Abstract
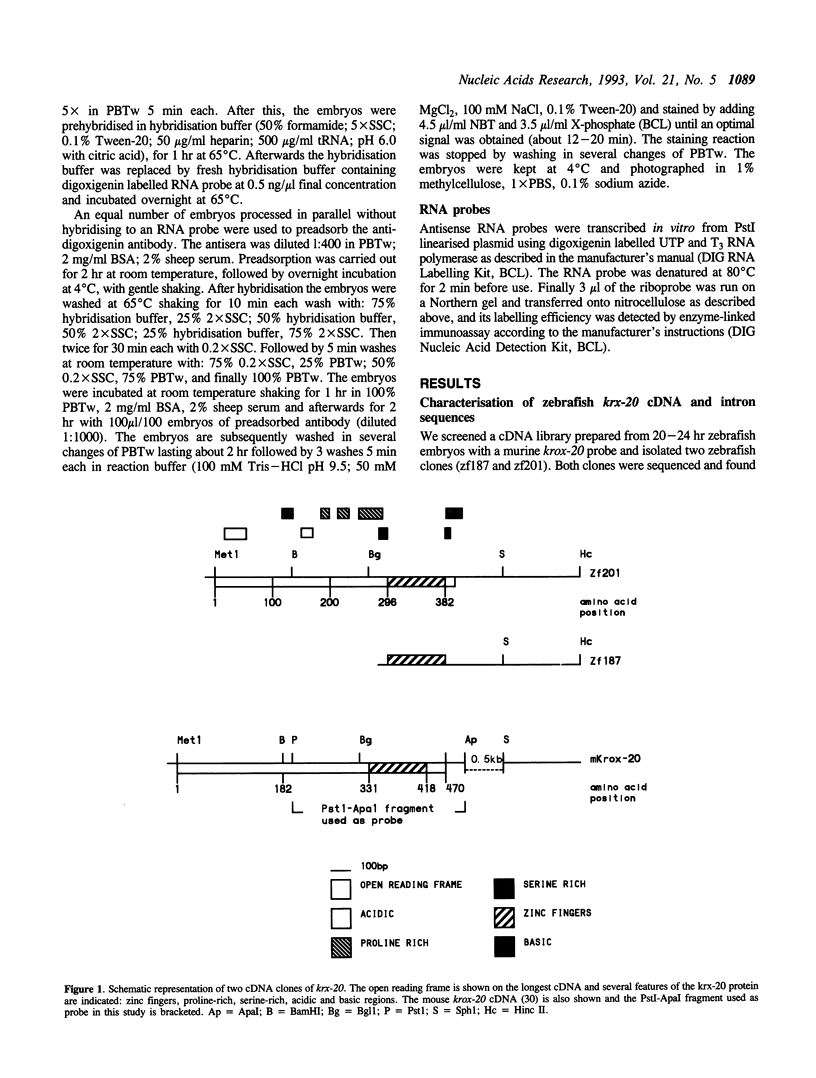
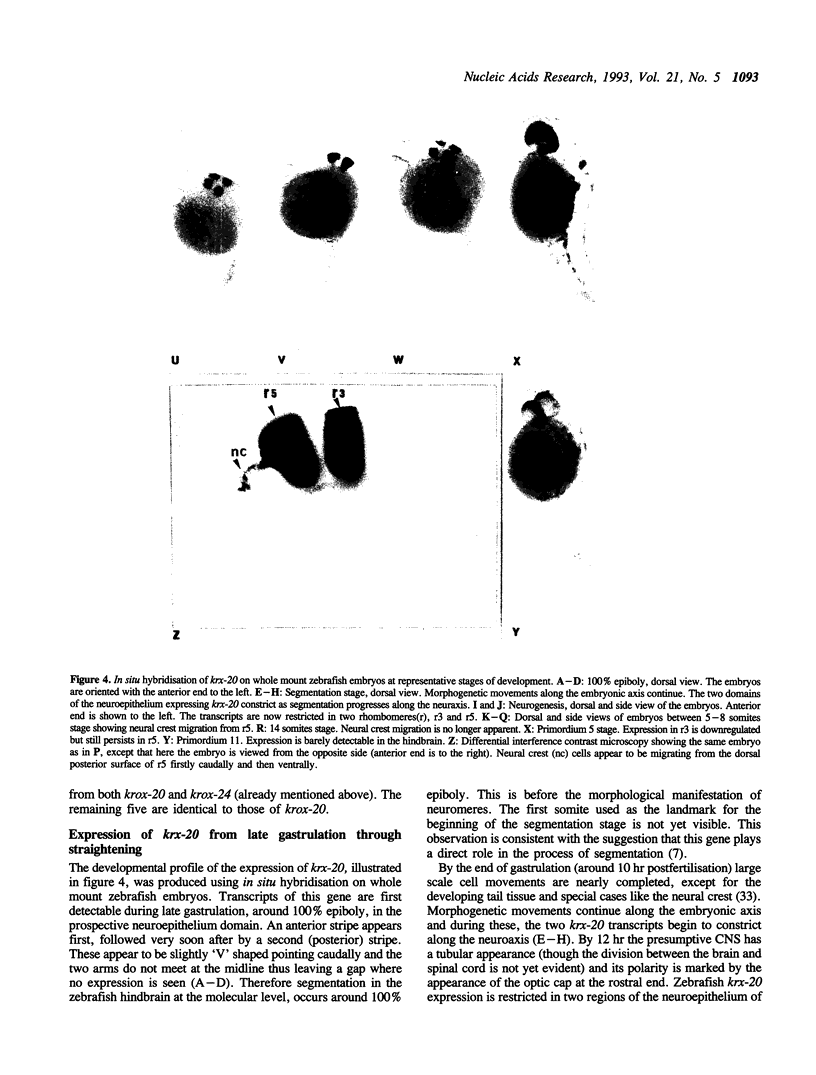
To begin to examine the function of genes that control early development in the hindbrain, we have screened an embryonic zebrafish cDNA library with a murine krox-20 gene probe that contained the conserved zinc finger regions. We have isolated two overlapping cDNAs, zf187 and zf201 which are homologues of the murine krox-20 gene. The N-terminal of the longest cDNA (zf201) contains two acidic regions identical to those of the murine krox-20. This indicates that the functional organisation of these proteins is probably conserved. Northern Blot analysis identified a single transcript of 2.0 kb. Wholemount in situ hybridisation established that expression of the zebrafish gene (krx-20) first appears at 100% epiboly as a single anterior domain of the prospective neuroepithelium, followed very soon after by a second more posterior domain. The alternating pattern of expression of this gene in rhombomeres(r) r3 and r5 is apparent by 12 hr post-fertilisation, that is prior to the morphological appearance of the rhombomeres. Around 14 hr neural crest migration begins from the dorsal surface of r5, moving caudally into r6 and then ventrally towards the pharyngeal arches. Crest migration is not apparent at or after 16 hr. No neural crest migration was observed from r3. Expression of krx-20 is down regulated firstly in r3 around 26 hr and later in r5 around 30 hr.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chakrabarti S., Streisinger G., Singer F., Walker C. Frequency of gamma-Ray Induced Specific Locus and Recessive Lethal Mutations in Mature Germ Cells of the Zebrafish, BRACHYDANIO RERIO. Genetics. 1983 Jan;103(1):109–123. doi: 10.1093/genetics/103.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Janssen-Timmen U., Mattéi M. G., Zerial M., Bravo R., Charnay P. Structure, chromosome location, and expression of the mouse zinc finger gene Krox-20: multiple gene products and coregulation with the proto-oncogene c-fos. Mol Cell Biol. 1989 Feb;9(2):787–797. doi: 10.1128/mcb.9.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Vesque C., Galliot B., Vigneron M., Dollé P., Duboule D., Charnay P. The segment-specific gene Krox-20 encodes a transcription factor with binding sites in the promoter region of the Hox-1.4 gene. EMBO J. 1990 Apr;9(4):1209–1218. doi: 10.1002/j.1460-2075.1990.tb08228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Zerial M., Lemaire P., Almendral J., Bravo R., Charnay P. A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J. 1988 Jan;7(1):29–35. doi: 10.1002/j.1460-2075.1988.tb02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B. A., Lau L. F., Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with "zinc finger" sequences. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly G., Le Douarin N. M. Head morphogenesis in embryonic avian chimeras: evidence for a segmental pattern in the ectoderm corresponding to the neuromeres. Development. 1990 Apr;108(4):543–558. doi: 10.1242/dev.108.4.543. [DOI] [PubMed] [Google Scholar]
- Couly G., Le Douarin N. M. Head morphogenesis in embryonic avian chimeras: evidence for a segmental pattern in the ectoderm corresponding to the neuromeres. Development. 1990 Apr;108(4):543–558. doi: 10.1242/dev.108.4.543. [DOI] [PubMed] [Google Scholar]
- Crosby S. D., Puetz J. J., Simburger K. S., Fahrner T. J., Milbrandt J. The early response gene NGFI-C encodes a zinc finger transcriptional activator and is a member of the GCGGGGGCG (GSG) element-binding protein family. Mol Cell Biol. 1991 Aug;11(8):3835–3841. doi: 10.1128/mcb.11.8.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp P., Nüsslein-Volhard C., Hopkins N. High-frequency germ-line transmission of plasmid DNA sequences injected into fertilized zebrafish eggs. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7953–7957. doi: 10.1073/pnas.88.18.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen J. S. Developmental neurobiology of the zebrafish. J Neurosci. 1991 Feb;11(2):311–317. doi: 10.1523/JNEUROSCI.11-02-00311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser S., Keynes R., Lumsden A. Segmentation in the chick embryo hindbrain is defined by cell lineage restrictions. Nature. 1990 Mar 29;344(6265):431–435. doi: 10.1038/344431a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Ripoll P., Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol. 1973 Oct 24;245(147):251–253. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J., Heitman J., Hall M. N. Nuclear protein localization. Biochim Biophys Acta. 1991 Mar 7;1071(1):83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Gilardi P., Schneider-Maunoury S., Charnay P. Krox-20: a candidate gene for the regulation of pattern formation in the hindbrain. Biochimie. 1991 Jan;73(1):85–91. doi: 10.1016/0300-9084(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Grunwald D. J., Kimmel C. B., Westerfield M., Walker C., Streisinger G. A neural degeneration mutation that spares primary neurons in the zebrafish. Dev Biol. 1988 Mar;126(1):115–128. doi: 10.1016/0012-1606(88)90245-x. [DOI] [PubMed] [Google Scholar]
- Hunt P., Wilkinson D., Krumlauf R. Patterning the vertebrate head: murine Hox 2 genes mark distinct subpopulations of premigratory and migrating cranial neural crest. Development. 1991 May;112(1):43–50. doi: 10.1242/dev.112.1.43. [DOI] [PubMed] [Google Scholar]
- Joseph L. J., Le Beau M. M., Jamieson G. A., Jr, Acharya S., Shows T. B., Rowley J. D., Sukhatme V. P. Molecular cloning, sequencing, and mapping of EGR2, a human early growth response gene encoding a protein with "zinc-binding finger" structure. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7164–7168. doi: 10.1073/pnas.85.19.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B. Genetics and early development of zebrafish. Trends Genet. 1989 Aug;5(8):283–288. doi: 10.1016/0168-9525(89)90103-0. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Kane D. A., Walker C., Warga R. M., Rothman M. B. A mutation that changes cell movement and cell fate in the zebrafish embryo. Nature. 1989 Jan 26;337(6205):358–362. doi: 10.1038/337358a0. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Schilling T. F., Hatta K. Patterning of body segments of the zebrafish embryo. Curr Top Dev Biol. 1991;25:77–110. doi: 10.1016/s0070-2153(08)60412-3. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Warga R. M., Schilling T. F. Origin and organization of the zebrafish fate map. Development. 1990 Apr;108(4):581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani S. C., Kirby M. L. Initial migration and distribution of the cardiac neural crest in the avian embryo: an introduction to the concept of the circumpharyngeal crest. Am J Anat. 1991 Jul;191(3):215–227. doi: 10.1002/aja.1001910302. [DOI] [PubMed] [Google Scholar]
- Langille R. M., Hall B. K. Role of the neural crest in development of the cartilaginous cranial and visceral skeleton of the medaka, Oryzias latipes (Teleostei). Anat Embryol (Berl) 1988;177(4):297–305. doi: 10.1007/BF00315836. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P. On the role of the engrailed+ gene in the internal organs of Drosophila. EMBO J. 1984 Dec 1;3(12):2839–2844. doi: 10.1002/j.1460-2075.1984.tb02217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G. Further studies of the engrailed phenotype in Drosophila. EMBO J. 1982;1(7):827–833. doi: 10.1002/j.1460-2075.1982.tb01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P., Revelant O., Bravo R., Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R. W., Varnum B. C., Herschman H. R. Cloning of tetradecanoyl phorbol ester-induced 'primary response' sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene. 1987;1(3):263–270. [PubMed] [Google Scholar]
- Lorens J. B. Rapid and reliable cloning of PCR products. PCR Methods Appl. 1991 Nov;1(2):140–141. doi: 10.1101/gr.1.2.140. [DOI] [PubMed] [Google Scholar]
- Lumsden A., Guthrie S. Alternating patterns of cell surface properties and neural crest cell migration during segmentation of the chick hindbrain. Dev Suppl. 1991;Suppl 2:9–15. [PubMed] [Google Scholar]
- Lumsden A., Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989 Feb 2;337(6206):424–428. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Lumsden A., Sprawson N., Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991 Dec;113(4):1281–1291. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Mével-Ninio M., Terracol R., Kafatos F. C. The ovo gene of Drosophila encodes a zinc finger protein required for female germ line development. EMBO J. 1991 Aug;10(8):2259–2266. doi: 10.1002/j.1460-2075.1991.tb07762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M. A., Bradley L. C., Wilkinson D. G. Conserved segmental expression of Krox-20 in the vertebrate hindbrain and its relationship to lineage restriction. Dev Suppl. 1991;Suppl 2:59–62. [PubMed] [Google Scholar]
- Noden D. M. Patterning of avian craniofacial muscles. Dev Biol. 1986 Aug;116(2):347–356. doi: 10.1016/0012-1606(86)90138-7. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Martin-Blanco E., Coleman K. G., Poole S. J., Ellis M. C., Kornberg T. B., Goodman C. S. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989 Sep 8;58(5):955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Patwardhan S., Gashler A., Siegel M. G., Chang L. C., Joseph L. J., Shows T. B., Le Beau M. M., Sukhatme V. P. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991 Jun;6(6):917–928. [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Streisinger G., Walker C., Dower N., Knauber D., Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature. 1981 May 28;291(5813):293–296. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Vielkind J. R., McMurray J. V., Westerfield M. Stable lines of transgenic zebrafish exhibit reproducible patterns of transgene expression. Development. 1990 Jul;109(3):577–584. doi: 10.1242/dev.109.3.577. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Vesque C., Charnay P. Mapping functional regions of the segment-specific transcription factor Krox-20. Nucleic Acids Res. 1992 May 25;20(10):2485–2492. doi: 10.1093/nar/20.10.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Colot H. V., Rosbash M. Sequence and structure of the serendipity locus of Drosophila melanogaster. A densely transcribed region including a blastoderm-specific gene. J Mol Biol. 1985 Nov 5;186(1):149–166. doi: 10.1016/0022-2836(85)90265-7. [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Nusslein-Volhard C., Kluding H. Krüppel, a gene whose activity is required early in the zygotic genome for normal embryonic segmentation. Dev Biol. 1984 Jul;104(1):172–186. doi: 10.1016/0012-1606(84)90046-0. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Chavrier P., Bravo R., Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989 Feb 2;337(6206):461–464. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., Cook M., Boncinelli E., Krumlauf R. Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature. 1989 Oct 5;341(6241):405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]