en.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornal CA, Metzler CW, Marrosu F, Ribiero-do-Valle LE, Jacobs BL. A subgroup of dorsal raphe serotonergic neurons in the cat is strongly activated during oral-buccal movements. Brain Res. 1996;716:123–133. doi: 10.1016/0006-8993(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, De Souza EB. Heterogeneity between brain and pituitary corticotropin-releasing factor receptors is due to differential glycosylation. Endocrinology. 1989;125:1877–1888. doi: 10.1210/endo-125-4-1877. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav Brain Res. 2003a;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002;22:1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003b;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311:427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- Hinkle RT, Donnelly E, Cody DB, Samuelsson S, Lange JS, Bauer MB, Tarnopolsky M, Sheldon RJ, Coste SC, Tobar E, Stenzel-Poore MP, Isfort RJ. Activation of the CRF 2 receptor modulates skeletal muscle mass under physiological and pathological conditions. Am J Physiol Endocrinol Metab. 2003;285:E889–E898. doi: 10.1152/ajpendo.00081.2003. [DOI] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol. 1986;243:363–380. doi: 10.1002/cne.902430307. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21:9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Varga V, Dahan L, Sik A. Serotonergic neuron diversity: Identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci U S A. 2006;103:1059–1064. doi: 10.1073/pnas.0508360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Veening JG, Kozicz T, Henckens M, Dederen J, Groenink L, van der GJ, Olivier B, Roubos EW. Distribution and expression of CRF receptor 1 and 2 mRNAs in the CRF over-expressing mouse brain. Brain Res. 2006;1072:46–54. doi: 10.1016/j.brainres.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Kostich WA, Chen A, Sperle K, Largent BL. Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol. 1998;12:1077–1085. doi: 10.1210/mend.12.8.0145. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Nair HP, Young LJ. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. J Comp Neurol. 2005;487:75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Chalmers DT, Liu C, De Souza EB. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995a;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995b;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Evans A, Gasser P, Hale MW, Staub DR, Shekhar A. Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. In: Monti J, Pandi-Perumal S, Jacobs BL, Nutt DJ, editors. Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Basel: Birkhauser; 2008. pp. 25–68. [Google Scholar]
- Lowry CA, Hale MW. Serotonin and the neurobiology of anxious states. In: Muller CP, Jacobs BL, editors. The Behavioural Neurobiology of Serotonin. Elsevier; 2010. [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Summers CH, Scholl JL, Renner KJ, Forster GL. Early life social isolation alters corticotropin-releasing factor responses in adult rats. Neuroscience. 2009;158:845–855. doi: 10.1016/j.neuroscience.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Miyata I, Shiota C, Chaki S, Okuyama S, Inagami T. Localization and characterization of a short isoform of the corticotropin-releasing factor receptor type 2alpha (CRF(2)alpha-tr) in the rat brain. Biochem Biophys Res Commun. 2001;280:553–557. doi: 10.1006/bbrc.2000.4112. [DOI] [PubMed] [Google Scholar]
- Miyata I, Shiota C, Ikeda Y, Oshida Y, Chaki S, Okuyama S, Inagami T. Cloning and characterization of a short variant of the corticotropin-releasing factor receptor subtype from rat amygdala. Biochem Biophys Res Commun. 1999;256:692–696. doi: 10.1006/bbrc.1999.0392. [DOI] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Evans AK, Getselter D, Spyroglou A, Hill A, Gil S, Tsoory M, Beuschlein F, Lowry CA, Vale W, Chen A. Urocortin-1 and −2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.115. [DOI] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Tsoory MM, Evans AK, Getselter D, Gil S, Lowry CA, Vale WW, Chen A. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc Natl Acad Sci U S A. 2010;107:19020–19025. doi: 10.1073/pnas.1013761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Brownstein M. Maps and Guide to Microdissection of the Rat Brain. New York: Elsever; 1988. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Fourth Edition. San Diego, CA: 1998. [Google Scholar]
- Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci U S A. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MH, Donaldson CJ, Chen R, Lewis KA, Vale WW. Cloning and functional expression of a rat brain corticotropin releasing factor (CRF) receptor. Endocrinology. 1993;133:3058–3061. doi: 10.1210/endo.133.6.8243338. [DOI] [PubMed] [Google Scholar]
- Perroud N, Neidhart E, Petit B, Vessaz M, Laforge T, Relecom C, La HR, Malafosse A, Guipponi M. Simultaneous analysis of serotonin transporter, tryptophan hydroxylase 1 and 2 gene expression in the ventral prefrontal cortex of suicide victims. Am J Med Genet B Neuropsychiatr Genet. 2010 doi: 10.1002/ajmg.b.31059. [DOI] [PubMed] [Google Scholar]
- Petit JM, Luppi PH, Peyron C, Rampon C, Jouvet M. VIP-like immunoreactive projections from the dorsal raphe and caudal linear raphe nuclei to the bed nucleus of the stria terminalis demonstrated by a double immunohistochemical method in the rat. Neurosci Lett. 1995;193:77–80. doi: 10.1016/0304-3940(95)11669-n. [DOI] [PubMed] [Google Scholar]
- Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277:289–295. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17:308–316. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Sydow S, Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (CRFR1) in the rat and mouse central nervous system. J Neurosci Res. 1998;54:507–521. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Heym J, Jacobs B. Activity of serotonin-containing neurons in nucleus centralis superior of freely moving cats. Exp Neurol. 1984;83:302–317. doi: 10.1016/S0014-4886(84)90100-6. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Crochet S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neuroscience. 2001;104:1141–1155. doi: 10.1016/s0306-4522(01)00103-8. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Schulz K, Rutz C, Westendorf C, Ridelis I, Vogelbein S, Furkert J, Schmidt A, Wiesner B, Schulein R. The pseudo signal peptide of the corticotropin-releasing factor receptor type 2a decreases receptor expression and prevents Gi-mediated inhibition of adenylyl cyclase activity. J Biol Chem. 2010;285:32878–32887. doi: 10.1074/jbc.M110.129627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Reiner PB, McGeer EG, Fibiger HC. Brainstem afferents to the magnocellular basal forebrain studied by axonal transport, immunohistochemistry, and electrophysiology in the rat. J Comp Neurol. 1988;267:433–453. doi: 10.1002/cne.902670311. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF(2)) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, van der KD, Verhofstad AA, Pellegrino A. Serotonergic and non-serotonergic projections from the nucleus raphe dorsalis to the caudate-putamen complex in the rat, studied by a combined immunofluorescence and fluorescent retrograde axonal labeling technique. Neurosci Lett. 1980;19:137–142. doi: 10.1016/0304-3940(80)90184-6. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Verhofstad AA, Joosten HW. Localization of serotonin in the central nervous system by immunohistochemistry: description of a specific and sensitive technique and some applications. Neuroscience. 1978;3:811–819. doi: 10.1016/0306-4522(78)90033-7. [DOI] [PubMed] [Google Scholar]
- Stout SC, Owens MJ, Lindsey KP, Knight DL, Nemeroff CB. Effects of sodium valproate on corticotropin-releasing factor systems in rat brain. Neuropsychopharmacology. 2001;24:624–631. doi: 10.1016/S0893-133X(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Tian JB, Shan X, Bishop GA, King JS. Presynaptic localization of a truncated isoform of the type 2 corticotropin releasing factor receptor in the cerebellum. Neuroscience. 2006;138:691–702. doi: 10.1016/j.neuroscience.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Turek VF, Ryabinin AE. Ethanol versus lipopolysaccharide-induced hypothermia: involvement of urocortin. Neuroscience. 2005;133:1021–1028. doi: 10.1016/j.neuroscience.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;39:245–270. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–463. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Lin SZ, Kuo JS, Huang HY, Tzeng SF, Liao CH, Chen DC, Chen WF. Urocortin modulates inflammatory response and neurotoxicity induced by microglial activation. J Immunol. 2007;179:6204–6214. doi: 10.4049/jimmunol.179.9.6204. [DOI] [PubMed] [Google Scholar]
- Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-Induced Redistribution of Corticotropin-Releasing Factor Receptor Subtypes in the Dorsal Raphe Nucleus. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J Comp Neurol. 2005;482:155–165. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Urocortin 1 in the dorsal raphe regulates food and fluid consumption, but not ethanol preference in C57BL/6J mice. Neuroscience. 2006;137:1439–1445. doi: 10.1016/j.neuroscience.2005.10.021. [DOI] [PubMed] [Google Scholar]