Abstract
RNAse P cleaves pre-tRNAs to liberate 5'-flanks and 5'-matured, 5'-phosphorylated tRNAs. It is not evident if the 2'-hydroxyls of the ribose moieties in the substrate are involved in the reaction. To study their influence in two different pre-tRNAs, we have modified specifically the 2'-hydroxyl groups at the cleavage site and in neighbouring positions. We have shown that these hydroxyls are important but not essential for the processing of these substrates by E. coli RNase P RNA (M1 RNA). The reduction in the catalytic efficiency was moderate for 2'-deoxy and severe for 2'-methoxy substitutions at the cleavage site. Additional effects of modifications in neighbouring positions were smaller. Based on our data we suggest that the modifications do not interfere with binding of the substrate, whereas they prevent an optimal steric arrangement for the hydrolysis reaction.
Full text
PDF
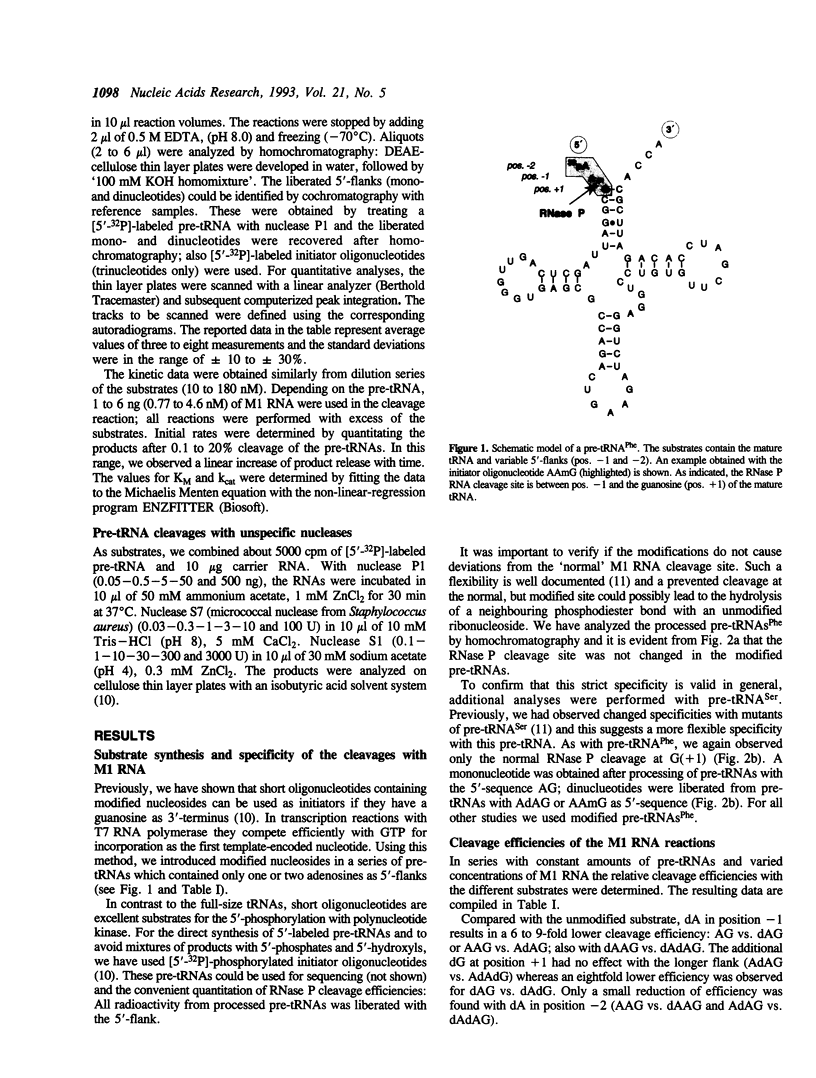
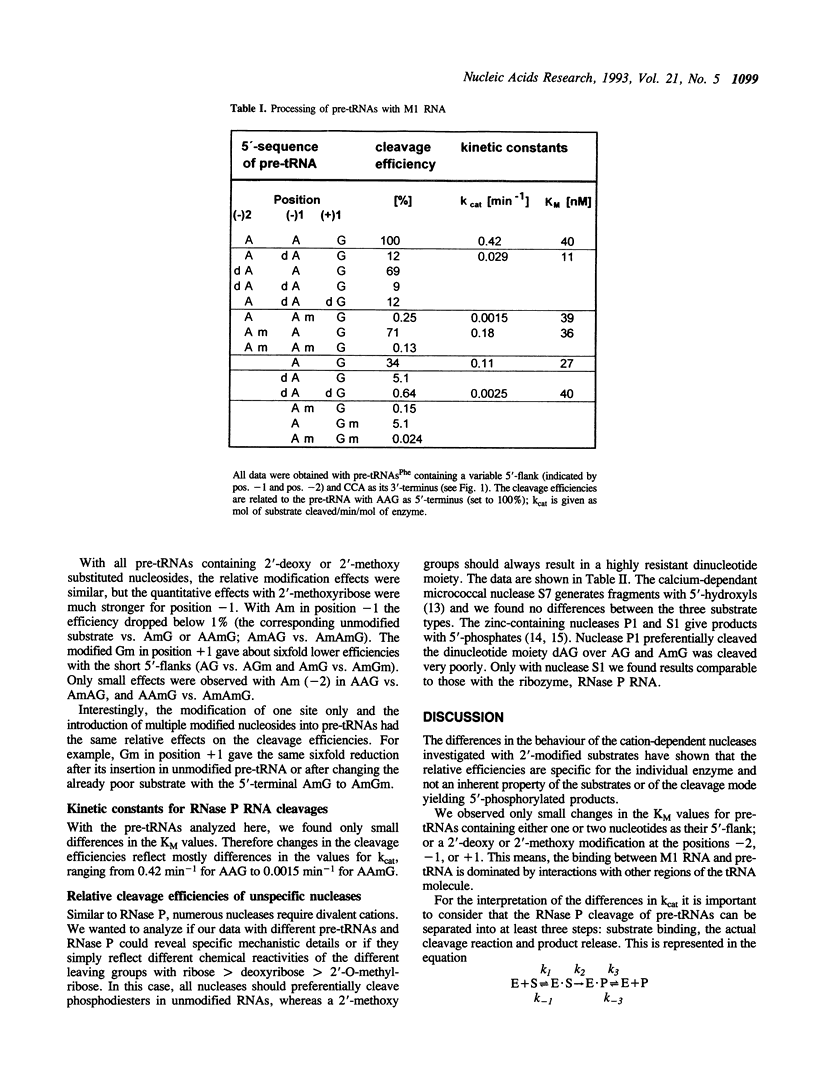


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S. Ribonuclease P. Postscript. J Biol Chem. 1990 Nov 25;265(33):20053–20056. [PubMed] [Google Scholar]
- Bass B. L., Cech T. R. Ribozyme inhibitors: deoxyguanosine and dideoxyguanosine are competitive inhibitors of self-splicing of the Tetrahymena ribosomal ribonucleic acid precursor. Biochemistry. 1986 Aug 12;25(16):4473–4477. doi: 10.1021/bi00364a001. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Staphylococcal nuclease: size and specificity of the active site. Science. 1968 Dec 27;162(3861):1491–1493. doi: 10.1126/science.162.3861.1491. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Altman S. External guide sequences for an RNA enzyme. Science. 1990 Aug 17;249(4970):783–786. doi: 10.1126/science.1697102. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Haydock K., Allen L., Altman S. Metal ion requirements and other aspects of the reaction catalyzed by M1 RNA, the RNA subunit of ribonuclease P from Escherichia coli. Biochemistry. 1986 Apr 8;25(7):1509–1515. doi: 10.1021/bi00355a006. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Lumelsky N., Altman S. Specific interactions in RNA enzyme-substrate complexes. Science. 1989 Dec 22;246(4937):1578–1584. doi: 10.1126/science.2480641. [DOI] [PubMed] [Google Scholar]
- Haydock K., Allen L. C. Molecular mechanism of catalysis by RNA. Prog Clin Biol Res. 1985;172A:87–98. [PubMed] [Google Scholar]
- Kahle D., Wehmeyer U., Char S., Krupp G. The methylation of one specific guanosine in a pre-tRNA prevents cleavage by RNase P and by the catalytic M1 RNA. Nucleic Acids Res. 1990 Feb 25;18(4):837–844. doi: 10.1093/nar/18.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai G., Yamamoto Y., Kamimura T., Masegi T., Sekine M., Hata T., Iimori T., Watanabe T., Miyazawa T., Yokoyama S. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2'-hydroxyl group. Biochemistry. 1992 Feb 4;31(4):1040–1046. doi: 10.1021/bi00119a012. [DOI] [PubMed] [Google Scholar]
- Krupp G., Kahle D., Vogt T., Char S. Sequence changes in both flanking sequences of a pre-tRNA influence the cleavage specificity of RNase P. J Mol Biol. 1991 Feb 20;217(4):637–648. doi: 10.1016/0022-2836(91)90522-8. [DOI] [PubMed] [Google Scholar]
- McClain W. H., Guerrier-Takada C., Altman S. Model substrates for an RNA enzyme. Science. 1987 Oct 23;238(4826):527–530. doi: 10.1126/science.2443980. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Smith D. Ribonuclease P: function and variation. J Biol Chem. 1990 Mar 5;265(7):3587–3590. [PubMed] [Google Scholar]
- Paolella G., Sproat B. S., Lamond A. I. Nuclease resistant ribozymes with high catalytic activity. EMBO J. 1992 May;11(5):1913–1919. doi: 10.1002/j.1460-2075.1992.tb05244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault J. P., Altman S. Important 2'-hydroxyl groups in model substrates for M1 RNA, the catalytic RNA subunit of RNase P from Escherichia coli. J Mol Biol. 1992 Jul 20;226(2):399–409. doi: 10.1016/0022-2836(92)90955-j. [DOI] [PubMed] [Google Scholar]
- Pitulle C., Kleineidam R. G., Sproat B., Krupp G. Initiator oligonucleotides for the combination of chemical and enzymatic RNA synthesis. Gene. 1992 Mar 1;112(1):101–105. doi: 10.1016/0378-1119(92)90309-d. [DOI] [PubMed] [Google Scholar]
- Reich C., Olsen G. J., Pace B., Pace N. R. Role of the protein moiety of ribonuclease P, a ribonucleoprotein enzyme. Science. 1988 Jan 8;239(4836):178–181. doi: 10.1126/science.3122322. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D., Burgin A. B., Haas E. S., Pace N. R. Influence of metal ions on the ribonuclease P reaction. Distinguishing substrate binding from catalysis. J Biol Chem. 1992 Feb 5;267(4):2429–2436. [PubMed] [Google Scholar]
- Vioque A., Arnez J., Altman S. Protein-RNA interactions in the RNase P holoenzyme from Escherichia coli. J Mol Biol. 1988 Aug 20;202(4):835–848. doi: 10.1016/0022-2836(88)90562-1. [DOI] [PubMed] [Google Scholar]
- Volbeda A., Lahm A., Sakiyama F., Suck D. Crystal structure of Penicillium citrinum P1 nuclease at 2.8 A resolution. EMBO J. 1991 Jul;10(7):1607–1618. doi: 10.1002/j.1460-2075.1991.tb07683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer F. H. Polyribonucleic acids as enzymes. Nature. 1986 Feb 13;319(6054):534–535. doi: 10.1038/319534a0. [DOI] [PubMed] [Google Scholar]




