Abstract
Developing and maintaining an exemplary research team is essential to the success of a quality clinical research program.
Staff is one of the most important, yet expensive, components of a research program. Of the total time and effort required to conduct a clinical trial, nurses and data managers each contribute > 30% of the time, whereas physicians account for only 9%.1 Although investigators appreciate the research team's value, they are often uncertain about how to properly develop and manage the team.
To help investigators develop the required knowledge and skills to run a clinical research program, Journal of Oncology Practice (JOP) chose to publish several articles on this topic. This article is part of the series on exemplary attributes of clinical trial sites, highlighting the attributes of sites conducting quality clinical trials.2
In the most recent issue of JOP is an original research article reporting on the Ontario Protocol Assessment (OPAL) tool, which is an evidence-based tool for assessing trial complexity and associated workload.3 The OPAL tool is an important addition to the field because it accounts for trial complexities that were not addressed in workload tools developed in previous decades.
Delegation of Research Tasks
A research site's principal investigator is responsible for oversight of clinical trials but can delegate research tasks to appropriately trained staff (investigator responsibilities are discussed in the previous article in the JOP series on exemplary attributes).4 Matching individuals with suitable tasks ensures that the program's resources are being used appropriately and that staff enjoy and remain challenged by their responsibilities.
Knowing what task can be delegated to individuals on the research team can be challenging because it varies on the basis of an individual's experience and licensure. A research team may include clinical research associates (CRAs), research nurses, data managers, and study coordinators. Depending on a program's organizational structure, the responsibilities assigned to each may differ greatly from one research program to another, both within and outside the United States. Tasks assigned to the staff include screening for potential study candidates, determining eligibility, coordinating the patient calendar, preparing documents for submission to institutional review boards, filing amendments, submitting safety data, conducting patient education, obtaining informed consent, and assessing potential adverse events. When resources permit, it is also helpful to have a manager or coordinator who supervises the program by overseeing quality assurance, staffing, budgeting, and site audits.5 This person will work closely with the primary investigator to ensure that all investigator responsibilities are being met. A common mistake to avoid is delegating tasks to someone who is overqualified, such as assigning the task of photocopying to a registered nurse. Poor delegation leads to inefficient use of the program's resources and may cause staff to become dissatisfied with their duties. The clinical research manager can help ensure that tasks are being delegated appropriately.
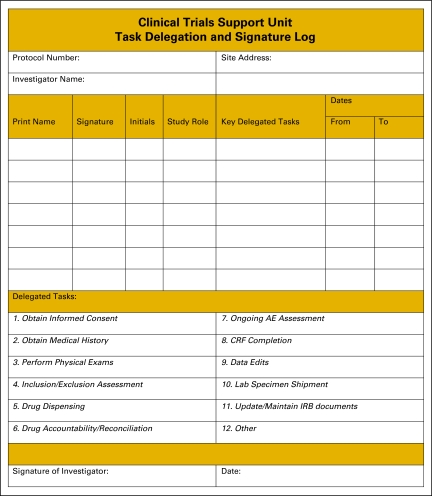
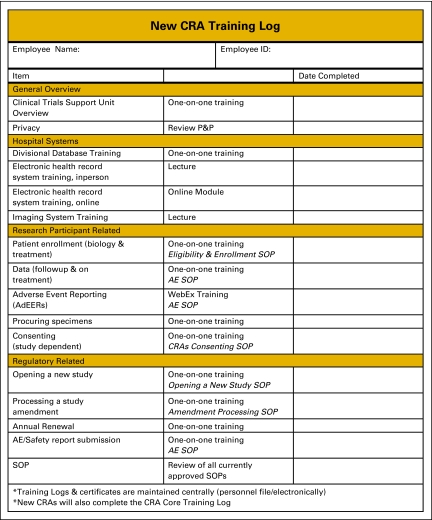
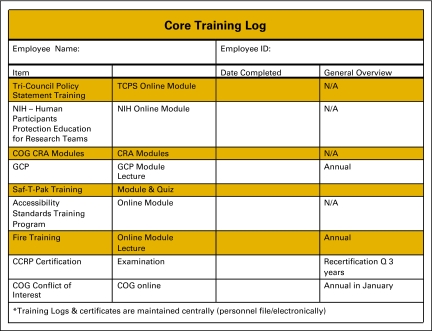
Once tasks have been delegated, many sites will use a delegation log to document which staff person has been delegated each specific study task (Figure 1). A delegation log should be completed for each study open at the site, because staff responsibilities often vary among trials. The site should also keep documentation of training modules that have been completed by each staff member. Training logs might be divided into (1) training that must be completed by new members of the research team (Figure 2), and ongoing training that must be maintained by the entire research team (Figure 3). Standard operating procedures (SOPs)6 that address training requirements can further document the process and ensure it is sustainable. In addition to pursuing basic research training requirements, CRAs and research nurses should be encouraged to pursue specialty certification because instruction about conducting clinical research is generally not taught in health science or nursing education programs. Certifications are offered by the Society of Clinical Research Associates7 and the Association of Clinical Research Professionals.8
Figure 1.
Clinical Trials Support Unit task delegation log. AE, adverse event; CRF, case report form; IRB, institutional review board. From Hospital for Sick Children, Toronto, Ontario, Canada. Adapted with permission.
Figure 2.
Training log for new clinical research associates (CRAs). P&P, policies and procedures; SOP, standard operating procedure; AE, adverse event. From Hospital for Sick Children, Toronto, Ontario, Canada. Adapted with permission.
Figure 3.
Training log for clinical trials core tasks. TCPS, Tri-Council Policy Statement; NIH, National Institutes of Health; COG, Children's Oncology Group; CRA, clinical research associate; GCP, good clinical practice; CCRP, certified clinical research professionals. From Hospital for Sick Children, Toronto, Ontario, Canada. Adapted with permission.
It is most effective when research staff are assigned solely to the research program. For example, if the research program can only support 0.5 full-time equivalent research associates/nurses, then the program should hire a part-time employee whose sole responsibility is to perform research. When one position is split between both research and clinical practice, the research tasks often get neglected.
The Extended Research Team
The research team extends well beyond the individuals who are directly employed by the research program. For example, infusion nurses are generally not on the payroll of the research program but are often responsible for administering investigational drugs and monitoring trial participants during infusion of the agent. The same is true of pharmacists, who may or may not be employed directly by the research program. It is important that pharmacists who support the research program receive training regarding drug accountability, because requirements vary greatly between standard practice and clinical research. There are also administrative roles that might be delegated to individuals external to the research program, such as billing and contracting. Patient navigators and advocates also play a critical role at many clinical research sites. Collaboration among all individuals who support the clinical research program will enable the full potential of the program to be realized.
Physician colleagues are vital to the success of the research program even when they are not listed as an investigator on the protocol. Surgeons, pathologists, radiologists, and many other specialists play an integral role in the conduct of quality clinical trials. It is important that all of these individuals are knowledgeable about the requirements of clinical trials to which they will be contributing, especially specific laboratory or diagnostic imaging requirements. The site's clinical trial implementation process should include collaboration with these specialists, who provide invaluable perspective regarding the feasibility and site-specific logistics of the protocol requirements.9 Outreach to primary care providers is also important. Every physician who interacts with a patient can influence the patients' understanding and desire to participate on clinical trials.
Because the direct benefit of clinical research may not be apparent to all medical professionals, efforts to promote support among staff are essential. Awareness about clinical trials needs to occur at all levels of the institution and should be incorporated in the mission and vision of the site. For some professionals, education and awareness activities can make a positive impact on their willingness to contribute. This can be done via in-person meetings in which individuals can connect with the research team and have their questions answered. Clinical professionals should also be educated about specific trials open at the site. For example, an informal lunch might be held to educate infusion nurses about current clinical trials open at the site and any special requirements associated with infusion of the investigational agent and potential adverse effects for which patients should be monitored.
Beyond education, there are several other ways to promote staff support of the research program, such as discussing how the research program can be a source of revenue for various departments. For example, although a special laboratory test may require increased effort on behalf of the staff in the laboratory, it might also represent a new way for the lab to generate income. Because private trial sponsors usually reimburse at cost, reimbursement for the tests might be higher than what is paid by third-party payers. Physician colleagues might also benefit from discussions about how the clinical research program can contribute to research that is important to them. For example, a pathologist who conducts basic or translational research might volunteer time to the clinical research program because specimens that are collected for the purpose of research ultimately enable the progress of the pathologists' research. Working collaboratively and understanding the opinions and needs of colleagues at the site will promote a culture of clinical research.
Promoting Staff Satisfaction
Even in a challenging economy, a number of things can be done to promote staff satisfaction and maintain, or improve, staff retention. Foremost, it is important that the program's leadership show respect and esteem for all staff. This can be done by listening to staff concerns, showing interest, and engaging staff in decision making. Engaging staff not only improves staff satisfaction, but it also helps the program make more informed decisions. For example, sites that engage staff in the selection of new trials promote a team culture within the site while also benefiting from perspectives that may be outside the physicians' scope, such as determining the feasibility of completing certain protocol-required testing at the site.
Encouraging staff to attend research meetings and participate in professional development activities also promotes staff satisfaction while maintaining quality within the program. Possible professional development activities may include attending meetings of the site's National Cancer Institute Cooperative Group affiliation or participating in sessions offered by a professional society. Professional development may also meet requirements for an individual's licensure or certification. A research program should develop a policy on how costs associated with continuing education will be managed, then budget accordingly. Costs associated with continuing education include registration fees, travel, and staff time away from the office.
Developing an internal promotion system is also a valuable way to retain employees. Some sites do this by creating different tiers within each position title. Under this system, a new CRA generally begins at an entry level and is responsible for tasks that are suited to their level of skill and training. As the individual progresses, they may be promoted to a midlevel position, and eventually to a senior-level position. A tiered system prevents new staff from becoming overwhelmed and ensures that senior staff remain engaged and challenged.
Professional accomplishments and recognition are also very important for staff satisfaction. When possible, sites should provide coauthorship opportunities to CRAs and nurses who had an integral role on the study. Staff may also play an important role in conceptualizing and developing certain research projects, such as cancer control studies or research regarding trial accrual. Even when coauthorship opportunities are not possible, leaders of the program should be certain to disseminate information about the outcome of any research to which the site's team contributed, such as articles reporting the results of multicenter trials that were open at the site.
Preparing for Staff Turnover
With adequate preparation and cross-training, staffing changes can have a minimal impact on the research program and provide an opportunity for the program to welcome new employees who bring additional skills and perspective to the team. An important way to prepare for staff turnover is to cross-train staff so more than one person knows how to conduct each task. This can be done both formally and informally; for example, double-checking colleagues' work for quality assurance is an informal way the entire team can become familiar with all projects being conducted at the site.
It is also important to fill open positions wisely. First, consider whether it is possible to promote from within the program to fill the position. When hiring new staff, be certain to look beyond their experience to determine whether an individual is going to be good fit for the program. Because of the expense and time needed to train new staff, it is important to find individuals whose attitude and work ethic meet the needs of the program.10 Also, a manager should use this staffing change as an opportunity to review position descriptions at the site and determine whether each position provides the opportunity for staff to make important contributions to the program. If this is not the case, then modifications should be made. No one person, including a new employee, should be delegated all the unpleasant tasks of the program. If this happens, dissatisfaction is likely to occur and can lead to frequent staff turnover.
Summary
Developing and maintaining an exemplary research team is essential to the success of a quality clinical research program. Functions such as regulatory compliance, protocol maintenance, patient care, tissue acquisition and transmittal, data collection and submission, and general administration are among the many tasks on which quality clinical research, protection of subjects' rights, and advancement of science depend. No single individual could expect to fulfill all of these tasks.
Effective management requires shared commitment to excellence, mutual respect for each team member's role, and effective communication. Once staff members are trained and data management systems are implemented, this infrastructure has to be maintained and refreshed continually. Ultimately, a leader of an effective research program must acknowledge the value of each of its members while promoting a culture of teamwork and commitment to delivering high-quality care to patients.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Susan Devine, SoCRA Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Allison R. Baer, Robin Zon, Susan Devine, Alan P. Lyss
Administrative support: Allison R. Baer
Collection and assembly of data: Allison R. Baer
Data analysis and interpretation: Allison R. Baer, Robin Zon, Susan Devine, Alan P. Lyss
Manuscript writing: Allison R. Baer, Robin Zon, Susan Devine, Alan P. Lyss
Final approval of manuscript: Allison R. Baer, Robin Zon, Susan Devine, Alan P. Lyss
References
- 1.Emanuel E, Schnipper L, Kamin D, et al. The costs of conducting clinical research. J Clin Oncol. Nov;:4145–4150. doi: 10.1200/JCO.2003.08.156. [DOI] [PubMed] [Google Scholar]
- 2.Zon R, Meropol N, Catalano R, et al. American Society of Clinical Oncology statement on minimum standards and exemplary attributes of clinical trial sites. J Clin Oncol. 2008:2562–2567. doi: 10.1200/JCO.2007.15.6398. [DOI] [PubMed] [Google Scholar]
- 3.Smuck B, Bettello P, Berghout K, et al. Ontario Protocol Assessment Level: Clinical trial complexity rating tool for oncology clinical trials. J Oncol Pract. 2011;7:80–84. doi: 10.1200/JOP.2010.000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baer AR, Devine S, Beardmore CD, et al. Clinical investigator responsibilities. J Oncol Pract. 2011;7:124–128. doi: 10.1200/JOP.2010.000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zon R. Role of the Oncology Team. Presented at the International Clinical Trials Workshop; January 27-28, 2011; Cairo, Egypt. http://www.semco-oncology.info/6-SEMCO_Download.html. [Google Scholar]
- 6.International Conference on Harmonisation, Good Clinical Practice (ICH GCP) 1.55 Standard Operating Procedures. http://ichgcp.net/?page_id_377.
- 7.Society of Clinical Research Associates. http://www.socra.org/
- 8.Association of Clinical Research Professionals. http://www.acrpnet.org/
- 9.American Society of Clinical Oncology. A Multidisciplinary Approach to Clinical Trials: The Role of Surgeons. http://www.asco.org/ASCOv2/Research+Resources/Research+Activities/Clinical+Trial+Resources/Clinical+Trial+Videos.